Simple Summary
Colorectal cancer is a leading cause of cancer-related death in the United States. Liquid biopsies, such as the detection of circulating tumor DNA (ctDNA), have been investigated as a biomarker for patients with colorectal cancer in terms of prognosis and recurrence, as well as their use to guide therapy. CtDNA may provide additional information to assist in the decision for the administration of chemotherapy after surgery as well as the early detection of cancer recurrence. However, further information is needed in order to fully utilize ctDNA as a therapeutic biomarker.
Abstract
Although the incidence of colorectal cancer (CRC) has decreased as a result of increased screening and awareness, it still remains a major cause of cancer-related death. Additionally, early detection of CRC recurrence by conventional means such as CT, endoscopy, and CEA has not translated into an improvement in survival. Liquid biopsies, such as the detection circulating tumor DNA (ctDNA), have been investigated as a biomarker for patients with CRC in terms of prognosis and recurrence, as well as their use to guide therapy. In this manuscript, we provide an overview of ctDNA as well as its utility in providing prognostic information, using it to guide therapy, and monitoring for recurrence in patients with CRC. In addition, we discuss the influence the site of disease may have on the ability to detect ctDNA in patients with metastatic CRC.
1. Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer and the second leading cause of cancer-related death in the United States (US), with over 100,000 cases of colon cancer and 45,000 cases of rectal cancer diagnosed annually [1]. As a result of increased screening efforts, the overall incidence of CRC has decreased. However, in patients less than 50 years old, the incidence of CRC has increased [1,2]. Metastatic disease is a leading cause of death in patients with CRC, and despite appropriate screening, 50–60% of patients eventually develop metastatic disease [3,4,5]. Additionally, 20 to 30% of patients present with synchronous liver metastases and 17% with peritoneal metastases (PM) [6,7,8,9]. Adjuvant chemotherapy has been demonstrated to improve disease-free survival (DFS) and overall survival (OS) in patients with localized resected CRC, but not all patients have been shown to benefit from adjuvant therapy [10,11]. In addition, there has been minimal evidence that intensive surveillance with clinical and endoscopic exams improved OS [12,13].
Many questions remain about the proper selection of patients and therapy so as to not overtreat patients at low risk of recurrence or undertreat those who are at a high recurrence risk. Biomarkers are of interest in many malignancies, including CRC, to offer prognostic information and help guide therapy [14,15]. For example, alfa-fetoprotein (AFP) has been used as a biomarker for hepatocellular carcinoma and is associated with aggressive tumor biology and high risk of tumor recurrence after potentially curative treatment such as liver transplantation [16]. Liquid biopsies, such as the detection of circulating tumor DNA (ctDNA), have been investigated as a biomarker for patients with CRC in terms of prognosis and recurrence, as well as their use to guide therapy. Herein, we provide an overview of ctDNA, as well as its utility in providing prognostic information, using it to guide therapy, and monitoring for recurrence. In addition, we discuss the influence the site of disease may have on the ability to detect ctDNA in patients with metastatic CRC.
2. Overview of Liquid Biopsies: ctDNA
Liquid biopsies involve the detection of tumor-derived biomarkers in patient fluid samples such as blood, urine, saliva, stool, cerebrospinal fluid, or bile. CtDNA or RNA, circulating tumor cells (CTC), and tumor derived exosomes, cytokines, and proteins are all of interest as liquid biopsy biomarkers [17,18] (Figure 1). Liquid biopsies can often be performed serially during a patient’s disease course and used to interpret disease biology, as well as changes in tumor biology and assess tumor response to therapy [18,19,20,21,22]. CtDNA is but a small fraction of circulating cell-free DNA (cfDNA) [23]. CtDNA, unlike cfDNA, is considered to be tumor-specific [24]. The ratio between ctDNA and cfDNA can greatly vary anywhere between 1 and 40% due to the characteristics of the primary tumor as well as the presence and location of metastatic disease [17,20,25].

Figure 1.
Utility of circulating tumor DNA in the treatment of colorectal cancer.
Liquid biopsies and biomarkers, such as ctDNA, are gaining significant interest in the management of patients with colorectal cancer in terms of screening, prognosis, treatment monitoring, and treatment guidance. Different techniques for the detection of ctDNA include real-time quantitative polymerase chain reaction (qPCR), digital droplet PCR (ddPCR), and next-generation sequencing (NGS).
Multiple techniques, such as real-time quantitative polymerase chain reaction (qPCR), digital droplet PCR (ddPCR), and next-generation sequencing (NGS), have been utilized for the detection and analysis of ctDNA [26]. The detection of ctDNA can either be targeted or untargeted, while DNA may enter the bloodstream through both active and passive mechanisms [27,28,29,30]. The targeted approach for ctDNA detection identifies previously determined genetic mutations, while an untargeted approach does not require prior knowledge of underlying genetic alterations [27]. Kloten et al. identified KRAS mutations in patients with CRC using Intplex PCR and ctDNA analysis, which demonstrated a 70% specificity and 50% sensitivity when compared to serum samples, with the tissue samples having a concordance of 66% [31]. Tumor DNA may enter the bloodstream due to migration from the primary tumor or metastatic site by tumor invasion, shedding, or mechanical stress [29,30]. Passive mechanisms include the release of DNA remnants through apoptosis and necrosis. Meanwhile, active mechanisms are not completely understood but may be due to the excretion of DNA through micro-vesicles containing fragments of ctDNA [29].
CfDNA has been found to be significantly higher in cancer patients as compared to healthy individuals, but serum levels may be influenced by a variety of factors [24]. Compared to healthy controls, CRC patients have higher levels of mutated DNA in their bloodstream as well as higher levels compared to other solid organ malignancies [32,33,34]. Additionally, ctDNA detection is also influenced by the stage of the disease. CtDNA has been shown to be detectable in approximately 46% of patients with stage I disease, 73% of patients with stages II–III, and 90% of patients with metastatic CRC [32,35]. As a result, there is considerable interest in utilizing ctDNA as a screening method for CRC in patients who are not willing to undergo colonoscopy [36]. On the other hand, carcinoembryonic antigen (CEA) has been the conventional tumor marker for evaluating patients with CRC for recurrence and therapeutic efficacy. Osumi et al. evaluated 110 patients with metastatic CRC undergoing chemotherapy to evaluate the correlation between ctDNA and CEA. The overall concordance rate between the ctDNA and CEA levels was 75.5% (83/110). Additionally, the correlation coefficient between ctDNA and CEA levels was lower in patients without liver and lymph node metastases (r = 0.18, p = 0.44) than in patients with liver metastasis (r = 0.48, p < 0.0001) [37].
3. Influence of Site of Metastasis on ctDNA
A majority of patients with CRC eventually develop metastatic disease. The influence of the site of metastatic disease has been investigated by its correlation with ctDNA. Bando et al. investigated the relationship between the site of metastasis and ctDNA in patients with single-organ CRC metastasis as part of the SCRUM-Japan GOZILA study. Of the 1187 patients with metastatic CRC enrolled in GOZILA, 138 patients were studied: 49 with liver metastasis, 15 with lymph node metastasis, 27 with peritoneal metastasis (PM), and 47 with lung metastasis. In this study, patients with lung metastases and PMs had significantly lower levels of ctDNA [38]. Similarly, in a retrospective analysis, Sullivan et al. identified patients with stage II-IV GI cancers treated at a single institution between 2015 and 2020 with available ctDNA results. PMs were associated with lower ctDNA levels independent of the primary tumor site (PM only: 12.1%; PM with visceral metastases: 26.8%; and visceral metastases only: 35.0%; p < 0.01) [39]. The authors hypothesized that PMs were associated with lower ctDNA levels than other metastatic sites due to the plasma-peritoneal barrier. Xue et al. performed a systematic review on the utility of ctDNA in CRC PM, including eight studies with 167 patients. The authors not only found that ctDNA can be isolated from both plasma and peritoneal fluid, but peritoneal fluid had higher mutation detection rates and was preferred for liquid biopsy [40]. Furthermore, as compared to patients with CRC PM, patients with CRC liver metastases have higher detectable ctDNA. Lee et al. investigated patients who underwent metastasectomies for metastatic CRC, where the detection rate of ctDNA was higher in patients with liver metastasis and tumors measuring ≥1 cm [41]. Therefore, more information is required to understand the utilization of ctDNA in the treatment of metastatic CRC, as different metastatic disease sites may influence ctDNA levels.
4. Prognostic Impact of ctDNA
The ability to provide prognostic information on recurrence and survival has been the backbone of cancer care. For example, in patients with melanoma, the use of a sentinel lymph node biopsy does not offer therapeutic benefits to patients but rather provides proper staging and diagnostic information to identify patients at high risk of recurrence where additional therapies may offer survival benefit [42,43]. Post-operative detection of ctDNA in patients with CRC has been found to be an independent marker of disease recurrence. In addition, compared to CT and CEA, ctDNA has a lead time of approximately 10 months [44]. In patients with stages I to III CRC, ctDNA detection 30 days post-operatively was associated with a higher likelihood of recurrence than patients who did not have detectable ctDNA (HR 72; 95% CI, 2.7–19.0, p < 0.001) [44]. Similarly, 150 patients with localized colon cancer were prospectively evaluated for ctDNA via NGS following surgery [45]. Again, detection of ctDNA was associated with poor DFS (HR, 17.56; log rank p = 0.0014). Furthermore, data indicate that patients with positive ctDNA prior to surgery not only have a higher risk of recurrence but also a shorter time to recurrence [46]. In addition to patients with positive ctDNA having worse outcomes after surgical resection, similar results are seen in patients with positive ctDNA after adjuvant chemotherapy. Reinert et al. found patients with positive ctDNA after adjuvant chemotherapy were 17 times more likely to have disease recurrence (HR 17.5; 95% CI, 54–56.5; p < 0.001). Additionally, ctDNA analysis found disease recurrence up to 16.5 months ahead of standard of care radiologic imaging (mean 8.7 months; range: 0.8–16.5 months) [47].
The use of ctDNA was also studied in patients with locally advanced rectal cancer. In 159 patients with locally advanced rectal cancer, Tie et al. measured plasma ctDNA pre-treatment, after chemoradiation, and after surgical resection. The three-year recurrence-free survival (RFS) was 33% for patients with detectable ctDNA vs. 87% for patients with no detectable ctDNA after surgery [48]. In a study by Vidal et al. of patients with locally advanced rectal cancer treated with fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) with or without aflibercept, followed by chemoradiation and surgery, ctDNA was detectable in 83% of patients at baseline and in 15% following total neoadjuvant therapy (TNT). There was found to be no association between ctDNA status and the pathologic response to TNT. However, detectable pre-operative ctDNA was significantly associated with systemic recurrence, shorter DFS (HR, 4; p = 0.033), and shorter OS (HR, 23; p < 0.0001) [49].
Kotani et al. reported data from the GALAXY study, which is the observational arm of the ongoing CIRCULATE-Japan study (UMIN000039205) that analyzed presurgical and postsurgical ctDNA in patients with stage II–IV resectable CRC. In this study, postoperative ctDNA positivity was associated with higher recurrence risk (HR 10.0, p < 0.0001) and was the most significant prognostic factor associated with recurrence risk in patients with stage II or III CRC (HR 10.82, p < 0.001). Additionally, postoperative positive ctDNA identified patients with stage II or III CRC who received benefit from adjuvant chemotherapy (HR 6.59, p < 0.0001) [50]. Similarly, Jones et al. performed a meta-analysis of 28 studies analyzing ctDNA in stage IV CRC patients, finding a strong correlation between measurable ctDNA after treatment with OS (HR 2.2, 95% CI 1.79–2.69, p < 0.00001) and progression-free survival (PFS) (HR 3.15, 95% CI 2.10–4.73, p < 0.00001). Furthermore, in patients with resectable disease treated with curative intent, detection of ctDNA offered a lead time of 10 months over radiological recurrence [51]. In patients with metastatic disease receiving chemotherapy, there was a high correlation between the ctDNA response and median survival [52]. In patients with metastatic CRC, Jia et al. examined serial changes of ctDNA in patients receiving first-line chemotherapy found that ctDNA reductions as early as prior to cycle 2 predicted responses after cycle 4 [53].
The treatment of metastatic CRC has evolved, with patients undergoing locoregional therapies for metastatic disease. Liver transplantation is now an accepted treatment strategy in well-selected patients with liver-confined, unresectable CRC liver metastasis. The utility of ctDNA in this population was assessed before (4 patients) and after transplant (6 patients). They found that four patients were negative for ctDNA following transplant, and two patients had persistently positive ctDNA after transplant. Three of four patients with positive ctDNA before transplant are ctDNA negative after transplant [54]. However, more studies are required to investigate ctDNA in the setting of liver transplantation.
Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is a therapeutic option for select patients with CRC PM. Beagan et al. explored the possibility of utilizing ctDNA to assist in selecting patients for CRS/HIPEC. Thirty patients eligible for CRS/HIPEC provided blood samples preoperatively and during follow-up if the procedure was completed. CtDNA was detected preoperatively in cfDNA samples from 33% of patients and was associated with a reduced disease-free survival (DFS) after CRS/HIPEC (median 6.0 months vs. median not reached, p = 0.016) [55]. Similarly, Dhiman et al. evaluated patients with CRC or appendiceal cancer with PM who underwent CRS/HIPEC and had post-resection ctDNA monitoring. One hundred thirty serial post-resection ctDNA assessments were performed in 33 patients who underwent complete CRS. Of the 19 patients with rising ctDNA levels, 90% recurred vs. 21% in the stable ctDNA group (n = 14, p < 0.001). Median DFS in the rising ctDNA cohort was 11 months and not reached in the stable group (p = 0.01). A rising ctDNA level was the most significant factor associated with DFS (HR: 3.67, 95% CI, 1.06–12.66, p = 0.03) [56].
5. Application of ctDNA to Guide Treatment
Traditionally, patients with stage II colon cancer do not receive adjuvant chemotherapy, while the vast majority of patients with stage III disease are recommended to receive three to six months of adjuvant chemotherapy [57]. Several studies have been conducted investigating the role of ctDNA to guide adjuvant treatment. The DYNAMIC study evaluated 455 patients with a ctDNA-guided approach to adjuvant chemotherapy. Patients with stage II CRC were randomly assigned to have treatment decisions guided by either ctDNA results or standard of care. In patients assigned to the ctDNA-guided arm, adjuvant chemotherapy was administered to patients with positive ctDNA 4 or 7 weeks after surgery. As a result, a lower percentage of patients in the ctDNA-guided arm received adjuvant chemotherapy. In addition, at 2-year follow-up, the RFS in the ctDNA-guided arm was non-inferior to the standard administration of adjuvant chemotherapy [58]. Furthermore, cost–utility analysis found a ctDNA-guided strategy increased quality-adjusted life-years and was shown to be a potentially cost-effective strategy towards reducing overtreatment in stage II colorectal cancer [59].
Traditional biopsies provide a static analysis on a tumor at a given time and location rather than providing information of tumor heterogeneity and changes occurring over time. In addition, there can be differences between the primary tumor and metastatic lesions, as tumors are often comprised of different clones [60,61]. CtDNA sequencing has allowed for repeated tumor mutational profiling through a noninvasive means. In a study by Kim et al., new mutations in samples from patients with progressive CRC were seen in 49.6% of treatments [62]. Additionally, patients with tumors that were RAS/BRAF wild-type were more likely to develop mutations causing progressive disease independent of anti-EGFR treatment with cetuximab [62].
Chemotherapy with anti-EGFR agents is standard first-line therapy in RAS-wild-type metastatic CRC. After treatment, the potential for conversion of RAS mutational status from RAS mutant to RAS wild-type has emerged, thus the concept of ‘NeoRAS wild-type’ [63]. RAS/BRAF mutations have been found to be associated with disease resistance detected after therapy, and an increase in RAS/BRAF ctDNA was associated with a shorter PFS [64]. Other studies have investigated the re-determination of the RAS mutational status in ctDNA at disease progression in RAS mutant metastatic CRC. The most commonly detected actionable mutations in “neo-RAS wild-type” were: PIK3CA (35.7%), RET (11.9%), IDH1 (9.5%), KIT (7%), EGFR (7%), MET (4.7%), ERBB2 (4.7%), and FGFR3(4.7%). OS and PFS were longer in patients with “neo-RAS wild-type” compared to those who remained RAS mutant [65]. Ciardiello et al. performed a further analysis of the CAVE and VELO studies, evaluating the percentage of patients with wild-type ctDNA tumors and the association of mutational status with time since the last anti-EGFR drug administration. The authors found no difference in the proportion of patients whose baseline plasma ctDNA was RAS/BRAF wild-type or mutated between 4 and 18 months since the last administration of anti-EGFR drugs. However, 38/44 patients with an anti-EGFR drug-free interval of 18 months or more had a ctDNA RAS/BRAF wild-type status, which supports the role of ctDNA assessment in improving treatment efficacy as the length of the anti-EGFR free interval is not sufficient for patient selection for further treatment [66]. Further investigation of RAS status in ctDNA as a useful biomarker of EGFR inhibitors for NeoRAS wild-type metastatic CRC is being explored (C-PROWESS trial) [63].
6. Treatment Monitoring
Detection of ctDNA may offer prognostic information in patients with CRC and identify patients at high risk for disease recurrence. Early detection of CRC recurrence by conventional means such as CT, endoscopy, and CEA has not translated into an improvement in survival [12,13]. CtDNA has been studied to investigate the recurrence of CRC and has been found to be more frequently positive than CEA (85% vs. 41%; p = 0.002) in CRC patients with disease recurrence seen on imaging. In addition, the time between ctDNA detection and radiologic disease recurrence has been found to be significantly shorter for ctDNA. This is also true when comparing ctDNA and CEA, leading to earlier detection (61 vs. 167 days, p = 0.04; ctDNA vs. CEA, respectively) [67]. Another study demonstrated that the lead time of ctDNA is approximately 8 months as compared to CEA [13]. Additionally, serial liquid biopsies allow response prediction prior to that obtained by conventional methods in patients with metastatic disease undergoing chemotherapy [68] (Figure 2). In a study by Zou et al., ctDNA was compared to CEA for the assessment of chemotherapy response in patients with metastatic CRC. In the study of thirty patients, 29 (97%) had detectable ctDNA, compared to 25 patients (83%) who were CEA positive. CtDNA not only predicted significantly more disease progression than CEA (16 (80%) vs. 6 (30%), respectively; p = 0.004) but also the rise in ctDNA occurred significantly earlier than CEA (p = 0.046) [69].
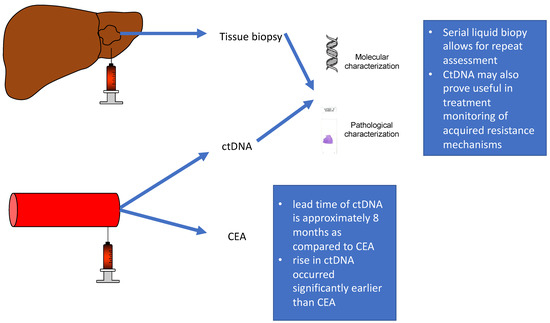
Figure 2.
Drawing comparing tissue biopsy and serum liquid biopsy, ctDNA.
Serial liquid biopsies allow response prediction prior to that obtained by conventional methods in patients with metastatic disease undergoing chemotherapy. CtDNA may also prove useful in treatment monitoring of acquired resistance mechanisms and thus change treatment strategy.
CtDNA may also prove useful in treatment monitoring of acquired resistance mechanisms, combinatorial strategies to delay resistance, and the potential for anti-EGFR rechallenge as acquired mutations appear as multiple concurrent subclonal alterations [70]. Several studies have demonstrated that repeated determination of mutational status in RAS/BRAF wild-type patients or patients who acquired resistance during the course of treatment had improvements in PFS and OS [71,72]. Nakamura et al. conducted a phase 2 trial to evaluate pertuzumab plus trastuzumab for metastatic CRC with human epidermal growth factor receptor 2 (HER2) amplification confirmed by tumor tissue biopsy or ctDNA. The authors found that decreased ctDNA three weeks after treatment was associated with a therapeutic response. Additionally, duel Her-2 blockade with pertuzumab and trastuzumab demonstrated similar efficacy with HER2 amplification in tissue or ctDNA, demonstrating that ctDNA genotyping can identify patients who benefit from dual-HER2 blockade as well as monitor treatment response [73].
The current NCCN guidelines highlight that ctDNA testing is not without drawbacks, where a positive result without evidence of disease is only helpful if the patient has therapeutic options with a reasonable chance to eradicate disease. Early knowledge of cancer recurrences without effective interventions could lead to significant distress. More details are needed on the timing of the assay and the value quantification of ctDNA in ongoing studies.
7. Future Directions
CtDNA has emerged as a useful adjunct in the treatment and screening of patients with CRC. However, the full extent of its utility has not been fully elucidated, with potential for a wider array of its use in guiding prognosis and therapeutic interventions. For example, a rise in ctDNA has been shown to precede radiologic recurrence or an increase in CEA in patients off therapy undergoing surveillance. Yet, currently, there are no high-level data to justify restarting treatment, such as chemotherapy, based on a rise in ctDNA alone. This may prove beneficial in treating patients before gross disease recurrence. However, one must consider potential for overtreatment and the ability to act upon the information. Additionally, as discussed, differences have been found in ctDNA elevation based on recurrence patterns in the lung, liver, and peritoneum. In patients at high risk for peritoneal recurrence, such as patients with perforated tumors or T4 tumors, ctDNA may not be an ideal monitoring modality. Additional studies are required and ongoing for the evaluation of the use of ctDNA in patients with metastatic disease (Table 1).

Table 1.
Ongoing Studies of ctDNA in the Management of Metastatic Colorectal Cancer.
Recently, four molecular subtypes of CRC have been identified, each of which has significant biological differences and thus the potential for subtype-based intervention [74]. The CMS1 subtype makes up 14% of cases and is microsatellite unstable with strong immune activation; CMS2 constitutes 37% of cases with marked WNT and MYC signaling activation; CMS3 (13% of cases) has metabolic dysregulation; and CMS4 (23% of cases) demonstrates prominent transforming growth factor–β (TGF-β) activation with stromal invasion and angiogenesis [74]. However, several limitations exist in transforming these discoveries into clinical practice, including the ability to classify tumors, as classification is reliant on gene expression profiles with associated costs, time, and expertise. A potential solution may be in the subtype-specific liquid biomarkers to classify patients, thus the potential to change therapeutic strategy [75].
8. Conclusions
Circulating tumor DNA has been studied in its application as a biomarker for patients with CRC in terms of prognosis and recurrence, as well as its use to guide therapy. Elevated ctDNA following surgical resection or systemic chemotherapy has been found to be associated with a poor prognosis. The additional use of ctDNA could also lead to an improvement in the distribution of healthcare resources by identifying patients more likely to respond to therapy as well as alternative and specific treatment regimens. CtDNA may prove to be beneficial in guiding adjuvant therapy in patients with a high-risk stage II or low risk stage III disease. We expect the use of ctDNA in CRC to gradually change the management and therapeutic options for eligible patients, and we believe its use will eventually be broadened to cancers of other organ systems.
Author Contributions
Conceptualization, M.K., B.L. and Z.J.B.; resources, M.K., B.L. and Z.J.B.; data curation, M.K., B.L. and Z.J.B.; writing—original draft preparation, M.K., B.L. and Z.J.B.; writing—review and editing, M.K., B.L. and Z.J.B.; visualization, M.K., B.L. and Z.J.B.; supervision, Z.J.B.; project administration, Z.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. 2011, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Yun, S.H.; Chun, H.K.; Lee, W.Y.; Yun, H.R.; Kim, J.; Kim, K.; Shim, Y.M. Pulmonary resection for metastases from colorectal cancer: Prognostic factors and survival. Int. J. Color. Dis. 2007, 22, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Yoo, P.S.; Lopez-Soler, R.I.; Longo, W.E.; Cha, C.H. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin. Color. Cancer 2006, 6, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Muratore, A.; Zorzi, D.; Bouzari, H.; Amisano, M.; Massucco, P.; Sperti, E.; Capussotti, L. Asymptomatic colorectal cancer with un-resectable liver metastases: Immediate colorectal resection or up-front systemic chemotherapy? Ann. Surg. Oncol. 2007, 14, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Inoue, Y.; Komeda, K.; Shimizu, T.; Asakuma, M.; Hirokawa, F.; Miyamoto, Y.; Okuda, J.; Takeshita, A.; Shibayama, Y.; et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010, 10, 27. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Bonnetain, F.; Chibaudel, B.; Lledo, G.; Hickish, T.; Tabernero, J.; Boni, C.; Bachet, J.B.; Teixeira, L.; et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: Subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J. Clin. Oncol. 2012, 30, 3353–3360. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Phelip, J.M.; Cany, L.; Barbier, E.; Manfredi, S.; Deguiral, P.; Faroux, R.; Baconnier, M.; Pezet, D.; Duchmann, J.; et al. 398O Effect of 5 years of imaging and CEA follow-up to detect recurrence of colorectal cancer (CRC)-PRODIGE 13 a FFCD phase III trial. Ann. Oncol. 2020, 31, S410. [Google Scholar] [CrossRef]
- Naidoo, M.; Gibbs, P.; Tie, J. ctDNA and Adjuvant Therapy for Colorectal Cancer: Time to Re-Invent Our Treatment Paradigm. Cancers 2021, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.B.; Brown, Z.J.; Pawlik, T.M. The Role of Biomarkers in the Management of Colorectal Liver Metastases. Cancers 2022, 14, 4602. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Patwardhan, S.; Bean, J.; Pawlik, T.M. Molecular diagnostics and biomarkers in cholangiocarcinoma. Surg. Oncol. 2022, 44, 101851. [Google Scholar] [CrossRef]
- Özdemir, F.; Baskiran, A. The Importance of AFP in Liver Transplantation for HCC. J. Gastrointest. Cancer 2020, 51, 1127–1132. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350. [Google Scholar] [CrossRef]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Vitiello, P.P.; Marsoni, S.; Siena, S.; Tabernero, J.; Trusolino, L.; Bernards, R.; Bardelli, A. Precision oncology in metastatic colorectal cancer-from biology to medicine. Nat. Rev. Clin. Oncol. 2021, 18, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Van Seventer, E.E.; Siravegna, G.; Hartwig, A.V.; Jaimovich, A.; He, Y.; Kanter, K.; Fish, M.G.; Fosbenner, K.D.; Miao, B.; et al. Minimal Residual Disease Detection using a Plasma-only Circulating Tumor DNA Assay in Patients with Colorectal Cancer. Clin. Cancer Res. 2021, 27, 5586–5594. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Beljanski, M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology 1989, 46, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Galarza Fortuna, G.M.; Dvir, K. Circulating tumor DNA: Where are we now? A mini review of the literature. World J. Clin. Oncol. 2020, 11, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore-Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e147. [Google Scholar] [CrossRef] [PubMed]
- Kirchweger, P.; Wundsam, H.V.; Rumpold, H. Circulating tumor DNA for diagnosis, prognosis and treatment of gastrointestinal malignancies. World J. Clin. Oncol. 2022, 13, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Ulz, P.; Geigl, J.B. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Postel, M.; Roosen, A.; Laurent-Puig, P.; Taly, V.; Wang-Renault, S.F. Droplet-based digital PCR and next generation sequencing for monitoring circulating tumor DNA: A cancer diagnostic perspective. Expert Rev. Mol. Diagn. 2018, 18, 7–17. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef]
- Luo, W.; Rao, M.; Qu, J.; Luo, D. Applications of liquid biopsy in lung cancer-diagnosis, prognosis prediction, and disease monitoring. Am. J. Transl. Res. 2018, 10, 3911–3923. [Google Scholar]
- Kloten, V.; Rüchel, N.; Brüchle, N.O.; Gasthaus, J.; Freudenmacher, N.; Steib, F.; Mijnes, J.; Eschenbruch, J.; Binnebösel, M.; Knüchel, R.; et al. Liquid biopsy in colon cancer: Comparison of different circulating DNA extraction systems following absolute quantification of KRAS mutations using Intplex allele-specific PCR. Oncotarget 2017, 8, 86253–86263. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Taniguchi, H.; Ikeda, M.; Bando, H.; Kato, K.; Morizane, C.; Esaki, T.; Komatsu, Y.; Kawamoto, Y.; Takahashi, N.; et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 2020, 26, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Vymetalkova, V.; Cervena, K.; Bartu, L.; Vodicka, P. Circulating Cell-Free DNA and Colorectal Cancer: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3356. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.L.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.S.; et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Geiger, S.; Keil, A.; Bias, H.; Schatz, P.; deVos, T.; Dhein, J.; Zimmermann, M.; Tauber, R.; Wiedenmann, B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Shinozaki, E.; Ooki, A.; Shimozaki, K.; Kamiimabeppu, D.; Nakayama, I.; Wakatsuki, T.; Ogura, M.; Takahari, D.; Chin, K.; et al. Correlation between circulating tumor DNA and carcinoembryonic antigen levels in patients with metastatic colorectal cancer. Cancer Med. 2021, 10, 8820–8828. [Google Scholar] [CrossRef]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; Kagawa, Y.; Denda, T.; Satoh, T.; Yamazaki, K.; et al. Effects of Metastatic Sites on Circulating Tumor DNA in Patients With Metastatic Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100535. [Google Scholar] [CrossRef]
- Sullivan, B.G.; Lo, A.; Yu, J.; Gonda, A.; Dehkordi-Vakil, F.; Dayyani, F.; Senthil, M. Circulating Tumor DNA is Unreliable to Detect Somatic Gene Alterations in Gastrointestinal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2023, 30, 278–284. [Google Scholar] [CrossRef]
- Xue, J.; Prabhakaran, S.; Prabhakaran, S.; Lim, W.M.; Guerra, G.; Heriot, A.; Kong, J.C. The utility of ctDNA in colorectal cancer with peritoneal metastases. ANZ J. Surg. 2023, 93, 506–509. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.S.; Chang, W.J.; Choi, J.Y.; Lim, A.; Kim, B.; Lee, S.B.; Lee, J.W.; Kim, S.H.; Kim, J.; et al. Clinical Implication of Liquid Biopsy in Colorectal Cancer Patients Treated with Metastasectomy. Cancers 2021, 13, 2231. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Schøler, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016, 65, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Benhaim, L.; Bouché, O.; Normand, C.; Didelot, A.; Mulot, C.; Le Corre, D.; Garrigou, S.; Djadi-Prat, J.; Wang-Renault, S.F.; Perez-Toralla, K.; et al. Circulating tumor DNA is a prognostic marker of tumor recurrence in stage II and III colorectal cancer: Multicentric, prospective cohort study (ALGECOLS). Eur. J. Cancer 2021, 159, 24–33. [Google Scholar] [CrossRef]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.; Wu, H.T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Wang, Y.; Li, L.; Christie, M.; Simons, K.; Elsaleh, H.; Kosmider, S.; Wong, R.; Yip, D.; et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut 2019, 68, 663–671. [Google Scholar] [CrossRef]
- Vidal, J.; Casadevall, D.; Bellosillo, B.; Pericay, C.; Garcia-Carbonero, R.; Losa, F.; Layos, L.; Alonso, V.; Capdevila, J.; Gallego, J.; et al. Clinical Impact of Presurgery Circulating Tumor DNA after Total Neoadjuvant Treatment in Locally Advanced Rectal Cancer: A Biomarker Study from the GEMCAD 1402 Trial. Clin. Cancer Res. 2021, 27, 2890–2898. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef]
- Jones, R.P.; Pugh, S.A.; Graham, J.; Primrose, J.N.; Barriuso, J. Circulating tumour DNA as a biomarker in resectable and irresectable stage IV colorectal cancer; a systematic review and meta-analysis. Eur. J. Cancer 2021, 144, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.; Andersen, R.F.; Hansen, T.F.; Jensen, L.H.; Faaborg, L.; Steffensen, K.D.; Thomsen, C.B.; Wen, S.W.C. Early ctDNA response to chemotherapy. A potential surrogate marker for overall survival. Eur. J. Cancer 2021, 149, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Sun, Z.; Gao, X.; Cheng, Y.; Zhou, Y.; Shen, C.; Chen, W.; Wang, X.; Shi, R.; Li, N.; et al. Serial Monitoring of Circulating Tumor DNA in Patients With Metastatic Colorectal Cancer to Predict the Therapeutic Response. Front. Genet. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Fujiki, M.; Hashimoto, K.; Quintini, C.; et al. Liquid Biopsy by ctDNA in Liver Transplantation for Colorectal Cancer Liver Metastasis. J. Gastrointest. Surg. 2023, 27, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. [Google Scholar] [CrossRef]
- Dhiman, A.; Kothary, V.; Witmer, H.D.D.; Bregio, C.; Sood, D.; Ong, C.T.; Polite, B.; Eng, O.S.; Shergill, A.; Turaga, K.K. Role of Tumor-informed Personalized ctDNA Assay in Informing Recurrence in Patients with Peritoneal Metastases from Colorectal and High-grade Appendix Cancer Undergoing Curative Intent Surgery. Ann. Surg. 2023, 278, 925–931. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- To, Y.H.; Degeling, K.; Kosmider, S.; Wong, R.; Lee, M.; Dunn, C.; Gard, G.; Jalali, A.; Wong, V.; Ijzerman, M.; et al. Circulating Tumour DNA as a Potential Cost-Effective Biomarker to Reduce Adjuvant Chemotherapy Overtreatment in Stage II Colorectal Cancer. Pharmacoeconomics 2021, 39, 953–964. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Geyer, F.C.; Weigelt, B.; Natrajan, R.; Lambros, M.B.; de Biase, D.; Vatcheva, R.; Savage, K.; Mackay, A.; Ashworth, A.; Reis-Filho, J.S. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J. Pathol. 2010, 220, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cha, Y.; Lim, Y.; Roh, H.; Kang, J.K.; Lee, K.H.; Kim, M.J.; Park, J.W.; Ryoo, S.B.; Kim, H.P.; et al. Mutational evolution after chemotherapy-progression in metastatic colorectal cancer revealed by circulating tumor DNA analysis. Int. J. Cancer 2023, 153, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Ishizuka, N.; Takashima, A.; Kumekawa, Y.; Nakano, D.; Shiozawa, M.; Denda, T.; Sawada, R.; Ouchi, K.; Wakatsuki, T.; et al. Multicentre single-arm phase II trial evaluating the safety and effiCacy of Panitumumab and iRinOtecan in NeoRAS Wild-type mEtaStatic colorectal cancer patientS (C-PROWESS trial): Study protocol. BMJ Open 2022, 12, e063071. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Fernández-Rodríguez, M.C.; Casadevall, D.; García-Alfonso, P.; Páez, D.; Guix, M.; Alonso, V.; Cano, M.T.; Santos, C.; Durán, G.; et al. Liquid Biopsy Detects Early Molecular Response and Predicts Benefit to First-Line Chemotherapy plus Cetuximab in Metastatic Colorectal Cancer: PLATFORM-B Study. Clin. Cancer Res. 2023, 29, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Magri, V.; Marino, L.; Belardinilli, F.; Di Nicolantonio, F.; De Renzi, G.; Caponnetto, S.; De Meo, M.; Giannini, G.; Santini, D.; et al. Genomic landscape and survival analysis of ctDNA “neo-RAS wild-type” patients with originally RAS mutant metastatic colorectal cancer. Front. Oncol. 2023, 13, 1160673. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Napolitano, S.; Famiglietti, V.; Esposito, L.; De Falco, V.; Di Liello, A.; Avallone, A.; Maiello, E.; Pietrantonio, F.; Cremolini, C.; et al. Pretreatment Plasma Circulating Tumor DNA RAS/BRAF Mutational Status in Refractory Metastatic Colorectal Cancer Patients Who Are Candidates for Anti-EGFR Rechallenge Therapy: A Pooled Analysis of the CAVE and VELO Clinical Trials. Cancers 2023, 15, 2117. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra392. [Google Scholar] [CrossRef]
- Holm, M.; Andersson, E.; Osterlund, E.; Ovissi, A.; Soveri, L.M.; Anttonen, A.K.; Kytölä, S.; Aittomäki, K.; Osterlund, P.; Ristimäki, A. Detection of KRAS mutations in liquid biopsies from metastatic colorectal cancer patients using droplet digital PCR, Idylla, and next generation sequencing. PLoS ONE 2020, 15, e0239819. [Google Scholar] [CrossRef]
- Zou, D.; Day, R.; Cocadiz, J.A.; Parackal, S.; Mitchell, W.; Black, M.A.; Lawrence, B.; Fitzgerald, S.; Print, C.; Jackson, C.; et al. Circulating tumor DNA is a sensitive marker for routine monitoring of treatment response in advanced colorectal cancer. Carcinogenesis 2020, 41, 1507–1517. [Google Scholar] [CrossRef]
- Topham, J.T.; O’Callaghan, C.J.; Feilotter, H.; Kennecke, H.F.; Lee, Y.S.; Li, W.; Banks, K.C.; Quinn, K.; Renouf, D.J.; Jonker, D.J.; et al. Circulating Tumor DNA Identifies Diverse Landscape of Acquired Resistance to Anti-Epidermal Growth Factor Receptor Therapy in Metastatic Colorectal Cancer. J. Clin. Oncol. 2023, 41, 485–496. [Google Scholar] [CrossRef]
- Khan, K.H.; Cunningham, D.; Werner, B.; Vlachogiannis, G.; Spiteri, I.; Heide, T.; Mateos, J.F.; Vatsiou, A.; Lampis, A.; Damavandi, M.D.; et al. Longitudinal Liquid Biopsy and Mathematical Modeling of Clonal Evolution Forecast Time to Treatment Failure in the PROSPECT-C Phase II Colorectal Cancer Clinical Trial. Cancer Discov. 2018, 8, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Rossini, D.; Dell’Aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Wang, W.; Kandimalla, R.; Huang, H.; Zhu, L.; Li, Y.; Gao, F.; Goel, A.; Wang, X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2019, 55, 37–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).