Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma
2. Classic Treatments
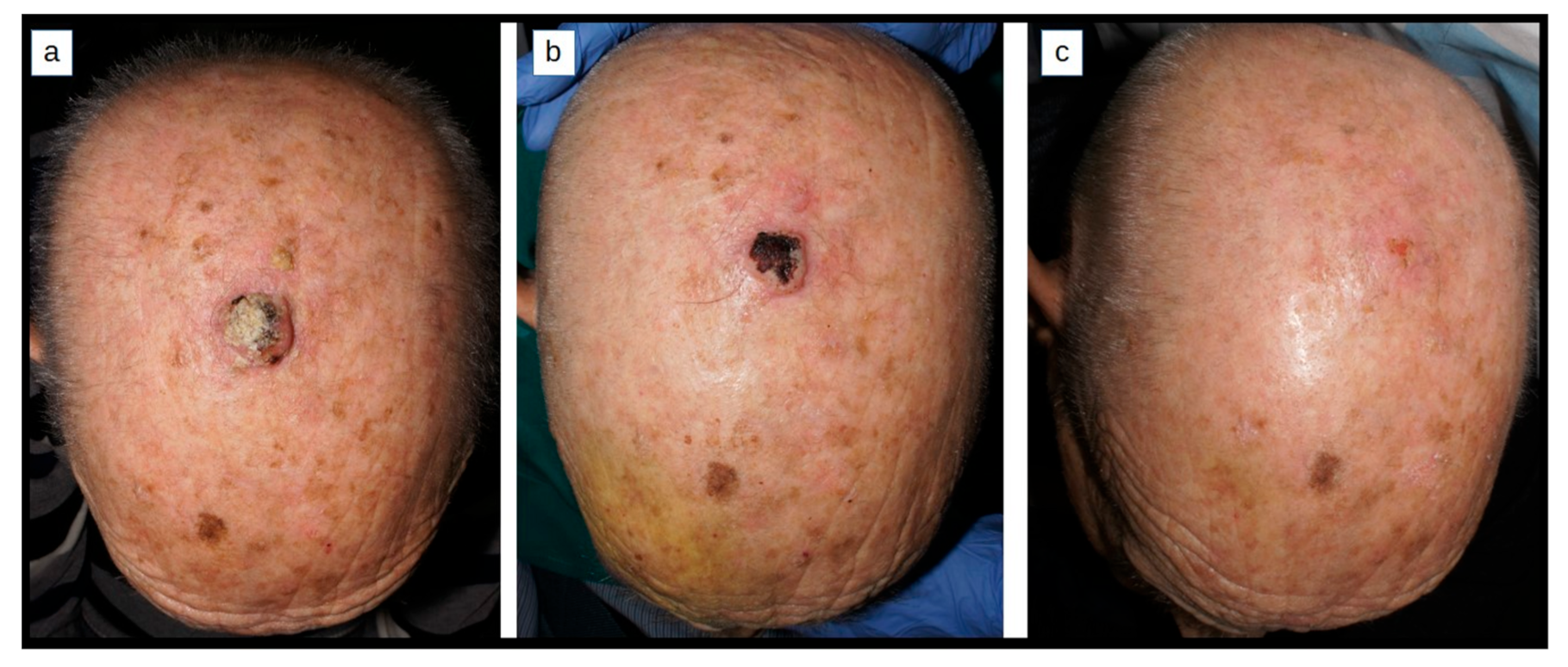
2.1. Methotrexate
2.2. 5-Fluorouracil
2.3. Bleomycin
2.4. Interferon
2.5. Interleukin 2 (IL-2)
2.6. OK-432 (Picibanil)
2.7. Photodynamic Therapy
2.8. Cryosurgery
2.9. Electrochemotherapy
3. Emerging Treatments and Future Directions
3.1. Intralesional Immunotherapy
3.2. Oncolytic Viruses
3.3. Cancer Vaccines
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Leiter, U. Cutaneous squamous cell carcinoma: State of the art, perspectives and unmet needs. JDDG J. Dtsch. Dermatol. Ges. 2023, 21, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Varra, V.; Woody, N.M.; Reddy, C.; Joshi, N.P.; Geiger, J.; Adelstein, D.J.; Burkey, B.B.; Scharpf, J.; Prendes, B.; Lamarre, E.D.; et al. Suboptimal Outcomes in Cutaneous Squamous Cell Cancer of the Head and Neck with Nodal Metastases. Anticancer Res. 2018, 38, 5825–5830. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death from Cutaneous Squamous Cell Carcinoma: A 10-Year, Single-Institution Cohort Study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Kus, K.J.B.; Ruiz, E.S. Non-Surgical Treatments for Keratinocyte Carcinomas. Adv. Ther. 2021, 38, 5635–5648. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Avci, P.; Bánvölgyi, A.; Lőrincz, K.; Szakonyi, J.; Gyöngyösi, N.; Fésűs, L.; Lee, G.; Wikonkál, N. Intralesional therapy for the treatment of keratoacanthoma. Dermatol. Ther. 2019, 32, e12872. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Intralesional methotrexate in dermatology: Diverse indications and practical considerations. Dermatol. Ther. 2021, 34, e14404. [Google Scholar] [CrossRef]
- Martorell-Calatayud, A.; Requena, C.; Nagore, E.; Sanmartín, O.; Serra-Guillén, C.; Botella-Estrada, R.; Sanz-Motilva, V.; Llombart, B.; Alcañiz-Moscardo, A.; Guillén-Barona, C. Ensayo clínico: La infiltración intralesional con metotrexato de forma neoadyuvante en la cirugía del queratoacantoma permite obtener mejores resultados estéticos y funcionales. Actas Dermo-Sifiliográficas 2011, 102, 605–615. [Google Scholar] [CrossRef]
- Moss, M.; Weber, E.; Hoverson, K.; Montemarano, A.D. Management of Keratoacanthoma: 157 Tumors Treated with Surgery or Intralesional Methotrexate. Dermatol. Surg. 2019, 45, 877–883. [Google Scholar] [CrossRef]
- Rambhia, S.; Rambhia, K.; Gulati, A. A rare case of multiple keratoacanthomas treated with oral acitretin and intralesional methotrexate. Indian Dermatol. Online J. 2016, 7, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Melton, J.L.; Nelson, B.R.; Stough, D.B.; Brown, M.D.; Swanson, N.A.; Johnson, T.M. Treatment of keratoacanthomas with intralesional methotrexate. J. Am. Acad. Dermatol. 1991, 25, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.; Gan, B.S. Intralesional methotrexate in keratoacanthoma of the nose. Br. J. Plast. Surg. 1995, 48, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Ahn, H.; Eom, J.W. A Case of Giant Keratoacanthoma of the Lower Lip Treated with Intralesional Methotrexate. Korean J. Otorhinolaryngol.-Head Neck Surg. 1997, 40, 1858–1862. [Google Scholar]
- Cuesta-Romero, C. Intralesional Methotrexate in Solitary Keratoacanthoma. Arch. Dermatol. 1998, 134, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Spieth, K.; Gille, J.; Kaufmann, R. Intralesional Methotrexate as Effective Treatment in Solitary Giant Keratoacanthoma of the Lower Lip. Dermatology 2000, 200, 317–319. [Google Scholar] [CrossRef]
- Remling, R.; Mempel, M.; Schnopp, N.; Abeck, D.; Ring, J. Intraläsionale Methotrexat-Injektionn: An effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Der Hautarzt 2000, 51, 612–614. [Google Scholar] [CrossRef]
- Kim, C.Y.; Yoon, T.J.; Kim, T.H. Treatment of Keratoacanthomas with Intralesional 5-Fluorouracil and Methotrexate. Korean J. Dermatol. 2001, 39, 1175–1178. [Google Scholar]
- You, D.O.; Youn, N.H.; Park, S.D. Successful treatment of two cases of keratoacanthomas with intralesional methotrexate. Korean J. Dermatol. 2002, 40, 555–558. [Google Scholar]
- de Visscher, J.G.; van der Wal, K.G.; Blanken, R.; Willemse, F. Treatment of giant keratoacanthoma of the skin of the lower lip with intralesional methotrexate: A case report. J. Oral Maxillofac. Surg. 2002, 60, 93–95. [Google Scholar] [CrossRef]
- Philip, R.C.; Keith, E.S.; Craig, F.T.; Bruce, R.N. Intralesional Methotrexate for Keratoacanthoma of the Nose. SKINmed Dermatol. Clin. 2005, 4, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.B.; Cho, H.M.; Park, J.H.; Son, S.W.; Kim, I.H. Two cases of giant keratoacanthoma treated with intralesional methotrexate. Korean J. Dermatol. 2006, 2006, 902–905. [Google Scholar]
- Yuge, S.; Godoy, D.A.S.; de Melo, M.C.C.; Sousa, D.S.; Soares, C.T. Keratoacanthoma centrifugum marginatum: Response to topical 5-fluorouracil. J. Am. Acad. Dermatol. 2006, 54, S218–S219. [Google Scholar] [CrossRef] [PubMed]
- Annest, N.M.; VanBeek, M.J.; Arpey, C.J.; Whitaker, D.C. Intralesional methotrexate treatment for keratoacanthoma tumors: A retrospective study and review of the literature. J. Am. Acad. Dermatol. 2007, 56, 989–993. [Google Scholar] [CrossRef]
- Basoglu, Y.; Metze, D.; Nashan, D.; Ständer, S. Keratoacanthoma with perineural invasion: An indicator for aggressive behavior? JDDG J. Dtsch. Dermatol. Ges. 2008, 6, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.P.; Cervino, A.L. Treatment of keratoacanthoma: Is intralesional methotrexate an option? Can J Plast Surg. 2011, 19, e15–e18. [Google Scholar] [CrossRef]
- Aubut, N.; Alain, J.; Claveau, J. Intralesional Methotrexate Treatment for Keratoacanthoma Tumors: A Retrospective Case Series. J. Cutan. Med. Surg. 2012, 16, 212–217. [Google Scholar] [CrossRef]
- Yoo, M.G.; Kim, I.-H. Intralesional Methotrexate for the Treatment of Keratoacanthoma: Retrospective Study and Review of the Korean Literature. Ann. Dermatol. 2014, 26, 172–176. [Google Scholar] [CrossRef]
- Panther, D.; Nino, T.; Macknet, K.D.; Macknet, C. Eruptive Keratoacanthomas as a Complication of Fractionated CO2 Laser Resurfacing and Combination Therapy with Imiquimod and Intralesional Methotrexate. Dermatol. Surg. 2015, 41, 172–175. [Google Scholar] [CrossRef]
- Veerula, V.L.; Ezra, N.; Aouthmany, M.; Graham, T.A.; Wolverton, S.E.; Somani, A.-K. Multiple keratoacanthomas occurring in surgical margins and de novo treated with intralesional methotrexate. Cutis 2016, 98, E12–E15. [Google Scholar]
- Rossi, A.M.; Park, B.; Qi, B.; Lee, E.H.; Busam, K.J.; Nehal, K.S. Solitary Large Keratoacanthomas of the Head and Neck: An Observational Study. Dermatol. Surg. 2017, 43, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Barros, B.; Helm, K.; Zaenglein, A.; Seiverling, E. Keratoacanthoma-Like Growths of Incontinentia Pigmenti Successfully Treated with Intralesional Methotrexate. Pediatr. Dermatol. 2017, 34, e203–e204. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, V.; Milani, M. Efficacy and Safety of Intralesional Methotrexate in the Treatment of a Large Keratoacanthoma of the Dorsal Hand in a 99-Year-Old Woman. Case Rep. Dermatol. 2018, 10, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, M.; Patrì, A.; Costa, C.; Megna, M.; Napolitano, M.; Fabbrocini, G.; Balato, N. Intralesional Methotrexate for the Treatment of Keratoacanthoma: The Neapolitan Experience. Dermatol. Ther. 2019, 9, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Saporito, R.C.; Harris, M.O.; Housewright, C.D. Intralesional methotrexate for treatment of keratoacanthoma and well-differentiated squamous cell carcinoma. J. Am. Acad. Dermatol. 2019, 81, AB85. [Google Scholar] [CrossRef]
- Doerfler, L.; Hanke, C.W. Treatment of Solitary Keratoacanthoma of the Nose with Intralesional Methotrexate and Review of the Literature. J. Drugs Dermatol. 2019, 18, 693–696. [Google Scholar]
- Smith, C.; Srivastava, D.; Nijhawan, R.I. Intralesional methotrexate for keratoacanthomas: A retrospective cohort study. J. Am. Acad. Dermatol. 2020, 83, 904–905. [Google Scholar] [CrossRef]
- Gualdi, G.; Caravello, S.; Frasci, F.; Giuliani, F.; Moro, R.; Fargnoli, M.C.; Ciciarelli, V.; Argenziano, G.; Giorgio, C.M.; Requena, C.; et al. Intralesional Methotrexate for the Treatment of Advanced Keratinocytic Tumors: A Multi-Center Retrospective Study. Dermatol. Ther. 2020, 10, 769–777. [Google Scholar] [CrossRef]
- Salido-Vallejo, R.; Cuevas-Asencio, I.; Garnacho-Sucedo, G.; González-Menchen, A.; Alcántara-Reifs, C.; De la Corte-Sánchez, S.; Vélez, A.; Moreno-Giménez, J. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: A comparative cohort study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1120–1124. [Google Scholar] [CrossRef]
- Bergón-Sendín, M.; Pulido-Pérez, A.; Suárez-Fernández, R. Neoadjuvant intralesional methotrexate in squamous cell carcinoma of the lip. Australas. J. Dermatol. 2019, 60, 158–160. [Google Scholar] [CrossRef]
- Bergón-Sendín, M.; Pulido-Pérez, A.; Rosell-Díaz, M.; Nieto-Benito, L.M.; Suárez-Fernández, R. Neoadjuvant intralesional methotrexate in small-sized cutaneous squamous cell carcinoma: A prospective study in 84 patients. Australas. J. Dermatol. 2021, 62, E308–E310. [Google Scholar] [CrossRef] [PubMed]
- Bergón-Sendín, M.; Pulido-Pérez, A.; Nieto-Benito, L.M.; Barchino-Ortiz, L.; Díez-Sebastián, J.; Suárez-Fernández, R. Effectiveness of neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: A prospective cohorts study. Dermatol. Ther. 2022, 35, e15233. [Google Scholar] [CrossRef] [PubMed]
- Bergón-Sendín, M.; Pulido-Pérez, A.; López, F.C.; Díez-Sebastián, J.; Suárez-Fernández, R. Cutaneous Ultrasound for Tumor Thickness Measurement in Squamous Cell Carcinoma: The Effect of Neoadjuvant Intralesional Methotrexate in 40 Patients. Dermatol. Surg. 2020, 46, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Gomez, A.; Calderón-Rocher, C.; Proy-Trujillo, H.; Moreno-Coutiño, G. Neoadjuvant Intralesional Methotrexate before Surgery in Squamous Cell Carcinoma. Dermatol. Surg. 2014, 40, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Moye, M.S.; Clark, A.H.; Legler, A.A.; Milhem, M.M.; Van Beek, M.J. Intralesional Methotrexate for Treatment of Invasive Squamous Cell Carcinomas in a Patient Taking Vemurafenib for Treatment of Metastatic Melanoma. J. Clin. Oncol. 2016, 34, e134–e136. [Google Scholar] [CrossRef] [PubMed]
- Vega-González, L.; Morales-Pérez, M.; Molina-Pérez, T.; Sereno-Gómez, B. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022, 24, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, B.M. Intralesional versus Intramuscular Methotrexate for Non-Melanoma Skin Cancers. 2022. Available online: https://clinicaltrials.gov/study/NCT05315128 (accessed on 4 August 2023).
- Elkholy, B.M.; El-Sayed, M.; Sola, M.A.; Bessar, H. Intralesional versus intramuscular methotrexate in the treatment of non-melanoma skin cancers. Preprint 2022. [Google Scholar] [CrossRef]
- Goebeler, M.; Lurz, C.; Kolve-Goebeler, M.E.; Bröcker, E.B. Pancytopenia after treatment of keratoacanthoma by single lesional methotrexate infiltration. Arch Dermatol. 2001, 137, 1104–1105. [Google Scholar]
- Maghfour, J.; Kuraitis, D.; Murina, A. Intralesional 5-Fluorouracil for Treatment of Non-Melanoma Skin Cancer: A Systematic Review. J. Drugs Dermatol. 2021, 20, 192–198. [Google Scholar] [CrossRef]
- Metterle, L.; Nelson, C.; Patel, N. Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): A review. J. Am. Acad. Dermatol. 2016, 74, 552–557. [Google Scholar] [CrossRef]
- Goette, D.K.; Odom, R.B. Successful treatment of keratoacanthoma with intralesional fluorouracil. J. Am. Acad. Dermatol. 1980, 2, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Marka, A.; Rodgers, D.; Castillo, L.Z.; Hoyt, B.; Chapman, M.; Carter, J. Dilute Intralesional 5-Fluorouracil for the Treatment of Squamous Cell Carcinomas and Keratoacanthomas: A Case Series. JDD 2023, 22, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.; Shah, M.; Schwartz, C.; Tanner, L.S.; Appel, J. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J. Am. Acad. Dermatol. 2021, 84, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
- Pugliano-Mauro, M. Trial of Intralesional 5-Fluorouracil (5FU) and Intralesional 5FU Combined with Topical Imiquimod in Patients with Squamous Cell Carcinoma (SCC) of the Lower Extremities. clinicaltrials.gov, 2023. Available online: https://clinicaltrials.gov/study/NCT03370406 (accessed on 4 August 2023).
- Gil-Lianes, J.; Morgado-Carrasco, D. RF—Intralesional 5-Fluorouracil in the Treatment of Nonmelanoma Skin Cancer. FR—Tratamiento intralesional del cáncer cutáneo no melanoma con 5-fluorouracilo. Actas Dermosifiliogr. 2023, 114, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Dando, E.E.; Lim, G.F.; Lim, S.J.; Kim, C.; Pugliano-Mauro, M. Intralesional 5-Fluorouracil for the Nonsurgical Management of Low-Risk, Invasive Squamous Cell Carcinoma. Dermatol. Surg. 2020, 46, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Manalo, I.F.; Lowe, M.C.; Nelson, K.C.; Chen, S.C. Triple therapy with intralesional 5-fluorouracil, chemowraps, and acitretin: A well-tolerated option for treatment of widespread cutaneous squamous cell carcinomas on the legs. JAAD Case Rep. 2019, 5, 1051–1054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamad, J.; Jolly, P.S. Debulking followed by intralesional 5-fluorouracil for the treatment of cutaneous squamous cell carcinoma and keratoacanthoma: A retrospective analysis. Dermatol. Ther. 2021, 34, e15139. [Google Scholar] [CrossRef]
- Khandpur, S.; Sharma, V.K. Successful treatment of multiple premalignant and malignant lesions in arsenical keratosis with a combination of acitretin and intralesional 5-fluorouracil. J. Dermatol. 2003, 30, 730–734. [Google Scholar] [CrossRef]
- Yumeen, S.; Kahn, B.J.; Leonard, A.; Shah, A.; Qureshi, A.A.; Saliba, E. When Surgery Won’t Cut It: Successful Management of Eruptive Squamous Atypia with Combination Medical Therapy. Cureus 2023, 15, e37694. [Google Scholar] [CrossRef]
- LaPresto, L.; Cranmer, L.; Morrison, L.; Erickson, C.P.; Curiel-Lewandrowski, C. A Novel Therapeutic Combination Approach for Treating Multiple Vemurafenib-Induced Keratoacanthomas: Systemic Acitretin and Intralesional Fluorouracil. JAMA Dermatol. 2013, 149, 279–281. [Google Scholar] [CrossRef]
- Hadley, J.C.; Tristani-Firouzi, P.; Florell, S.F.; Bowen, G.M.; Hadley, M.L. Case Series of Multiple Recurrent Reactive Keratoacanthomas Developing at Surgical Margins. Dermatol. Surg. 2009, 35, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Helm, F.; Milgrom, H.; Stoll, H.L.; Traenkle, H.L. Tumors of the skin. II. Keratoacanthoma; local effect of 5-fluorouracil. Skin 1962, 1, 153–156. [Google Scholar] [PubMed]
- Odom, R.B.; Goette, D.K. Treatment of Keratoacanthomas with Intralesional Fluorouracil. Arch. Dermatol. 1978, 114, 1779–1783. [Google Scholar] [CrossRef]
- Kurtis, B.; Rosen, T. Treatment of Cutaneous Neoplasms by Intralesional Injections of 5-Fluorouracil (5-FU). J. Dermatol. Surg. Oncol. 1980, 6, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, S.W.; Gentry, R.H.; Patterson, J.W.; May, D.L. Treatment of multiple keratoacanthomas with intralesional fluorouracil. J. Am. Acad. Dermatol. 1982, 7, 126–129. [Google Scholar] [CrossRef]
- Parker, C.M.; Hanke, C.W. Large keratoacanthomas in difficult locations treated with intralesional 5-fiuorouracil. J. Am. Acad. Dermatol. 1986, 14 Pt 1, 770–777. [Google Scholar] [CrossRef]
- Bergin, D.J.; Lapins, N.A.; Deffer, T.A. Intralesional 5-Fluorouracil for Keratoacanthoma of the Eyelid. Ophthalmic Plast. Reconstr. Surg. 1986, 2, 201–204. [Google Scholar] [CrossRef]
- Singal, A.; Mohanty, S.; Bhattacharya, S.N.; Baruah, M.C.; Singh, N. Unusual Multiple Keratoacanthoma in a Child Successfully Treated with 5-Fluorouracil. J. Dermatol. 1997, 24, 546–548. [Google Scholar] [CrossRef]
- Leonard, A.L.; Hanke, C.W. Treatment of giant keratoacanthoma with intralesional 5-fluorouracil. J. Drugs Dermatol. 2006, 5, 454–456. [Google Scholar]
- Que, S.K.T.; Compton, L.A.; Schmults, C.D. Eruptive squamous atypia (also known as eruptive keratoacanthoma): Definition of the disease entity and successful management via intralesional 5-fluorouracil. J. Am. Acad. Dermatol. 2019, 81, 111–122. [Google Scholar] [CrossRef]
- Dominiak, N.; Hayes, B.; Swick, J.; Leach, B.; Maize, J.; Ralston, J. Keratoacanthoma centrifugum marginatum: A diagnostic and therapeutic challenge. JAAD Case Rep. 2016, 2, 206–208. [Google Scholar] [CrossRef]
- Hemperly, S.; Branch, S.; Purcell, S.M. Field Cancerization with Multiple Keratoacanthomas Successfully Treated with Topical and Intralesional 5-Fluorouracil. CUTIS 2020, 105, E12–E14. [Google Scholar] [CrossRef]
- Seger, E.W.; Tarantino, I.S.; Neill, B.C.; Wang, T. Successful non-operative treatment of eruptive keratoacanthomas refractory to excision. Dermatol. Online J. 2020, 26, 18. [Google Scholar] [CrossRef]
- Ahmed, F.; Memon, N.; Haque, A. Multiple Keratoacanthomas Following Moderna Messenger RNA-1273 COVID-19 Vaccination Resolved With 5-Fluorouracil Treatment: Case Report. JMIR Dermatol. 2022, 5, e41739. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.; Miller, B.H.; Swinehart, J.M.; Shavin, J.S.; Georgouras, K.E.; Jenner, D.A.; Griffin, E.; Korey, A.; Orenberg, E.K. Intratumoral chemotherapy with fluorouracil/ epinephrine injectable gel: A nonsurgical treatment of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1998, 38, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Morse, L.G.; Kendrick, C.; Hooper, D.; Ward, H.; Parry, E. Treatment of Squamous Cell Carcinoma with Intralesional 5-Fluorouracil. Dermatol. Surg. 2003, 29, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, D.M.; Cognetta, A.B.; Pynes, L.T.; Paredes, A.A.; Sweeney, T.J.; Dolson, D.J. Treatment of a giant squamous cell carcinoma on the dominant thumb with intralesional 5-fluorouracil. J. Am. Acad. Dermatol. 2011, 65, 219–221. [Google Scholar] [CrossRef]
- Mackey, M.; Shahsavari, A.; Mackey, V.T. Intralesional 5-Fluorouracil in the Treatment of Lower Leg Squamous Cell Carcinoma. J. Drugs Dermatol. 2018, 17, 1241–1243. [Google Scholar]
- Luu, W.; McRae, M.Y. Intralesional 5-fluorouracil as a management for cutaneous squamous cell carcinomas: A rural Australian retrospective case series. Australas. J. Dermatol. 2023, 64, 556–559. [Google Scholar] [CrossRef]
- Sayama, S.; Tagami, H. Treatment of keratoacanthoma with intralesional bleomycin. Br. J. Dermatol. 1983, 109, 449–452. [Google Scholar] [CrossRef]
- Tanigaki, T.; Endd, H. A Case of Squamous Cell Carcinoma Treated by Intralesional Injection of Oil Bleomycin. Dermatology 1985, 170, 302–305. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, C.; Losada, A.; Cruces, M.J. Keratoacanthoma centrifugum marginatum: Treatment with intralesional bleomycin. J. Am. Acad. Dermatol. 1997, 37, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, A.; Pianigiani, E.; Taddeucci, P.; Lorenzini, G.; Fimiani, M.; Biagioli, M. Guess what! Keratoacanthoma treated with in-tralesional bleomycin. Eur. J. Dermatol. 1999, 9, 403–405. [Google Scholar] [PubMed]
- Khanna, N.R.; Gerriets, V. Interferon. In Advances in Virus Research; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK555932/ (accessed on 10 September 2023).
- Grob, J.; Suzini, F.; Richard, M.; Weiller, M.; Zarour, H.; Noe, C.; Munoz, M.; Bonerandi, J. Large keratoacanthomas treated with intralesional interferon alfa-2a. J. Am. Acad. Dermatol. 1993, 29, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, L.; Hindson, T.; Wacks, H. Treatment of neoplastic skin lesions with intralesional interferon. J. Am. Acad. Dermatol. 1989, 20, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-K.; Son, H.-S.; Lee, J.-B.; Jang, H.-S.; Kwon, K.-S. Intralesional interferon alfa-2b treatment of keratoacanthomas. J. Am. Acad. Dermatol. 2004, 51, S177–S180. [Google Scholar] [CrossRef]
- Anadolu, R.; Akay, B.N.; Bodamyali, P.; Akyol, A. Giant keratoacanthoma-type low-grade squamous cell carcinoma of the upper lip: Response to intralesional interferon alpha-2B. J. Dermatol. Treat. 2011, 22, 239–240. [Google Scholar] [CrossRef]
- Good, L.M.; Miller, M.D.; High, W.A. Intralesional agents in the management of cutaneous malignancy: A review. J. Am. Acad. Dermatol. 2011, 64, 413–422. [Google Scholar] [CrossRef]
- Hanlon, A.; Kim, J.; Leffell, D.J. Intralesional interferon alfa-2b for refractory, recurrent squamous cell carcinoma of the face. J. Am. Acad. Dermatol. 2013, 69, 1070–1072. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499840/ (accessed on 10 September 2023).
- Vidovic, D.; Simms, G.A.; Pasternak, S.; Walsh, M.; Peltekian, K.; Stein, J.; Helyer, L.K.; Giacomantonio, C.A. Case Report: Combined Intra-Lesional IL-2 and Topical Imiquimod Safely and Effectively Clears Multi-Focal, High Grade Cutaneous Squamous Cell Cancer in a Combined Liver and Kidney Transplant Patient. Front. Immunol. 2021, 12, 678028. [Google Scholar] [CrossRef]
- Slobbe, L. Drugs that act on the immune system. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2012; Volume 34, pp. 609–651. [Google Scholar] [CrossRef]
- Akeda, T.; Yamanaka, K.; Kitagawa, H.; Kawabata, E.; Tsuda, K.; Kakeda, M.; Omoto, Y.; Habe, K.; Isoda, K.; Kurokawa, I.; et al. Intratumoral injection of OK-432 suppresses metastatic squamous cell carcinoma lesion inducing interferon-γ and tumour necrosis factor-α. Clin. Exp. Dermatol. 2012, 37, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, E.; Apalla, Z.; Ioannides, D. Complete resolution of a squamous cell carcinoma of the skin using intralesional 5-aminolevulinic acid photodynamic therapy intralesional PDT for SCC: Intralesional PDT for SCC. Photodermatol. Photoimmunol. Photomed. 2010, 26, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-N.; Pan, S.-C.; Lee, J.Y.-Y.; Wong, T.-W. Successful treatment of cutaneous squamous cell carcinoma with intralesional cryosurgery: Case report. Medicine 2016, 95, e4991. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Clover, A.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.; Tosti, G.; Martinoli, C.; Spadola, G.; Cataldo, F.; Verrecchia, F.; Baldini, F.; Mosconi, M.; Soteldo, J.; Tedeschi, I.; et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: A novel therapeutic approach: ECT for skin tumors. Dermatol. Ther. 2010, 23, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Díez, D.V.; Lario, A.R.-V.; Marugán, L.T. [Translated article] RF—Electrochemotherapy in the Treatment of Primary and Secondary Skin Tumors. Actas Dermosifiliogr. 2022, 113, T817–T818. [Google Scholar] [CrossRef]
- Montuori, M.; Santurro, L.; Feliziani, A.; De Sanctis, F.; Ricciardi, E.; Gaudio, D.; Campione, E.; Bianchi, L.; Silvi, M.B.; Rossi, P. Electrochemotherapy for basocellular and squamocellular head and neck cancer: Preliminary experience in Day Surgery Unit. Ital. J. Dermatol. Venereol. 2018, 153, 19–25. [Google Scholar] [CrossRef]
- Ribero, S.; Balagna, E.; Baduel, E.S.; Picciotto, F.; Sanlorenzo, M.; Fierro, M.T.; Quaglino, P.; Macripo, G. Efficacy of electrochemotherapy for eruptive legs keratoacanthomas: Efficacy of electrochemotherapy. Dermatol. Ther. 2016, 29, 345–348. [Google Scholar] [CrossRef]
- Pasquali, P.; Spugnini, E.P.; Baldi, A. Successful Treatment of a Keratoacanthoma with Electrochemotherapy: A Case Report. Dermatol. Ther. 2018, 8, 143–146. [Google Scholar] [CrossRef]
- De Giorgi, V.; Scarfì, F.; Saqer, E.; Gori, A.; Tomassini, G.M.; Covarelli, P. The use of cisplatin electrochemotherapy in nonmelanoma skin cancers: A single-center study. Dermatol. Ther. 2020, 33, e13547. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Bertino, G.; Groselj, A.; Campana, L.G.; Kunte, C.; Schepler, H.; Gehl, J.; Muir, T.; Clover, J.A.P.; Quaglino, P.; Kis, E.; et al. Electrochemotherapy for the treatment of cutaneous squamous cell carcinoma: The INSPECT experience (2008–2020). Front. Oncol. 2022, 12, 951662. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Testori, A.; Curatolo, P.; Quaglino, P.; Mocellin, S.; Framarini, M.; Borgognoni, L.; Ascierto, P.; Mozzillo, N.; Guida, M.; et al. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumors. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Di Monta, G.; Caracò, C.; Simeone, E.; Grimaldi, A.M.; Marone, U.; Di Marzo, M.; Vanella, V.; Festino, L.; Palla, M.; Mori, S.; et al. Electrochemotherapy efficacy evaluation for treatment of locally advanced stage III cutaneous squamous cell carcinoma: A 22-cases retrospective analysis. J. Transl. Med. 2017, 15, 82. [Google Scholar] [CrossRef]
- Solari, N.; Spagnolo, F.; Ponte, E.; Quaglia, A.; Lillini, R.; Battista, M.; Queirolo, P.; Cafiero, F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: A series of 39 patients treated with palliative intent. J. Surg. Oncol. 2014, 109, 270–274. [Google Scholar] [CrossRef]
- Kreuter, A.; van Eijk, T.; Lehmann, P.; Fischer, M.; Horn, T.; Assaf, C.; Schley, G.; Herbst, R.; Kellner, I.; Weisbrich, C.; et al. Electrochemotherapy in advanced skin tumors and cutaneous metastases—A retrospective multicenter analysis. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 308–315. [Google Scholar] [CrossRef]
- Campana, L.G.; Mali, B.; Sersa, G.; Valpione, S.; Giorgi, C.A.; Strojan, P.; Miklavcic, D.; Rossi, C.R. Electrochemotherapy in non-melanoma head and neck cancers: A retrospective analysis of the treated cases. Br. J. Oral Maxillofac. Surg. 2014, 52, 957–964. [Google Scholar] [CrossRef]
- Serša, G.; Štabuc, B.; Čemažar, M.; Jančar, B.; Miklavčič, D.; Rudolf, Z. Electrochemotherapy with cisplatin: Potentiation of local cisplatin antitumour effectiveness by application of electric pulses in cancer patients. Eur. J. Cancer 1998, 34, 1213–1218. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1147–1154. [Google Scholar] [CrossRef]
- Jamsek, C.; Sersa, G.; Bosnjak, M.; Groselj, A. Long term response of electrochemotherapy with reduced dose of bleomycin in elderly patients with head and neck non-melanoma skin cancer. Radiol. Oncol. 2020, 54, 79–85. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; Balzani, A.; Pagnotta, A.; Tati, E.; Orlandi, F.; D’autilio, M.F.L.M.; Cervelli, V.; Gentile, P. Malignant Skin Cancer Excision in Combined Therapy with Electro-Chemotherapy and Dermal Substitute. Curr. Oncol. 2021, 28, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, M.; Cortese, A.; Pantaleo, G.; Parascandalo, S.; Amato, M. A relapsed lower-lip squamous cell carcinoma treated with curative electrochemotherapy in an elderly patient. Minerva Dent. Oral Sci. 2018, 67, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, M.; Mestre, J.S.; Cortese, A.; Murphy, D.; Parascandolo, S.; Razzano, S. Long term effectiveness of electrochemotherapy for the treatment of lower lip squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 2017, 46, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Pichi, B.; Moretto, S.; Petruzzi, G.; Zocchi, J.; Mazzola, F.; Manciocco, V.; Marchesi, P.; Pellini, R. Surgeons reaching satisfying outcomes without surgery: Nasal dorsum skin cancer recurrence treated with electrochemotherapy. Anti-Cancer Drugs 2020, 31, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, J.; Farricha, V.; Carvalhal, S.; Moura, C.; Abecasis, N. Electrochemotherapy, a local treatment for squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Dermatol. Ther. 2020, 33, e14093. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, A.; Rotunno, R.; El Hachem, M.; Latorre, S.; Cozza, R.; Curatolo, P. Electrochemotherapy, a potential new treatment for the management of squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa: Report of three cases. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1195–1196. [Google Scholar] [CrossRef]
- Sommerlad, M.; Lock, A.; Moir, G.; McGregor, J.; Bull, R.; Cerio, R.; Harwood, C. Linear porokeratosis with multiple squamous cell carcinomas successfully treated by electrochemotherapy. Br. J. Dermatol. 2016, 175, 1342–1345. [Google Scholar] [CrossRef]
- Regeneron Pharmaceuticals. A Phase 1 Study of Pre-Operative Cemiplimab (REGN2810), Administered Intralesionally, for Patients with Cutaneous Squamous Cell Carcinoma (CSCC) or Basal Cell Carcinoma (BCC). 2023. Available online: https://clinicaltrials.gov/study/NCT03889912 (accessed on 9 August 2023).
- Regeneron Pharmaceuticals. A Multicenter, Open-Label, Phase 2 Study of Intratumoral Vidutolimod (CMP-001) in Combination with Intravenous Cemiplimab in Subjects with Selected Types of Advanced or Metastatic Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT04916002 (accessed on 9 August 2023).
- CureVac. Phase I Study of Intratumoral CV8102 in Patients with Advanced Melanoma, Squamous Cell Carcinoma of the Skin, Squamous Cell Carcinoma of the Head and Neck, or Adenoid Cystic Carcinoma. 2021. Available online: https://clinicaltrials.gov/study/NCT03291002 (accessed on 9 August 2023).
- Eisai Inc. An Open-Label, Multicenter Phase 1/1b Study of Intratumorally Administered STING Agonist E7766 in Subjects with Advanced Solid Tumors or Lymphomas—INSTAL-101. 2023. Available online: https://clinicaltrials.gov/study/NCT04144140 (accessed on 9 August 2023).
- Philogen, S.A.A. Phase II Study of Intratumoral Administration of L19IL2/L19TNF in Non-Melanoma Skin Cancer Patients with Presence of Injectable Lesions. 2022. Available online: https://clinicaltrials.gov/study/NCT04362722 (accessed on 4 August 2023).
- Philogen, S.A.A. Phase II Study of L19IL2/L19TNF in Patients with Skin Cancers Amenable to Intralesional Treatment. 2023. Available online: https://clinicaltrials.gov/study/NCT05329792 (accessed on 4 August 2023).
- AbbVie. A Multicenter, Phase 1, Open-Label, Dose-Escalation Study of ABBV-927 and ABBV-181, an Immunotherapy, in Subjects with Advanced Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT02988960 (accessed on 9 August 2023).
- Tyligand Bioscience (Shanghai) Limited. A Phase I/II Study to Evaluate the Safety, Tolerability, Pharmacokinetic Profile, and Preliminary Efficacy of TSN222 in Subjects with Advanced Solid Tumors or Lymphoma. 2023. Available online: https://clinicaltrials.gov/study/NCT05842785 (accessed on 9 August 2023).
- Sanofi. A Phase 1 First-in-Human Dose Escalation and Expansion Study for the Evaluation of Safety, Pharmacokinetics, Pharmacodynamics and Anti-Tumor Activity of SAR441000 Administered Intratumorally as Monotherapy and in Combination with Cemiplimab in Patients with Advanced Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT03871348 (accessed on 9 August 2023).
- Intensity Therapeutics, Inc. A Phase 1/2 Safety Study of Intratumorally Administered INT230-6 in Adult Subjects with Advanced Refractory Cancers. 2023. Available online: https://clinicaltrials.gov/study/NCT03058289 (accessed on 4 August 2023).
- Ibrahim, S. A Phase 1B Study of Intralesional Injection of RP1 in Patients with Resectable Cutaneous SCC. 2023. Available online: https://clinicaltrials.gov/study/NCT05858229 (accessed on 4 August 2023).
- Curiel, C.N.; Stratton, D.; Cui, H.; Roe, D.; Tiwari, H.A.; Sundararajan, S. A single arm phase 2 study of talimogene laherparepvec in patients with low-risk invasive cutaneous squamous cell cancer. interim analysis. J. Clin. Oncol. 2022, 40, e21583. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme LLC. A Phase 1b/2 Clinical Study of Intratumoral Administration of V937 in Combination with Pem-brolizumab (MK-3475) in Participants with Advanced/Metastatic Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT04521621 (accessed on 9 August 2023).
- Turnstone Biologics, Corp. A Phase 1/2a, Multicenter, Open-Label Trial of TBio-6517, an Oncolytic Vaccinia Virus, Adminis-tered Alone and in Combination with Pembrolizumab, in Patients with Advanced Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT04301011 (accessed on 9 August 2023).
- Morphogenesis, Inc. Phase 1 Trial of IFx-Hu2.0 to Evaluate Safety in Patients with Skin Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT04925713 (accessed on 4 August 2023).
- Morphogenesis, Inc. Phase 1 Trial of Intralesional Immunotherapy with IFx-Hu2.0 Vaccine in Patients with Advanced Non-Melanoma Skin Cancers. 2023. Available online: https://clinicaltrials.gov/study/NCT04160065 (accessed on 4 August 2023).
- Eigentler, T.; Bauernfeind, F.G.; Becker, J.C.; Brossart, P.; Fluck, M.; Heinzerling, L.; Krauss, J.; Mohr, P.; Ochsenreither, S.; Schreiber, J.S.; et al. A phase I dose-escalation and expansion study of intratumoral CV8102 as single-agent or in combination with anti-PD-1 antibodies in patients with advanced solid tumors. J. Clin. Oncol. 2020, 38 (Suppl. 15), 3096. [Google Scholar] [CrossRef]
- Tyligand Bioscience Receives Clinical Trial Clearance from U.S. FDA and China NMPA for Dual Function Immune Agonist TSN222—Tyligand Bioscience. Available online: https://en.tyligand.com.cn/tyligand-news/news-release/tyligand-tsn222-ind-approval (accessed on 14 August 2023).
- Bechter, O.; Utikal, J.; Baurain, J.-F.; Massard, C.; Sahin, U.; Derhovanessian, E.; Ozoux, M.-L.; Marpadga, R.; Imedio, E.-R.; Acquavella, N.; et al. 391 A first-in-human study of intratumoral SAR441000, an mRNA mixture encoding IL-12sc, interferon alpha2b, GM-CSF and IL-15sushi as monotherapy and in combination with cemiplimab in advanced solid tumors. J. Immunother. Cancer. 2020, 8 (Suppl. 3). [Google Scholar] [CrossRef]
- Thomas, J.S.; El-Khoueiry, A.B.; Olszanski, A.J.; Azad, N.S.; Whalen, G.F.; Hanna, D.L.; Ingham, M.; Camacho, L.H.; Mahmood, S.; Bender, L.H.; et al. Effect of intratumoral INT230-6 on tumor necrosis and promotion of a systemic immune response: Results from a multicenter phase 1/2 study of solid tumors with and without pembrolizumab (PEM) [Intensity IT-01; Merck KEYNOTE-A10]. J. Clin. Oncol. 2022, 40 (Suppl. 16), 2520. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Offner, M.N.; Hamid, O.; Zumsteg, Z.S.; Gharavi, N.M. Complete and Sustained Remission of Metastatic Cutaneous Squamous Cell Carcinoma in a Liver Transplant Patient Treated with Talimogene Laherparepvec. Dermatol. Surg. 2021, 47, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Kim, N.H.; Thosani, M.K.; Moser, J.C. Use of Talimogene Laherparepvec to Treat Cutaneous Squamous Cell Carcinoma in a Renal Transplant Patient. Case Rep. Dermatol. 2023, 15, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lebhar, J.; Jacobs, J.; Faraz, K.; Salama, A.K.; Mosca, P.J. Liver transplant patient with in-transit squamous cell carcinoma treated with talimogene laherparepvec. JAAD Case Rep. 2023, 40, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ramelyte, E.; Pawlik, L.; Turko, P.; Sella, F.; Prieto, N.M.R.; Stäger, R.; Maul, J.-T.; Dummer, R. Intralesional oncolytic virotherapy with talimogene laherparepvec in patients with cutaneous lymphomas and non-melanoma skin cancers. J. Clin. Oncol. 2023, 41 (Suppl. 16), 9581. [Google Scholar] [CrossRef]
- Replimune Inc. A Randomized, Controlled, Open-Label, Phase 2 Study of Cemiplimab as a Single Agent and in Combination with RP1 in Patients with Advanced Cutaneous Squamous Cell Carcinoma. 2023. Available online: https://clinicaltrials.gov/study/NCT04050436 (accessed on 4 August 2023).
- Nichols, A.J.; Gonzalez, A.; Clark, E.S.; Khan, W.N.; Rosen, A.C.; Guzman, W.; Rabinovitz, H.; Badiavas, E.V.; Kirsner, R.S.; Ioannides, T. Combined Systemic and Intratumoral Administration of Human Papillomavirus Vaccine to Treat Multiple Cutaneous Basaloid Squamous Cell Carcinomas. JAMA Dermatol. 2018, 154, 927–930. [Google Scholar] [CrossRef]
- Wilson, A.; Cowan, T.; Marucci, D.; Murrell, D. Intralesional 9-valent human papillomavirus as a treatment for facial squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E1064–E1065. [Google Scholar] [CrossRef]
- IFx-Hu2.0: Morphogenesis Inc. Available online: https://www.morphogenesis-inc.com/programs-pipelines/ifx-hu2-0 (accessed on 10 September 2023).
- Brohl, A.S.; Markowitz, J.; Eroglu, Z.; Silk, A.W.; Hyngstrom, J.R.; Khushalani, N.I.; Tarhini, A.A.; De-Aquino, D.B.; Abraham, E.; Tsai, K.Y.; et al. Phase 1b trial of IFx-Hu2.0, a novel personalized cancer vaccine, in checkpoint inhibitor resistant merkel cell carcinoma and cutaneous squamous cell carcinoma. J. Clin. Oncol. 2023, 41 (Suppl. 16), 9534. [Google Scholar] [CrossRef]

| Author, Year | n | Location | MTX Administration | Outcome | Adverse Events Related to ilMTX |
|---|---|---|---|---|---|
| Melton et al., 1991 [12] | 9 | Face/scalp (7) > trunk (1) and hand (1) | 1–2 injections; cumulative dose: 5.0–37.5 mg of MTX | 100% resolution | Discomfort with injection |
| Hurst et al., 1995 [13] | 2 | Nose | 1–3 injections; cumulative dose: 25–75 mg of MTX | 100% resolution | None reported |
| Hong et al., 1997 [14] | 1 | Lower lip | 3 injections; cumulative dose: 75 mg of MTX | 100% resolution | None reported |
| Cuesta-Romero et al., 1998 [15] | 6 | Nose (5) > Cheek (1) | 1–4 injections; cumulative dose: 12.5–62.5 mg of MTX | 100% resolution | None reported |
| Spieth et al., 2000 [16] | 1 | Lower lip | 5 injections; cumulative dose: 25 mg of MTX | 100% resolution | Moderate pain with injection |
| Remling et al., 2000 [17] | 1 | Nose | 2 injections; cumulative dose: 21.25 mg of MTX | 100% resolution | None reported |
| Kim et al., 2001 [18] | 1 | Nose | 2 injections; cumulative dose not reported | 100% resolution | None reported |
| You et al., 2022 [19] | 2 | Thigh and cheek | 2–6 injections; cumulative dose: 15–45 mg of MTX | 100% resolution | None reported |
| De Visscher et al., 2002 [20] | 1 | Lower lip | 2 injections; cumulative dose: 50 mg of MTX | 100% resolution | None reported |
| Cohen et al., 2005 [21] | 1 | Nose | 3 injections; cumulative dose: 19.5 mg of MTX | 100% resolution | None reported |
| Shin et al., 2006 [22] | 2 | Lower lip and forehead | 6 injections; cumulative dose: 21–120 mg of MTX | 100% resolution | None reported |
| Yuge et al., 2006 [23] | 1 | Leg (KA marginatum centrifugum) | 3 injections; cumulative dose: 37.5 mg of MTX | Failure; resolution with topical 5-FU. | None reported |
| Annest et al., 2007 [24] | 18 | Face/scalp (10) > leg (4) and hand (4) | 1–3 injections; cumulative dose: 2.0–87.5 mg of MTX | 83% resolution | None reported |
| Basoglu et al., 2008 [25] | 1 | Upper lip | 10 injections; cumulative dose: 85 mg | Recurrence after 3 months (neurotropic KA) | None reported |
| After CO2 laser | |||||
| Martorell-Calatayud et al., 2011 [9] | 10 | Face (7) > dorsum of hand (3) | 1 neoadjuvant injection prior to surgery; 0.3–0.5 mL of 25 mg/mL of MTX | Tumor size reduction 50–80%, 100% surgical direct wound closure | None reported |
| Patel et al., 2011 [26] | 9 | Face (5) > leg (3) > arm (1) | 1–4 injections; 12.5–25.0 mg of MTX | 88.9% resolution | Transient injection pain (n = 1) |
| Aubut et al., 2012 [27] | 46 | Head (35) > other | 1–4 injections; cumulative dose: 2–25 mg of MTX | 74% resolution | None reported |
| 11/12 non-responders SCC histology on surgical excision | |||||
| Yoo et al., 2014 [28] | 5 | Nose (2) > cheek, chin, lower lip | 2–7 injections; cumulative dose: 25–90 mg of MTX | 80% resolution and 20% excision after reduction | None reported |
| Panther et al., 2015 [29] | 1 | 5 lesions on the face | 1 injection; 0.2–0.3 mL of 12.5 mg/mL of MTX per lesion | 100% resolution | None reported |
| After CO2 laser | with topical imiquimod | ||||
| Veerula et al., 2016 [30] | 1 | Leg, over scar of SCC (KA marginatum centrifugum-like, isotopic and isomorphic response) | 3 injections; cumulative dose: 37.5 mg of MTX | 100% resolution | None reported |
| Rambhia et al., 2017 [11] | 1 | Multiple KAs on the legs, buttocks, elbow, and hand | 2 injections; 0.3–2.0 mL of 12.5 mg/mL of MTX | Shrinkage of lesions | Pain at site of injection |
| with acitretin | Later treated with topical 5-FU and tazarotene | ||||
| Rossi et al., 2017 [31] | 14 | Head and neck | 1–3 injections; 12.5–25.0 mg of MTX | 71.4% resolution | None reported |
| Barros et al., 2017 [32] | 1 | KA-like lesions of incontinentia pigmenti on the leg | 3 injections; 12.5–43.75 mg of MTX | 100% resolution | Injection discomfort |
| Della Valle et al., 2018 [33] | 1 | Dorsum of hand | 2 injections; cumulative dose: 40 mg of MTX | 100% resolution | None reported |
| Moss et al., 2019 [10] | 54 | 73 tumors | 1–4 injections; cumulative dose: 1.3–31.3 mg of MTX | 88% resolution | None reported |
| Leg (60) > arm (9) > trunk (2) > head and neck (2) | |||||
| Scalvenzi et al., 2019 [34] | 11 | 13 lesions | 4–8 injections; 20 mg of MTX (≤2 cm, n = 6) or 25 mg of MTX (>2 cm, n = 5) | 100% resolution | None reported |
| Face (6) > trunk (3) > ears (2) and hands (2) | |||||
| Saporito et al., 2019 [35] | 3 | KA and well-differentiated SCC | 2–3 injections of 25 mg/mL of MTX | 100% resolution | None reported |
| Doerfler et al., 2019 [36] | 1 | Nose | 3 injections; cumulative dose: 75 mg of MTX | 100% resolution | None reported |
| Smith et al., 2020 [37] | 29 | 69 lesions | Mean: 2 injections; mean cumulative dose: 39 mg of MTX | 95.7% resolution | None reported |
| Leg (62) > arm (7) | |||||
| Gualdi et al., 2020 [38] | 12 | Not reported | 4–6 injections (different protocols, not separated by type of tumor); cumulative mean dose: 133.29 mg of MTX | 92% resolution | Yes; anemia the most frequent (5/35) |
| Author, Year | n | Location | MTX Administration | Outcome | Adverse Events Related to ilMTX |
|---|---|---|---|---|---|
| Plascencia-Gómez et al., 2014 [44] | 1 | Lower lip | 3 injections of MTX, 1 week apart before surgery; cumulative dose: 75 mg of MTX | 80% tumor size reduction | None reported |
| Moye et al., 2014 [45] | 1 | Multiple SCCs (face, thigh, back, and calf) Patient with melanoma receiving vemurafenib | 4 injections of MTX, 3–4 weeks apart; approximately 1.9–12.5 mg of MTX per injection | All tumors decreased in size | Tolerable pain with injection |
| Salido-Vallejo et al., 2016 [39] | 43 | Cheek (12) > scalp (8) > temple (6) > lower lip (5), arm (5) > nose (3), ear (3) > leg (1) | 1 injection of 25 mg/mL MTX before surgery; mean: 0.74 mL (0.1–1.3 mL) | Reduction in tumor size (mean: 42.6%) compared to surgery alone; greater reduction in lower lip tumors | Discomfort during MTX infiltration (60.47%) |
| Bergón-Sendín et al., 2018 [40] | 10 | Lower lip | 2 injections of 20 mg MTX, 1 week apart before scheduled surgery; cumulative dose: of MTX 40 mg | 100% response Mean decrease in diameter: 68.2% | None reported |
| Bergón-Sendín et al., 2019 [43] | 40 | Face (50%) > extremities (22.5%) > scalp (17.5%) > trunk (2.5%) | 2 injections of 50 mg/mL MTX, 1 week apart before surgery; mean cumulative dose: 37.6 mg | Clinical and sonographic response in 92.5% | None reported |
| Bergón-Sendín et al., 2020 [41] | 84 | Not reported | 2 injections of MTX before scheduled surgery, 1 week apart; mean cumulative dose: 36.9 mg of MTX | 100% tumor size reduction; 46/84 complete clinical and histopathological response | None reported |
| Gualdi et al., 2020 [38] | 21 | Not reported | 4–6 injections (different protocols, not separated by type of tumor); mean cumulative dose: 133.29 mg of MTX | 47.6% resolution | Yes; anemia the most frequent (5/35) |
| Bergón-Sendín et al., 2021 [42] | 100 | Face (50) > scalp (18) > limbs (16) > lip/ear (14) > trunk (2) | 2 injections of MTX, 1 week apart before scheduled surgery; mean cumulative dose: 37.72 mg of MTX | 93% tumor size reduction; less complex reconstructions than surgery-only group | None reported |
| Vega-González et al., 2022 [46] | 1 | Lower lip | 3 injections of MTX monthly; cumulative dose: 75 mg of MTX | 100% resolution | None reported |
| Author, Year | n | Location | 5-FU Administration | Outcome | Adverse Events Related to il5-FU |
|---|---|---|---|---|---|
| Klein et al., 1962 [64] | 2 | Ear, nose | 0.1 mL of 5% il5FU, twice daily or every other day; 7–34 injections | 100% resolution | Not reported |
| Odom et al., 1978 [65] | 14 | 26 KAs on face and upper extremities | 0.2–0.5 mL of 5% il5-FU, once a week for 2–8 weeks (mean: 2.8 injections) | 96.2% resolution | Mild pain during injection, slight irritation, and necrotic involution of KAs |
| Goette et al., 1980 [52] | 30 | 41 KAs (arms > face > shins) | 0.5–1.0 mL of 5% il5FU, 1 week apart; up to 5 injections (mean: 3 injections) | 97.5% resolution | Mild pain during injection, slight irritation, and necrotic involution of KAs |
| Kurtis et al., 1980 [66] | 3 | Upper lip, medial epicanthus, neck | Cumulative dose of 3.55–11.7 mL of 5% il5FU in 6–12 injections | 100% resolution | Necrotic involution of KA |
| Eubanks et al., 1982 [67] | 1 | Multiple KAs on the arms, also over scar | 0.1–0.2 mL of 5% il5FU per lesion, 1 week apart; 5–9 injections | 100% resolution | None reported |
| Parker et al., 1986 [68] | 5 | Face | 1–3 mL of 5% il5FU, 1–4 weeks apart; 2–6 injections | 100% resolution | None reported |
| Bergin et al., 1986 [69] | 1 | Eyelid | 0.5 and 0.25 mL of 5% il5FU, 1 week apart | 100% resolution | Minimal pain with injection and edema |
| Singal et al., 1997 [70] | 1 | Multiple lesions on buttocks and legs (probably Ferguson-Smith type) | 0.2–0.3 mL of 5% il5-FU, 1 week apart for 3 weeks (only larger lesions) | Nearly complete resolution | None reported |
| Leonard et al., 2006 [71] | 1 | Nose | 8 injections of 5-FU (50 mg/mL), 1–2 weeks apart for 14 weeks | 100% resolution | None reported |
| Hadley et al., 2009 [63] | 3 | Multiple KAs over scar | 1 to 2 mL of 5% il5-FU, 1 week apart for 8 weeks with acitretin in 1 case | 2/3 resolution 1/3 discontinued | 1 case of shortness of breath during injection |
| LaPresto et al., 2013 [62] | 1 | 13 lesions (cheek, shoulder, chest, back, and leg) Patient with melanoma receiving vemurafenib | 1 injection of 5% il5-FU, 2.5 mL (mean: 0.2 mL per lesion) with acitretin | Nearly complete resolution and significant reduction in size | None reported |
| Que et al., 2018 [72] | 30 | 136 lesions (eruptive KA) Legs (80%) > arms (10%) and arms/legs (10%) | Median of 2 injections (1–8) of 0.5 mL (0.1–1.0) of 5% il5-FU per lesion every 3 weeks (2.0–8.5) | Focal koebnerizing eruptive KA: 91% complete resolution; diffuse KA type: 53% complete resolution | Mild; cutaneous dyspigmentation and shallow erosions |
| Dominiak et al., 2016 [73] | 1 | Leg (type KA centrifugum marginatum) | 2 injections of 0.6 mL of 5% il-5FU, 3 weeks apart | 100% resolution | Discomfort with injection |
| Hemperly et al., 2020 [74] | 1 | 2 lesions on arm (field cancerization, previous radiation) | 4–5 injections of 5% il5-FU, 1 week apart; cumulative doses: 22.5 mg and 32.5 mg of 5FU, respectively | 100% resolution | Tolerable pain during injection |
| Seger et al., 2020 [75] | 1 | Arm (eruptive KA over scar) | 4 injections of 5-FU, every 3–4 weeks; total of 5 mL of 5-FU | 100% resolution | None reported |
| Hamad et al., 2021 [59] | 46 | Not separated by tumor type; >legs | Mean of 1.6 injections of 0.1–1.0 mL of 5% 5-FU (max. 1 mL per 3 lesions), 2–8 weeks apart Prior debulking | 97.8% resolution | Temporary alopecia, nausea, fatigue, and dyspigmentation |
| Ahmed et al., 2022 [76] | 1 | 11 lesions (eruptive KA) on the legs after COVID-19 vaccine | 1.5 mL of 5% 5-FU per lesion, once every week for 3 weeks | 100% resolution | None reported |
| Yumeen et al., 2023 [61] | 1 | 2 lesions (eruptive KA) Patient with IgG immunodeficiency | 1 injection; dose not reported with acitretin and topical 5-FU/imiquimod | 100% resolution | None reported |
| Marka et al., 2023 [53] | 7 | 40 KAs, >legs | 2–13 injections of dilute 5-FU (10.0–16.7 mg/mL), 1 week to one month apart; mean dose per visit: 3.3–32.5 mg of 5-FU | 97.5% resolution | None reported |
| Author, Year | n | Location | 5-FU Administration | Outcome | Adverse Events Related to il5-FU |
|---|---|---|---|---|---|
| Kraus et al., 1998 [77] | 23 | Head and neck (10) > upper extremities (9) > trunk (4) | 4–6 injections of 3% 5-FU gel, 1 week apart; median cumulative dose: 3.7 mL (0.6–6) | 96% complete resolution | None reported |
| Morse et al., 2003 [78] | 1 | Face | 8 injections of 5-FU, 1 week apart; weekly dose: 0.8–2.4 ml | 100% resolution | None reported |
| Khandpur et al., 2003 [60] | 1 | 3 SCCs on thumb, toe, and ankle | Injection of 1 mL (per lesion) of 5FU 0.05 mg/mL, 4 days per week for 4 weeks | 100% resolution | None reported |
| Patient with arsenical keratosis | with acitretin | ||||
| Reisinger et al., 2011 [79] | 1 | Thumb | 6 injections of 5% 5-FU, 1 week apart; cumulative dose: 600 mg of 5-FU | 100% resolution | None reported |
| Mackey et al., 2018 [80] | 1 | 5 SCCs and 1 atypical squamous proliferation on the legs | 6 injections of 0.6 mL of 5% 5-FU per lesion, 1 week apart; cumulative dose: 180 mg per lesion | 100% resolution | None reported |
| Manalo et al., 2019 [58] | 2 | Multiple lesions on the legs | 4–12 injections of 0.1–0.5 mg 5FU, 1 week apart | 100% resolution | Ulcer at injection sites; infected ulcer |
| with chemowraps of 5% 5-FU and acitretin | |||||
| Dando et al., 2020 [57] | 3 | 7 lesions on the legs (6) and arm (1) | 1–2 injections of 0.3–1.0 mL 5% 5-FU | 85.7% resolution | Local pain (1) and mild pruritus (1) |
| Hamad et al., 2021 [59] | 230 | Not classified by tumor type; >legs | Mean of 1.6 injections of 0.1–1.0 mL of 5% 5-FU (max. 1 mL per 3 lesions), 2–8 weeks apart | 83% resolution in invasive SCC; higher resolution in well-differentiated SCC (90%) | Temporary alopecia, nausea, fatigue, and dyspigmentation |
| Prior debulking | |||||
| Maxfield et al., 2021 [54] | 148 | 172 lesions (7/172 KA subtype), >lower extremity (37%) | Mean of 1.25 injections of 0.2–2.0 mL 5% 5-FU per lesion, 4 weeks apart | 92% resolution | n = 5, mostly local site reactions; 1 patient with headache, dizziness, and nausea |
| Marka et al., 2023 [53] | 4 | 10 lesions, >legs | 1–6 injections of dilute 5-FU (10.0–16.7 mg/mL), 1 week to 1 month apart; mean dose per visit: 8.3–13.4 mg of 5-FU | 90% resolution | None reported |
| Luu et al., 2023 [81] | 15 | 23 lesions, >legs | Average of 4 injections (1–35) of 75 mg (50–150 mg) of dilute 5% 5-FU | 87% resolution and 8.7% size reduction | Ulceration (6) and infection (4) |
| Drug | Treatment | Mechanism of Action | Condition | Current Status | Phase | NCT Code | |
|---|---|---|---|---|---|---|---|
| Immunotherapy | Cemiplimab | Alone | Anti-PD1 | cSCC | Recruiting | Phase 1 | NCT03889912 [124] |
| Cemiplimab with vidutolimod | Anti-PD1 with CpG-A TLR9 | Advanced or mcSCC | Recruiting | Phase 2 | NCT04916002 [125] | ||
| CV8102 | Alone or with anti-PD1 | TLR7/8 and RIG-I | Advanced cSCC | Active, not recruiting | Phase 1 | NCT03291002 [126] | |
| E7766 | Alone | STING agonist | Advanced solid tumors | Completed | Phase 1/1b | NCT04144140 [127] | |
| Daromun | Alone | L19IL2/L19TNF | Non-metastatic cSCC and KA | Recruiting | Phase 2 | NCT05329792 [128] | |
| NCT04362722 [129] | |||||||
| Giloralimab | Alone or with budigalimab | Anti-CD40 | Advanced solid tumors | Active, not recruiting | Phase 1 | NCT02988960 [130] | |
| TSN222 | Alone | Bifunctional small molecule | Unresectable locally advanced or metastatic solid tumors | Not yet recruiting | Phase 1/2 | NCT05842785 [131] | |
| SAR441000 | Alone or with cemiplimab | mRNA mixture encoding IL-12 single chain, interferon alpha-2b, GM-CSF, and IL-15sushi | Advanced solid tumors | Active, not recruiting | Phase 1 | NCT03871348 [132] | |
| INT230-6 | Alone or with anti-PD1 | Cell permeation enhancer and cisplatin and vinblastin | Advanced or metastatic cSCC | Completed | Phase 1/2 | NCT03058289 [133] | |
| Oncolytic viruses | RP1 | Alone (neoadjuvant) | Oncolytic modified herpes simplex 1 | Resectable cSCC | Not yet recruiting | Phase 1b | NCT05858229 [134] |
| Alone or with cemiplimab | Oncolytic modified herpes simplex 1 | lacSCC and mcSCC | Active, not recruiting | Phase 2 | NCT04050436 [135] | ||
| Gebasaxturev | With pembrolizumab | Oncolytic coxsackievirus A21 | Advanced or metastatic SCC | Completed | Phase 1b/2 | NCT04521621 [136] | |
| TBio-6517 | With pembrolizumab | Oncolytic vaccinia virus | Locally advanced or metastatic SCC | Active, not recruiting | Phase 1/2a | NCT04301011 [137] | |
| Cancer vaccines | IFx-Hu2.0 | Alone | Bacterial protein emm55 | cSCC | Completed | Phase 1 | NCT04925713 [138] |
| Alone | Advanced nonmelanoma skin cancer | Recruiting | Phase 1 | NCT04160065 [139] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Hernández, G.; Cañueto, J. Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma. Cancers 2024, 16, 158. https://doi.org/10.3390/cancers16010158
Baeza-Hernández G, Cañueto J. Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma. Cancers. 2024; 16(1):158. https://doi.org/10.3390/cancers16010158
Chicago/Turabian StyleBaeza-Hernández, Gloria, and Javier Cañueto. 2024. "Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma" Cancers 16, no. 1: 158. https://doi.org/10.3390/cancers16010158
APA StyleBaeza-Hernández, G., & Cañueto, J. (2024). Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma. Cancers, 16(1), 158. https://doi.org/10.3390/cancers16010158







