Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
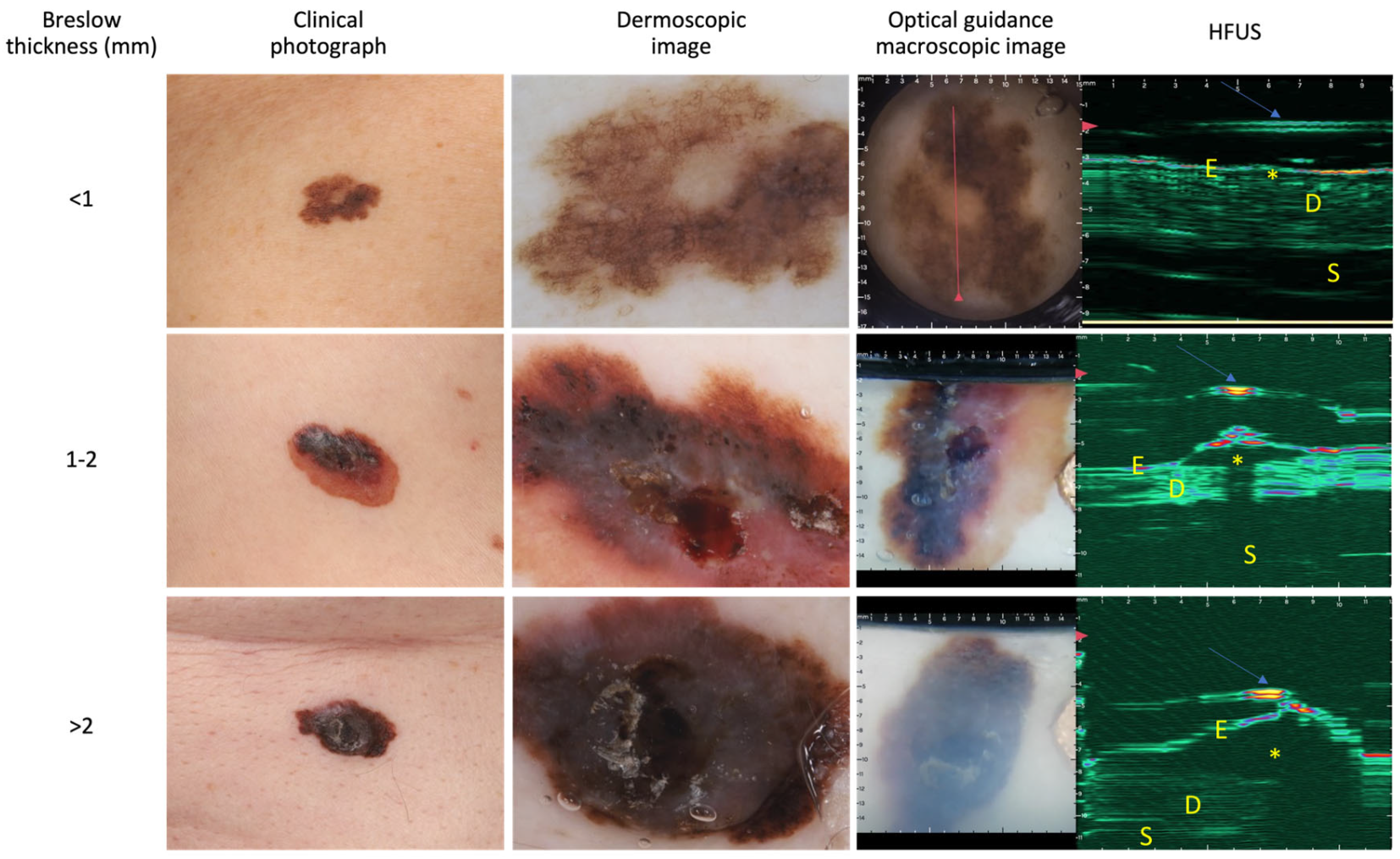
2.2. OG-HFUS Device and Image Analysis
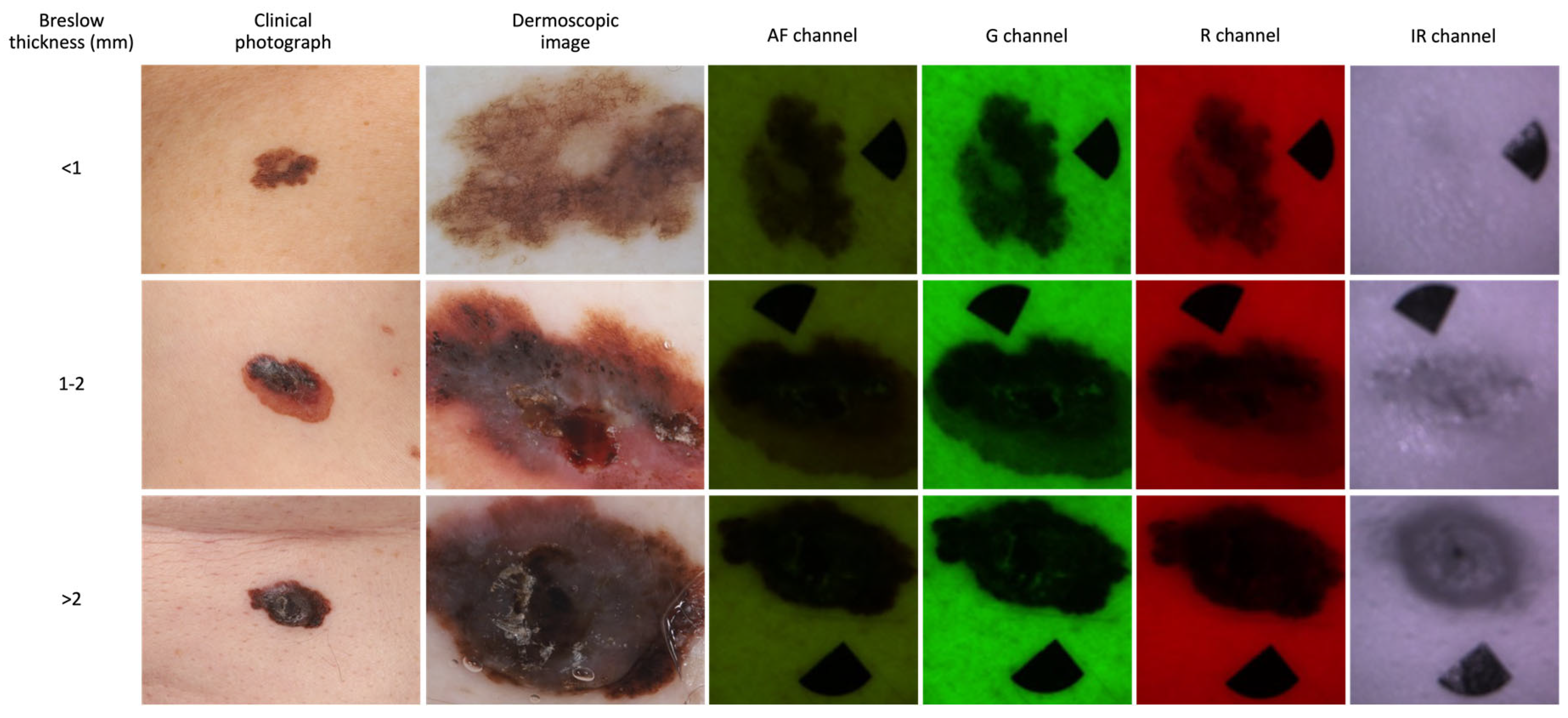
2.3. MSI Device and Image Analysis
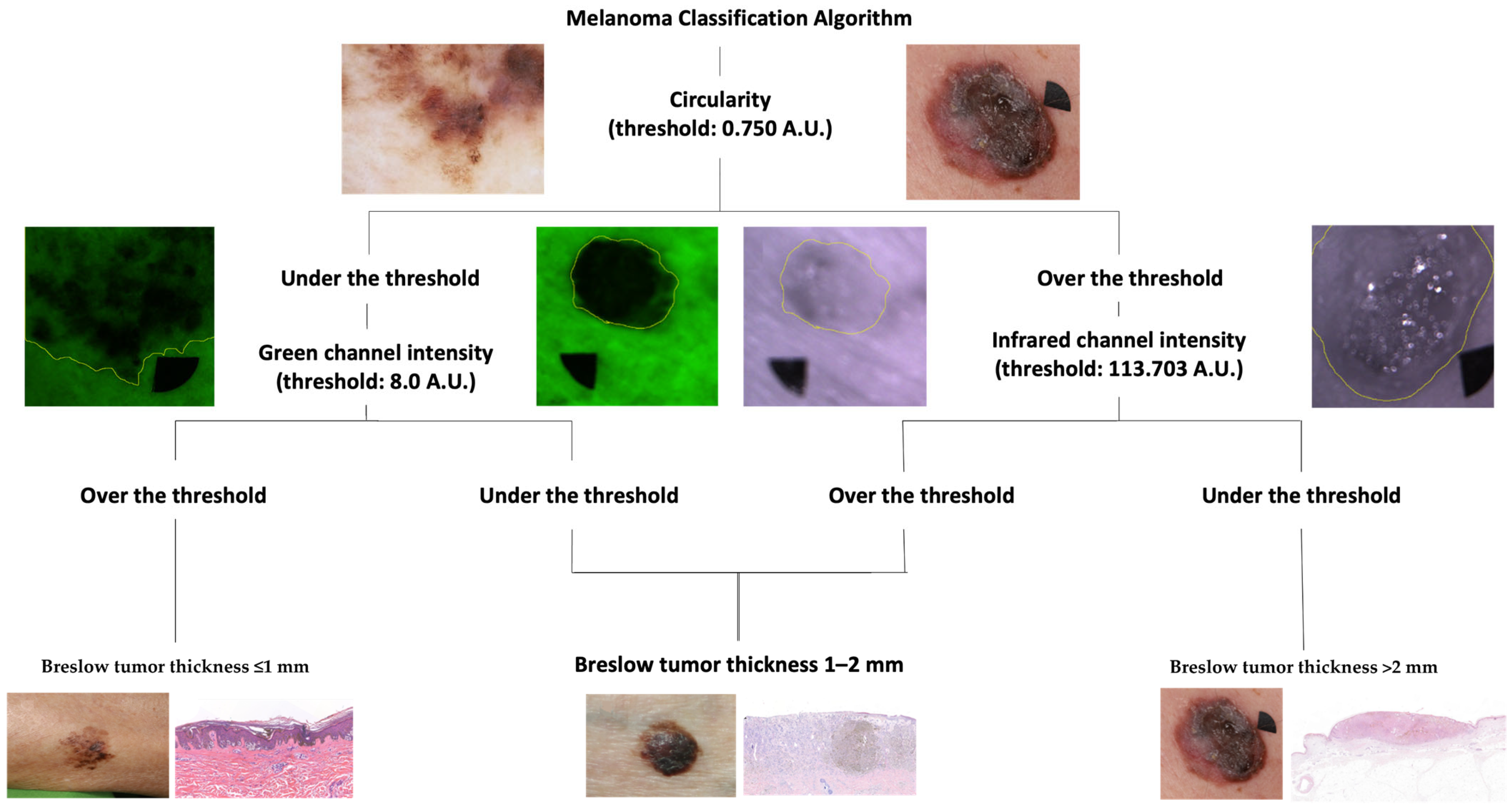
2.4. Melanoma Classification Algorithm
2.5. Statistical Analysis
3. Results
3.1. Patient Data
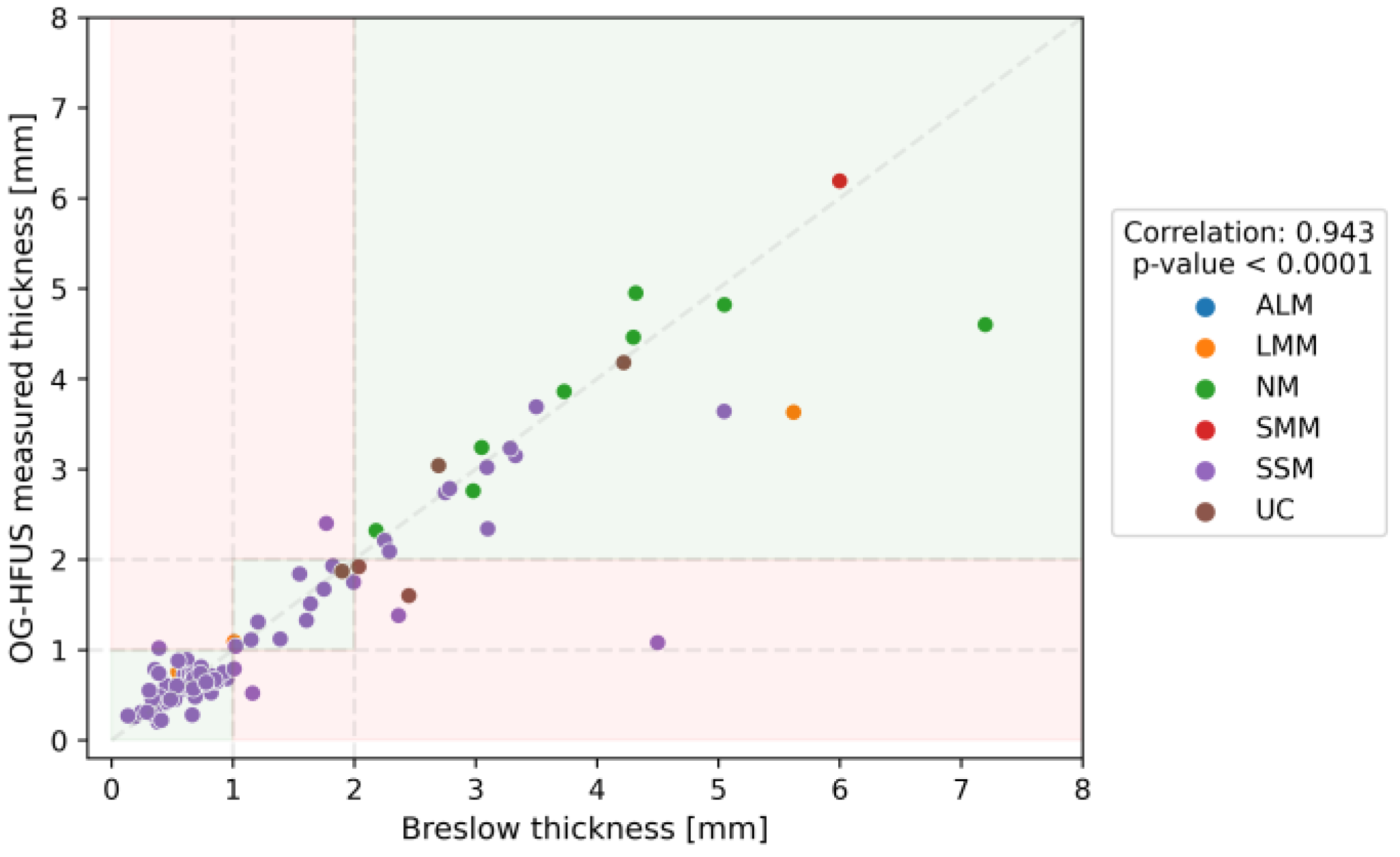
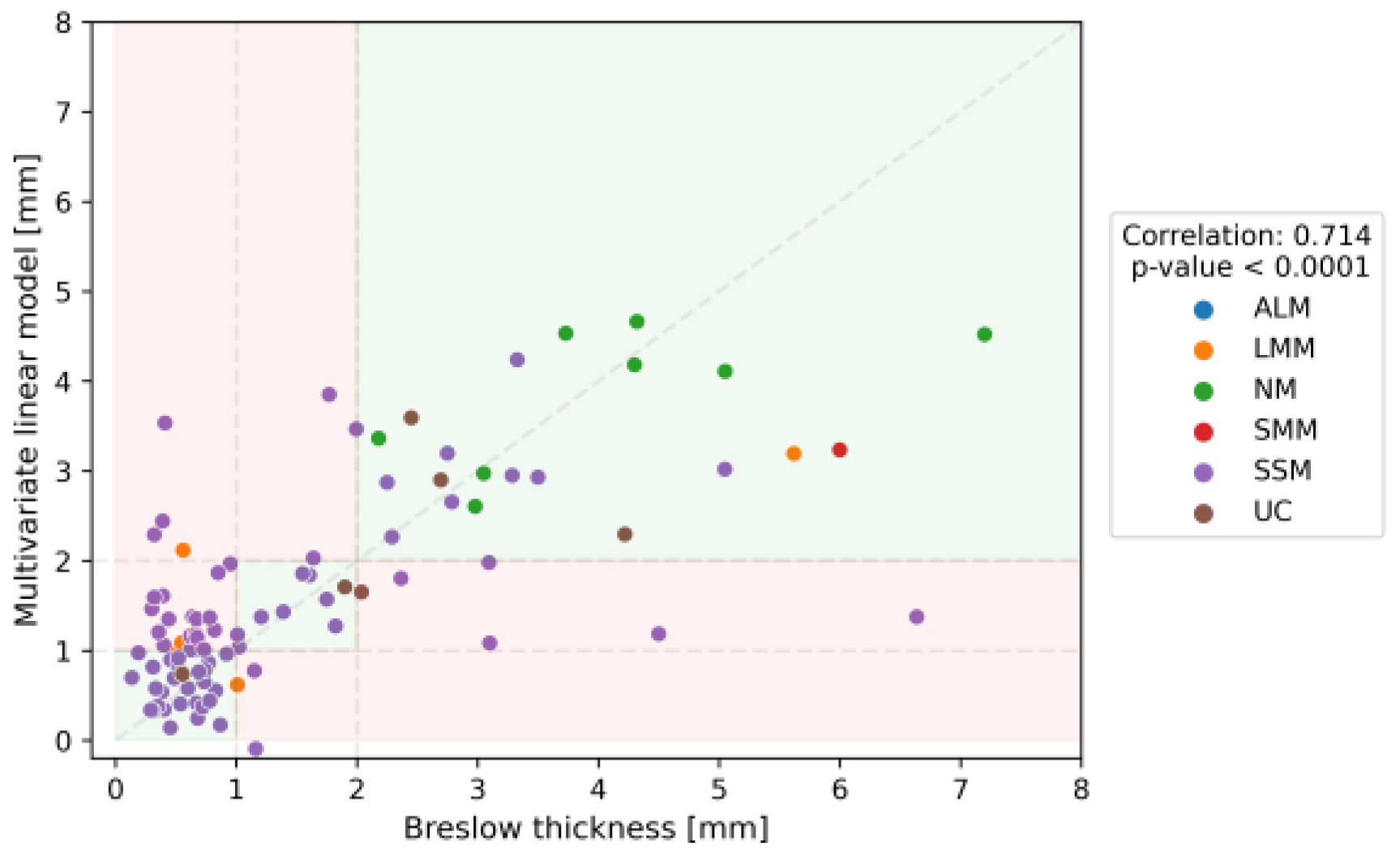
3.2. Diagnostic Accuracy of the OG-HFUS
3.3. Diagnostic Accuracy of the MSI
3.4. Comparative Analysis of Imaging Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, J.A.; Fisher, D.E. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Higgins, H.W.; Qureshi, A.A. Familial risk of melanoma and links with other cancers. Melanoma Manag. 2015, 2, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Hassidim, R.; Ostrowski, S.M.; Fisher, D.E. The Complex Interplay between Nevi and Melanoma: Risk Factors and Precursors. Int. J. Mol. Sci. 2023, 24, 3541. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in acral and mucosal melanoma: Current status and future directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Boldrick, J.C.; Jung, S.Y.; Egbert, B.M.; Harvell, J.D. Increasing incidence of lentigo maligna melanoma subtypes: Northern California and national trends 1990–2000. J. Investig. Dermatol. 2005, 125, 685–691. [Google Scholar] [CrossRef]
- Li, H.; Colantonio, S.; Dawson, A.; Lin, X.; Beecker, J. Sunscreen application, safety, and sun protection: The evidence. J. Cutan. Med. Surg. 2019, 23, 357–369. [Google Scholar] [CrossRef]
- Glenn, B.A.; Lin, T.; Chang, L.C.; Okada, A.; Wong, W.K.; Glanz, K.; Bastani, R. Sun protection practices and sun exposure among children with a parental history of melanoma. Cancer Epidemiol. Biomark. Prev. 2015, 24, 169–177. [Google Scholar] [CrossRef]
- Dobbinson, S.; Wakefield, M.; Hill, D.; Girgis, A.; Aitken, J.F.; Beckmann, K.; Reeder, A.I.; Herd, N.; Fairthorne, A.; Bowles, K.-A. Prevalence and determinants of Australian adolescents’ and adults’ weekend sun protection and sunburn, summer 2003–2004. J. Am. Acad. Dermatol. 2008, 59, 602–614. [Google Scholar] [CrossRef]
- D’souza, C.; Kramadhari, N.; Skalkos, E.; Dutton, T.; Bailey, J. Sun safety knowledge, practices and attitudes in rural Australian farmers: A cross-sectional study in Western New South Wales. BMC Public Health 2021, 21, 731. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, M.; Wang, S.; Li, X.; Sun, Y. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun. 2020, 40, 154–166. [Google Scholar] [CrossRef]
- Echle, A.; Rindtorff, N.T.; Brinker, T.J.; Luedde, T.; Pearson, A.T.; Kather, J.N. Deep learning in cancer pathology: A new generation of clinical biomarkers. Br. J. Cancer 2021, 124, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Puig, S.; Zalaudek, I.; Sera, F.; Corona, R.; Alsina, M.; Barbato, F.; Carrera, C.; Ferrara, G.; Guilabert, A.; et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J. Clin. Oncol. 2006, 24, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Jolivot, R.; Benezeth, Y.; Marzani, F. Skin parameter map retrieval from a dedicated multispectral imaging system applied to dermatology/cosmetology. Int. J. Biomed. Imaging 2013, 2013, 26. [Google Scholar] [CrossRef] [PubMed]
- Berson, M.; Grégoire, J.; Gens, F.; Rateau, J.; Jamet, F.; Vaillant, L.; Tranquart, F.; Pourcelot, L. High frequency (20 MHz) ultrasonic devices: Advantages and applications. Eur. J. Ultrasound 1999, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Themstrup, L.; Jemec, G. Optical coherence tomography in dermatology. G. Ital. Dermatol. Venereol. 2015, 150, 603–615. [Google Scholar] [PubMed]
- Vestergaard, M.; Macaskill, P.; Holt, P.; Menzies, S. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef]
- Argenziano, G.; Soyer, H.P.; Chimenti, S.; Talamini, R.; Corona, R.; Sera, F.; Binder, M.; Cerroni, L.; De Rosa, G.; Ferrara, G.; et al. Dermoscopy of pigmented skin lesions: Results of a consensus meeting via the Internet. J. Am. Acad. Dermatol. 2003, 48, 679–693. [Google Scholar] [CrossRef]
- Kittler, H.; Guitera, P.; Riedl, E.; Avramidis, M.; Teban, L.; Fiebiger, M.; Weger, R.A.; Dawid, M.; Menzies, S. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch. Dermatol. 2006, 142, 1113–1119. [Google Scholar] [CrossRef]
- Zherdeva, L.A.; Bratchenko, I.A.; Myakinin, O.O.; Moryatov, A.A.; Kozlov, S.V.; Zakharov, V.P. In vivo hyperspectral imaging and differentiation of skin cancer. In Proceedings of the SPIE/COS Photonics Asia 2016, Beijing, China, 12–14 October 2016. [Google Scholar]
- Kuzmina, I.; Diebele, I.; Asare, L.; Kempele, A.; Abelite, A.; Jakovels, D.; Spigulis, J. Multispectral imaging of pigmented and vascular cutaneous malformations: The influence of laser treatment. In Proceedings of the Laser Applications in Life Sciences 2010, Oulu, Finland, 9–11 June 2010. [Google Scholar]
- Kuzmina, I.; Diebele, I.; Jakovels, D.; Spigulis, J.; Valeine, L.; Kapostinsh, J.; Berzina, A. Towards noncontact skin melanoma selection by multispectral imaging analysis. J. Biomed. Opt. 2011, 16, 060502. [Google Scholar] [CrossRef]
- Ilișanu, M.-A.; Moldoveanu, F.; Moldoveanu, A. Multispectral Imaging for Skin Diseases Assessment—State of the Art and Perspectives. Sensors 2023, 23, 3888. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, I.C.; Nasution, A.M.; Chandra, T.G. A new LED-based multispectral imaging system for blood and melanin content estimation: The validation. AIP Conf. Proc. 2019, 2193, 050017. [Google Scholar]
- Grossman, D.; Huntsman Cancer Institute; Hand, M.; Chien, A. Screening and Non-Invasive Evaluative Devices for Melanoma Detection A Comparison of Commercially Available Devices and Dermoscopic Evaluation. Clin. Dermatol. Ther. 2015, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Bozsányi, S.; Varga, N.N.; Farkas, K.; Bánvölgyi, A.; Lőrincz, K.; Lihacova, I.; Lihachev, A.; Plorina, E.V.; Bartha, Á.; Jobbágy, A.; et al. Multispectral Imaging Algorithm Predicts Breslow Thickness of Melanoma. J. Clin. Med. 2022, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Bozsányi, S.; Boostani, M.; Farkas, K.; Hamilton-Meikle, P.; Varga, N.N.; Szabó, B.; Vasanits, F.; Kuroli, E.; Meznerics, F.A.; Lőrincz, K.; et al. Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study. J. Clin. Med. 2023, 12, 6910. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of High-Frequency Ultrasonography in the Diagnosis of Melanoma: Mini Review. Front. Oncol. 2021, 11, 673026. [Google Scholar] [CrossRef] [PubMed]
- Serrone, L.; Solivetti, F.M.; Thorel, M.F.; Eibenschutz, L.; Donati, P.; Catricalà, C. High frequency ultrasound in the preoperative staging of primary melanoma: A statistical analysis. Melanoma Res. 2002, 12, 287–290. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Mogensen, M.; Joergensen, T.M.; Nürnberg, B.M.; Morsy, H.A.; Thomsen, J.B.; Thrane, L.; Jemec, G.B. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: Observer-blinded evaluation by dermatologists and pathologists. Dermatol. Surg. 2009, 35, 965–972. [Google Scholar] [CrossRef]
- Wang, T.; Mallidi, S.; Qiu, J.; Ma, L.L.; Paranjape, A.S.; Sun, J.; Kuranov, R.V.; Johnston, K.P.; Milner, T.E. Comparison of pulsed photothermal radiometry, optical coherence tomography and ultrasound for melanoma thickness measurement in PDMS tissue phantoms. J. Biophotonics 2010, 4, 335–344. [Google Scholar] [CrossRef]
- Welzel, J. Optical coherence tomography in dermatology: A review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, C.; Baja, A.-V.; Gargano, L.; Rossi, G.; Ardigò, M.; Soda, G.; Boostani, M.; Kiss, N.; Pellacani, G. Optical Coherence Tomography as a Valuable Tool for the Evaluation of Cutaneous Kaposi Sarcoma Treated with Imiquimod 5% Cream. Diagnostics 2023, 13, 2901. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, N.D.; Petrova, G.A.; Nikulin, N.K.; Snopova, L.B.; Chumakov, Y.P.; Nasonova, V.A.; Gelikonov, V.M.; Gelikonov, G.V.; Kuranov, R.V.; Sergeev, A.M.; et al. In vivo optical coherence tomography imaging of human skin: Norm and pathology. Ski. Res. Technol. 2000, 6, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Csány, G.; Gergely, L.H.; Kiss, N.; Szalai, K.; Lőrincz, K.; Strobel, L.; Csabai, D.; Hegedüs, I.; Marosán-Vilimszky, P.; Füzesi, K.; et al. Preliminary Clinical Experience with a Novel Optical–Ultrasound Imaging Device on Various Skin Lesions. Diagnostics 2022, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Lihachev, A.; Derjabo, A.; Ferulova, I.; Lange, M.; Lihacova, I.; Spigulis, J. Autofluorescence imaging of basal cell carcinoma by smartphone RGB camera. J. Biomed. Opt. 2015, 20, 120502. [Google Scholar] [CrossRef] [PubMed]
- Bliznuks, D.; Jakovels, D.; Saknite, I.; Spigulis, J. Mobile platform for online processing of multimodal skin optical images: Using online Matlab server for processing remission, fluorescence and laser speckle images, obtained by using novel handheld device. In Proceedings of the 2015 International Conference on BioPhotonics (BioPhotonics), Florence, Italy, 20–22 May 2015. [Google Scholar]
- Spigulis, J. Multispectral, fluorescent and photoplethysmographic imaging for remote skin assessment. Sensors 2017, 17, 1165. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Balch, C.M.; Soong, S.-J.; Gershenwald, J.E.; Reintgen, D.S.; Cascinelli, N.; Urist, M.; McMasters, K.M.; Ross, M.I.; Kirkwood, J.M.; Atkins, M.B.; et al. Prognostic Factors Analysis of 17,600 Melanoma Patients: Validation of the American Joint Committee on Cancer Melanoma Staging System. J. Clin. Oncol. 2001, 19, 3622–3634. [Google Scholar] [CrossRef]
- Cassileth, B.R.; Lusk, E.J.; Tenaglia, A.N. Patients’ perceptions of the cosmetic impact of melanoma resection. Plast. Reconstr. Surg. 1983, 71, 73–75. [Google Scholar] [CrossRef]
- Tamošiūnas, M.; Plorina, E.V.; Lange, M.; Derjabo, A.; Kuzmina, I.; Bļizņuks, D.; Spigulis, J. Autofluorescence imaging for recurrence detection in skin cancer postoperative scars. J. Biophotonics 2020, 13, e201900162. [Google Scholar] [CrossRef]
- Koshenkov, V.P.; Broucek, J.; Kaufman, H.L. Surgical management of melanoma. In Melanoma; Springer: Berlin/Heidelberg, Germany, 2016; pp. 149–179. [Google Scholar]
- Chaput, L.; Laurent, E.; Pare, A.; Sallot, A.; Mourtada, Y.; Ossant, F.; Vaillant, L.; Patat, F.; Machet, L. One-step surgical removal of cutaneous melanoma with surgical margins based on preoperative ultrasound measurement of the thickness of the melanoma. Eur. J. Dermatol. 2018, 28, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Lauwers-Cances, V.; Lourari, S.; Laurent, J.; Konstantinou, M.; Lagarde, J.; Krief, B.; Batatia, H.; Lamant, L.; Paul, C. High-frequency ultrasonography but not 930 nm optical coherence tomography reliably evaluates melanoma thickness in vivo: A prospective validation study. Br. J. Dermatol. 2014, 171, 799–805. [Google Scholar] [CrossRef]
- Pellacani, G.; Seidenari, S. Preoperative Melanoma Thickness Determination by 20-MHz Sonography and Digital Videomicroscopy in Combination. Arch. Dermatol. 2003, 139, 293–298. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, N.; Welzel, J.; Schuh, S.; Themstrup, L.; Ulrich, M.; Jemec, G.B.E.; Holmes, J.; Kaleci, S.; Chester, J.; Bigi, L.; et al. The vascular morphology of melanoma is related to Breslow index: An in vivo study with dynamic optical coherence tomography. Exp. Dermatol. 2018, 27, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Huzaira, M.; Rius, F.; Rajadhyaksha, M.; Anderson, R.R.; González, S. Topographic Variations in Normal Skin, as Viewed by In Vivo Reflectance Confocal Microscopy. J. Investig. Dermatol. 2001, 116, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Caltabiano, R.; Broggi, G.; Di Natale, A.; Micali, G. Discoid lupus erythematosus: Reflectance confocal microscopy features correlate with horizontal histopathological sections. Ski. Res. Technol. 2018, 25, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Elias, M.; Busam, K.; Rajadhyaksha, M.; Marghoob, A. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br. J. Dermatol. 2005, 153, 1031–1036. [Google Scholar] [CrossRef]
- Yélamos, O.; Cordova, M.; Blank, N.; Kose, K.; Dusza, S.W.; Lee, E.; Rajadhyaksha, M.; Nehal, K.S.; Rossi, A.M. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017, 153, 1278–1284. [Google Scholar] [CrossRef]
- Verzì, A.E.; Broggi, G.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of lentigo maligna with horizontal and vertical histopathologic correlations. J. Cutan. Pathol. 2023, 50, 118–122. [Google Scholar] [CrossRef]
- Perez-Anker, J.; Puig, S.; Alos, L.; García, A.; Alejo, B.; Cinotti, E.; Cano, C.O.; Tognetti, L.; Lenoir, C.; Monnier, J.; et al. Morphological evaluation of melanocytic lesions with three-dimensional line-field confocal optical coherence tomography: Correlation with histopathology and reflectance confocal microscopy. A pilot study. Clin. Exp. Dermatol. 2022, 47, 2222–2233. [Google Scholar] [CrossRef]
- Barragán-Estudillo, Z.F.; Brito, J.; Chavez-Bourgeois, M.; Alejo, B.; Alos, L.; García, A.P.; Puig, S.; Malvehy, J.; Carrera, C. Dermoscopy and Reflectance Confocal Microscopy to Estimate Breslow Index and Mitotic Rate in Primary Melanoma. Dermatol. Pract. Concept. 2022, 12, e2022174. [Google Scholar] [CrossRef] [PubMed]
- Kukk, A.F.; Wu, D.; Gaffal, E.; Panzer, R.; Emmert, S.; Roth, B. Multimodal system for optical biopsy of melanoma with integrated ultrasound, optical coherence tomography and Raman spectroscopy. J. Biophotonics 2022, 15, e202200129. [Google Scholar] [CrossRef]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Cano, C.O.; Diet, G.; Perez-Anker, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. G. Ital. Dermatol. Venereol. 2023, 158, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kaikaris, V.; Samsanavičius, D.; Maslauskas, K.; Rimdeika, R.; Valiukevičienė, S.; Makštienė, J.; Pundzius, J. Measurement of melanoma thickness—Comparison of two methods: Ultrasound versus morphology. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 796–802. [Google Scholar] [CrossRef]
- Botar-Jid, C.M.; Cosgarea, R.; Bolboacă, S.D.; Şenilă, S.C.; Lenghel, L.M.; Rogojan, L.; Dudea, S.M. Assessment of Cutaneous Melanoma by Use of Very- High-Frequency Ultrasound and Real-Time Elastography. Am. J. Roentgenol. 2016, 206, 699–704. [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Cappabianca, S. A preliminary study for quantitative assessment with HFUS (High-Frequency Ultrasound) of nodular skin melanoma breslow thickness in adults before surgery: Interdisciplinary team experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar]
- Oranges, T.; Janowska, A.; Scatena, C.; Faita, F.; Lascio, N.D.; Izzetti, R.; Fidanzi, C.; Romanelli, M.; Dini, V. Ultra-High Frequency Ultrasound in Melanoma Management: A New Combined Ultrasonographic–Histopathological Approach. J. Ultrasound Med. 2023, 42, 99–108. [Google Scholar] [CrossRef]
| Subtype | Lesion (n) | Distribution Ratio (%) |
|---|---|---|
| SSM | 69 | 68.32 |
| NM | 8 | 7.92 |
| SSM sec. Nod. | 10 | 9.90 |
| LMM sec. Nod. | 1 | 0.99 |
| LMM | 6 | 5.94 |
| ALM | 1 | 0.99 |
| UC | 4 | 3.96 |
| Naevoid | 2 | 1.98 |
| Breslow (mm) | Patients (n) | MSE | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cohen’s Kappa (κ) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| <1 | 56 | 0.034 | 98.2 | 95.2 | 96.5 | 97.6 | 0.937 | 0.867–1.000 |
| 1–2 | 15 | 0.080 | 80.0 | 94.0 | 70.6 | 96.3 | 0.701 | 0.503–0.900 |
| >2 | 27 | 1.02 | 85.2 | 98.6 | 95.8 | 94.6 | 0.868 | 0.755–0.980 |
| Total | 98 | 0.31 | 91.8 | 96.0 | 91.8 | 96.0 | 0.858 | 0.763–0.952 |
| Breslow (mm) | Patients (n) | MSE | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Cohen’s Kappa (κ) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| <1 | 56 | 0.64 | 55.4 | 93.0 | 91.2 | 61.5 | 0.457 | 0.286–0.627 |
| 1–2 | 15 | 0.61 | 60.0 | 67.9 | 25.0 | 90.5 | 0.177 | −0.052–0.406 |
| >2 | 28 | 3.36 | 78.6 | 90.1 | 75.9 | 91.4 | 0.680 | 0.517–0.842 |
| Total | 99 | 1.41 | 62.6 | 81.3 | 62.6 | 81.3 | 0.440 | 0.298–0.583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, N.N.; Boostani, M.; Farkas, K.; Bánvölgyi, A.; Lőrincz, K.; Posta, M.; Lihacova, I.; Lihachev, A.; Medvecz, M.; Holló, P.; et al. Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study. Cancers 2024, 16, 157. https://doi.org/10.3390/cancers16010157
Varga NN, Boostani M, Farkas K, Bánvölgyi A, Lőrincz K, Posta M, Lihacova I, Lihachev A, Medvecz M, Holló P, et al. Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study. Cancers. 2024; 16(1):157. https://doi.org/10.3390/cancers16010157
Chicago/Turabian StyleVarga, Noémi Nóra, Mehdi Boostani, Klára Farkas, András Bánvölgyi, Kende Lőrincz, Máté Posta, Ilze Lihacova, Alexey Lihachev, Márta Medvecz, Péter Holló, and et al. 2024. "Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study" Cancers 16, no. 1: 157. https://doi.org/10.3390/cancers16010157
APA StyleVarga, N. N., Boostani, M., Farkas, K., Bánvölgyi, A., Lőrincz, K., Posta, M., Lihacova, I., Lihachev, A., Medvecz, M., Holló, P., Paragh, G., Wikonkál, N. M., Bozsányi, S., & Kiss, N. (2024). Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study. Cancers, 16(1), 157. https://doi.org/10.3390/cancers16010157









