Real-World Effectiveness, Safety, and Health-Related Quality of Life in Patients Receiving Adjuvant Nivolumab for Melanoma in Belgium and Luxembourg: Results of PRESERV MEL †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessments
2.3. Statistical Analysis
3. Results
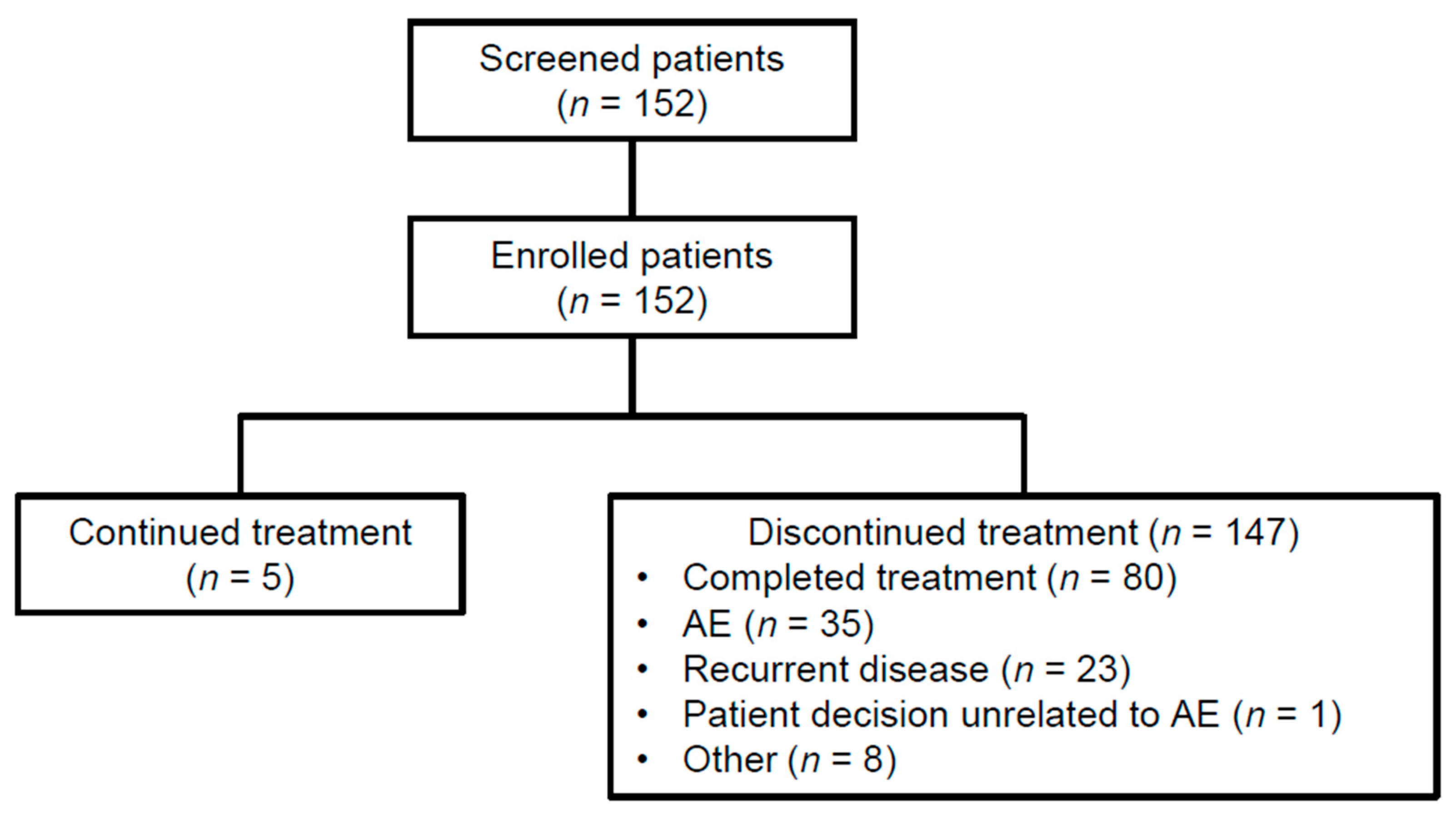
3.1. Patient Disposition and Baseline Characteristics
3.2. Treatment Exposure
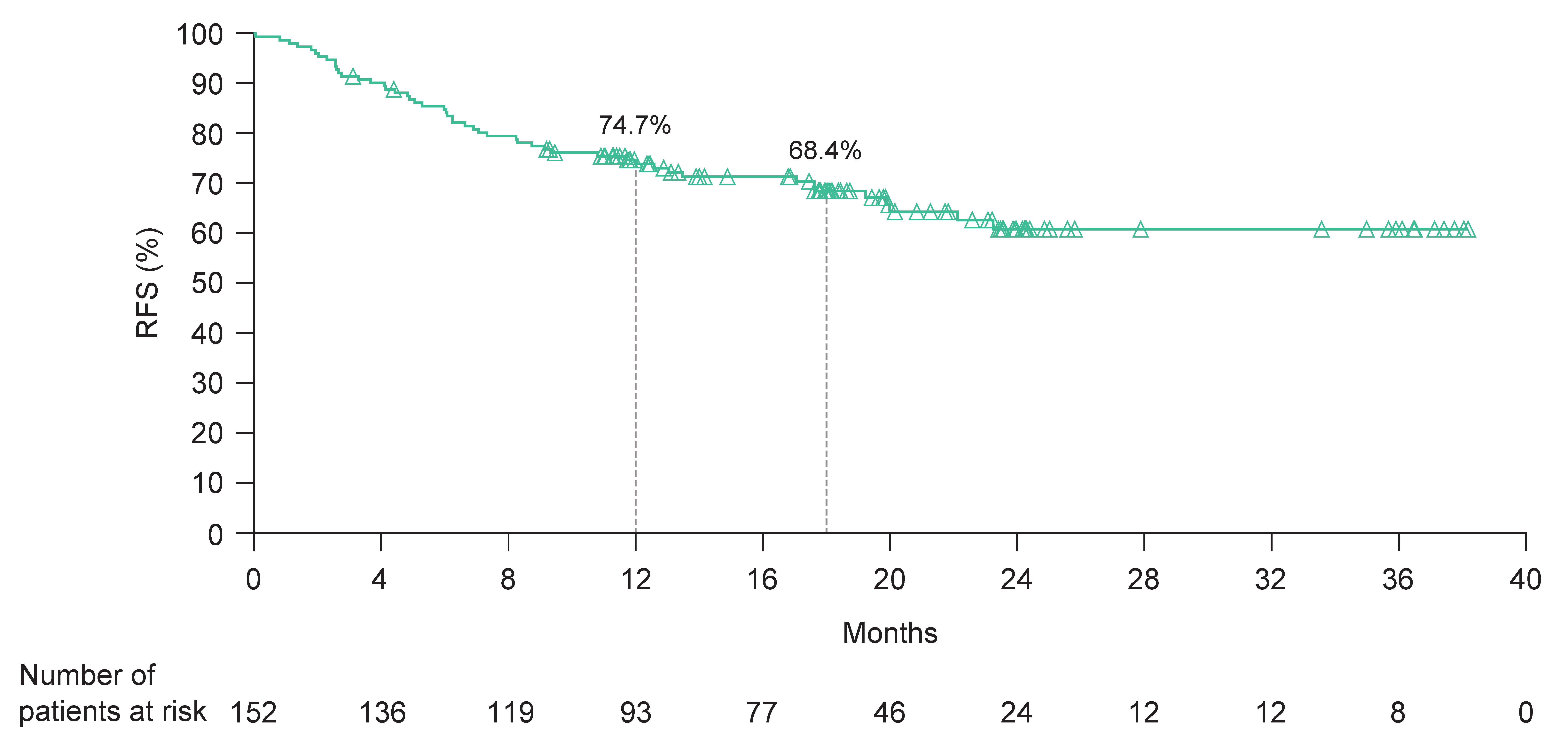
3.3. Effectiveness
3.4. Safety
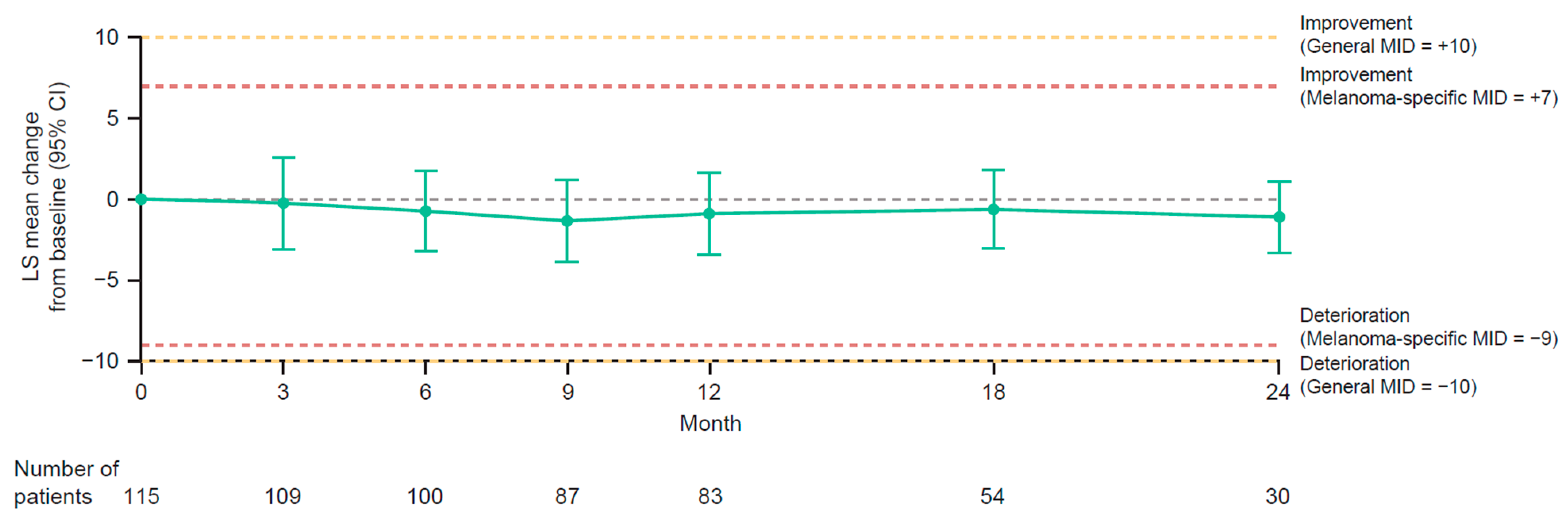
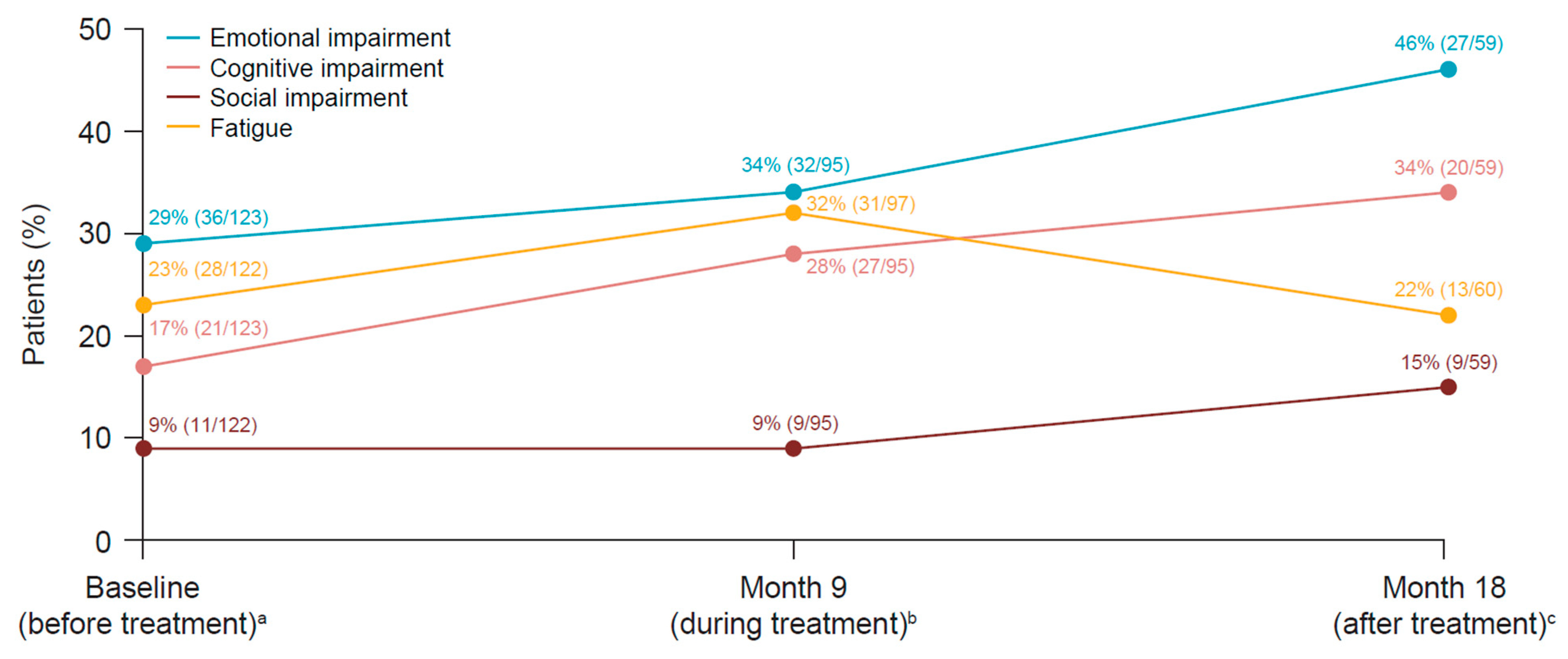
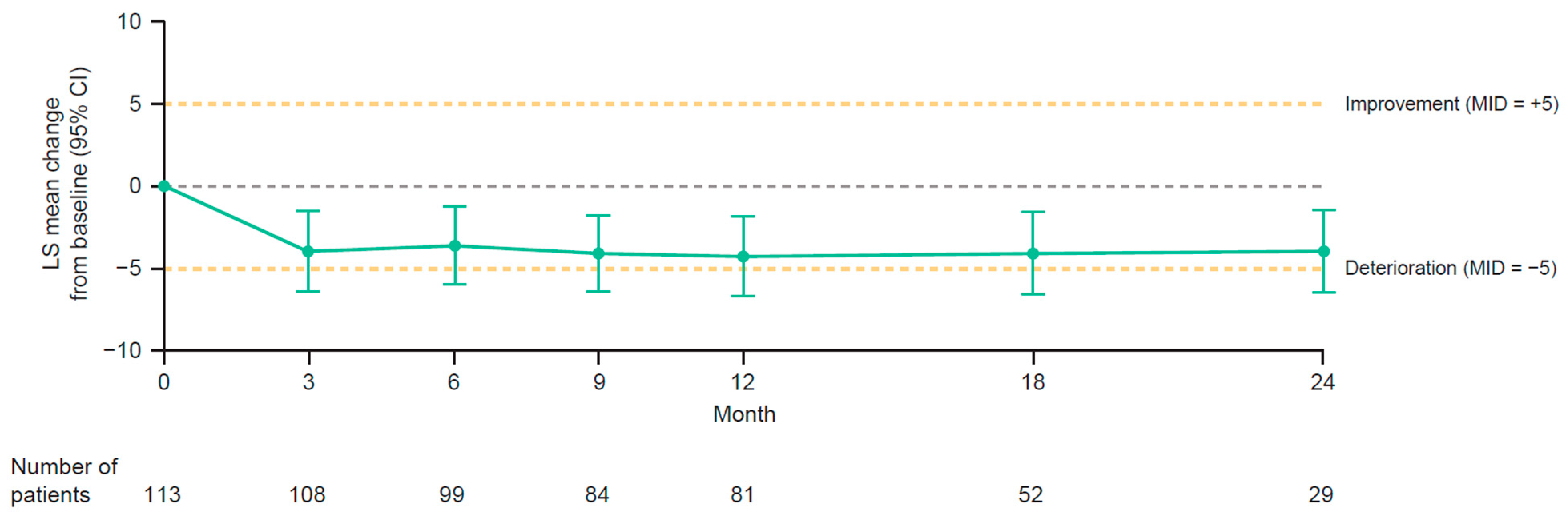
3.5. Health-Related Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Golgas, M.; Arance, A.M.; Dalle, S.D.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Gogas, H.J.; Sun, X.; Yip, C.; Taylor, F.; Braverman, J.; Lobo, M.; Thakkar, P.K.; Moshyk, A.; Larkin, J.; et al. Association of health-related quality of life and treatment safety with nivolumab in patients with resected stage IIIB–C or stage IV melanoma: Analysis of CheckMate 238 4-year follow-up data. In Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual, 4–8 June 2021. Poster 9574. [Google Scholar]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant therapy of nivolumab combined with ipilimumab versus nivolumab alone in patients with resected stage IIIB-D or stage IV melanoma (CheckMate 915). J. Clin. Oncol. 2022, 41, 517. [Google Scholar] [CrossRef] [PubMed]
- OPDIVO® (nivolumab) [Summary of Product Characteristics]. EPAR—European Medicines Agency. Published 16 July 2015. Updated 13 September 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf (accessed on 15 June 2022).
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Neyns, B.; Willemot, L.; McDonald, L.; Amandi, A.; Vouk, K.; Rorive, A. Real-world outcomes of nivolumab in adjuvant melanoma in Belgium and Luxembourg (PRESERV MEL). In Proceedings of the Society for Melanoma Research (SMR) International Congress, New Orleans, LA, USA, 28–31 October 2021. Poster #53. [Google Scholar] [CrossRef]
- Rogiers, A.; Willemot, L.; McDonald, L.; Van Campenhout, H.; Vouk, K.; Berchem, G.; Jacobs, C.; Rorive, A.; Neyns, B. PRESERV MEL: Real-world outcomes and health-related quality of life in patients receiving adjuvant nivolumab for melanoma in Belgium and Luxembourg. In Proceedings of the European Society for Medical Oncology 47th Congress—ESMO 2022, Paris, France, 9–13 September 2022. [Google Scholar]
- US Food & Drug Administration. Guidance for Industry—Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. December 2009. Available online: www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed on 18 August 2022).
- Rogiers, A.; Leys, C.; De Cremer, J.; Awada, G.; Schembri, A.; Theuns, P.; De Ridder, M.; Neyns, B. Health-related quality of life, emotional burden, and neurocognitive function in the first generation of metastatic melanoma survivors treated with pembrolizumab: A longitudinal pilot study. Support. Care Cancer 2020, 28, 3267–3278. [Google Scholar] [CrossRef]
- Rogiers, A.; Leys, C.; Lauwyck, J.; Schembri, A.; Awada, G.; Schwarze, J.K.; De Cremer, J.; Theuns, P.; Maruff, P.; De Ridder, M.; et al. Neurocognitive function, psychosocial outcome, and health-related quality of life of the first-generation metastatic melanoma survivors treated with ipilimumab. J. Immunol. Res. 2020, 2020, 2192480. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Rogiers, A.; Jozwiak, K.; Boers-Sonderen, M.J.; van den Eertwegh, A.J.; Hospers, G.A.; de Groot, J.W.B.; Aarts, M.J.B.; Kapiteijn, E.; Ten Tije, A.J.; et al. Health-related quality of life of long-term advanced melanoma survivors treated with anti-CTLA-4 immune checkpoint inhibition compared to matched controls. Acta Oncol. 2021, 60, 69–77. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Cormier, J.N.; Ross, M.I.; Gershenwald, J.E.; Lee, J.E.; Mansfield, P.F.; Camacho, L.H.; Kim, K.; Webster, K.; Cella, D.; Palmer, J.L. Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer 2008, 112, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P. Modeling valuations for EuroQol health states. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- EQ-5D-3L User Guide. Available online: https://euroqol.org/publications/user-guides/ (accessed on 12 December 2022).
- Musoro, J.Z.; Bottomley, A.; Coens, C.; Eggermont, A.M.; King, M.T.; Cocks, K.; Sprangers, M.A.; Groenvold, M.; Velikova, G.; Flechtner, H.-H.; et al. Interpreting European Organisation for Research and Treatment for Cancer Quality of life Questionnaire core 30 scores as minimally importantly different for patients with malignant melanoma. Eur. J. Cancer 2018, 104, 169–181. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Bharmal, M.; Nolte, S.; Henry-Szatkowski, M.; Hennessy, M.; Schlichting, M. Update on the psychometric properties and minimal important difference (MID) thresholds of the FACT-M questionnaire for use in treatment-naïve and previously treated patients with metastatic Merkel cell carcinoma. Health Qual. Life Outcomes 2020, 18, 145. [Google Scholar] [CrossRef]
- Askew, R.L.; Xing, Y.; Palmer, J.L.; Cella, D.; Moye, L.A.; Cormier, J.N. Evaluating minimal important differences for the FACT-Melanoma quality of life questionnaire. Value Health 2009, 12, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5, 70, Correction in Health Qual. Life Outcomes 2010, 8, 4. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Loth, F.L.; Aaronson, N.K.; Arraras, J.I.; Caocci, G.; Efficace, F.; Groenvold, M.; van Leeuwen, M.; Petersen, M.A.; Ramage, J.; et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. 2020, 118, 1–8. [Google Scholar] [CrossRef]
- Nolte, S.; Liegl, G.; Petersen, M.A.; Aaronson, N.K.; Costantini, A.; Fayers, P.M.; Groenvold, M.; Bholzner, B.; Johnson, C.D.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15, 386 persons across 13 European countries, Canada and the United States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- König, H.H.; Bernert, S.; Angermeyer, M.C.; Matschinger, H.; Martinez, M.; Vilagut, G.; Haro, J.M.; de Girolamo, G.; de Graaf, R.; Kovess, V.; et al. Comparison of population health status in six European countries: Results of a representative survey using the EQ-5D questionnaire. Med. Care 2009, 47, 255–261. [Google Scholar] [CrossRef]
- For a Healthy Belgium, National Health Survey, Belgium. 2018. Available online: https://www.healthybelgium.be/en/health-status/life-expectancy-and-quality-of-life/quality-of-life (accessed on 30 May 2023).
- Larkin, J.; Weber, J.; Del Vecchio, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab (CheckMate 238 trial): Reassessment of 4-year efficacy outcomes in patients with stage III melanoma per AJCC-8 staging criteria. Eur. J. Cancer 2022, 173, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Samlowski, W.; Robert, N.J.; Poretta, T.; Moshyk, A.; Rajkumar, J.; Salvatore, A.; Stwalley, B.; Nwokeji, E. Real-world outcomes of patients with resected stage IIIA melanoma treated with adjuvant nivolumab. In Proceedings of the Society for Immunotherapy of Cancer (SITC) Annual Meeting, Virtual, 9–14 November 2020. Poster 220. [Google Scholar]
- Moser, J.; Pavlick, A.C.; Poretta, T.; Sakkal, L.A.; Moshyk, A.; Gu, J.; Zhang, Y.; Amin, A. Real-world outcomes of patients with resected stage IIIA melanoma treated with adjuvant nivolumab or monitored with observation. In Proceedings of the Society for Melanoma Research (SMR) International Congress, Virtual, 28–31 October 2021. Poster 51. [Google Scholar]
- Moser, J.C.; Pavlick, A.C.; Poretta, T.; Sakkal, L.A.; Moshyk, A.; Hao, Y.; Zhang, Y.; Palaia, J.; Amin, A. Real-world outcomes of patients with resected stage IIIA melanoma treated with adjuvant nivolumab or monitored with observation. In Proceedings of the 19th International Congress of the Society for Melanoma Research (SMR), Edinburgh, UK, 17–20 October 2022. [Google Scholar]
- Pavlick, A.C.; Amin, A.; Moser, J.C.; Poretta, T.; Sakkal, L.A.; Moshyk, A.; Klink, A.J.; Schuler, T.; Feinberg, B. Outcomes in patients with resected stage IIIA melanoma treated with adjuvant nivolumab or monitored with observation: A real-world study. In Proceedings of the 19th International Congress of the Society for Melanoma Research (SMR), Edinburgh, UK, 17–20 October 2022. [Google Scholar]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant therapy with nivolumab versus ipilimumab after complete resection of stage III/IV melanoma: Updated results from a phase 3 trial (CheckMate 238). In Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, USA, 1–5 June 2018. abstract 9502. [Google Scholar]
- Long, G.V.; Del Vecchio, M.; Weber, J.; Hoeller, C.; Grob, J.-J.; Mohr, P.; Grabbe, S.; Dutriaux, C.; Chiarion-Sileni, V.; Mackiewicz, J.; et al. Adjuvant therapy with nivolumab versus placebo in patients with resected stage IIB/C melanoma (CheckMate 76K). In Proceedings of the 19th International Congress of the Society for Melanoma Research (SMR), Edinburgh, UK, 17–20 October 2022. [Google Scholar]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.S.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Queirolo, P.; Specchia, C.; Cocorocchio, E.; Ferrucci, P.; Patanè, D.; Saponara, M.; et al. Association of anticancer immune checkpoint inhibitors with patient-reported outcomes assessed in randomized clinical trials: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e2226252. [Google Scholar] [CrossRef]
- Malkhasyan, K.A.; Zakharia, Y.; Milhem, M. Quality-of-life outcomes in patients with advanced melanoma: A review of the literature. Pigment. Cell Melanoma Res. 2017, 30, 511–520. [Google Scholar] [CrossRef]
- Mamoor, M.; Postow, M.A.; Lavery, J.A.; Baxi, S.S.; Khan, N.; Mao, J.J.; Rogak, L.J.; Sidlow, R.; Thom, B.; Wolchok, J.A.; et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J. Immunother. Cancer 2020, 8, e000260. [Google Scholar] [CrossRef]
- Kiss, I.; Kuhn, M.; Hrusak, K.; Buchler, T. Incidence of fatigue associated with immune checkpoint inhibitors in patients with cancer: A meta-analysis. ESMO Open 2022, 7, 100474. [Google Scholar] [CrossRef]
- Khan, M.A.; Florou, V.; Swami, U. Immunotherapy and fatigue: What we know and what we don’t know. Oncotarget 2021, 12, 719–720. [Google Scholar] [CrossRef]
- Schagen, S.B.; Tsvetkov, A.S.; Compter, A.; Wefel, J.S. Cognitive adverse effects of chemotherapy and immunotherapy: Are interventions within reach? Nat. Rev. Neurol. 2022, 18, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Bartels, F.; Strönisch, T.; Farmer, K.; Rentzsch, K.; Kiecker, F.; Finke, C. Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann. Oncol. 2019, 30, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Lai-Kwon, J.; Inderjeeth, A.J.; Lisy, K.; Sandhu, S.; Rutherford, C.; Jefford, M. Impact of immune checkpoint inhibitors and targeted therapy on health-related quality of life of people with stage III and IV melanoma: A mixed-methods systematic review. Eur. J. Cancer 2023, 184, 83–105. [Google Scholar] [CrossRef]
- Rogiers, A.; Boekhout, A.; Schwarze, J.K.; Awada, G.; Blank, C.U.; Neyns, B. Long-term survival, quality of life, and psychosocial outcomes in advanced melanoma patients treated with immune checkpoint inhibitors. J. Oncol. 2019, 2019, 5269062. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Adjuvant Nivolumab (n = 152) |
|---|---|
| Median age, years (range) | 60.0 (29–85) |
| Male, n (%) | 80 (53) |
| Primary melanoma subtype, n (%) a | |
| Superficial spreading | 69 (45) |
| Nodular | 37 (24) |
| Acral lentiginous | 6 (4) |
| Lentigo maligna | 2 (1) |
| Not otherwise specified | 29 (19) |
| Missing | 9 (6) |
| Primary melanoma location, n (%) | |
| Limbs | 86 (57) |
| Trunk | 47 (31) |
| Head/neck | 10 (7) |
| Missing | 9 (6) |
| Primary melanoma resection, n (%) | 143 (94) |
| SLNB performed, n (%) | 127 (84) |
| ≥1 positive sentinel lymph node, n (%) | |
| Yes | 93 (61) |
| No | 31 (20) |
| Missing | 28 (18) |
| Complete lymph node dissection, n (%) | |
| Yes | 53 (35) |
| No | 97 (64) |
| Missing | 2 (1) |
| Complete lymph node dissection in patients with non-occult disease, n (%) | |
| Yes | 21 (14) |
| No | 18 (12) |
| Clinically occult only lymph node involvement, n (%) | 74 (49) |
| Resected stage at baseline (per AJCC-8), n (%) | |
| IIIA | 28 (18) |
| IIIB | 41 (27) |
| IIIC | 51 (34) |
| IIID | 6 (4) |
| IV | 17 (11) |
| Other | 8 (5) |
| Missing | 1 (1) |
| Tumor ulceration patients with stage III disease, n (%) | |
| Yes | 48 (32) |
| No | 69 (45) |
| Missing | 9 (6) |
| SLN metastasis with longest diameter < 1 mm, n (%) | |
| Yes | 13 (9) |
| No | 63 (41) |
| Missing | 51 (34) |
| Stage IIIA with a longest diameter of the sentinel lymph node metastasis < 1 mm, n (%) | |
| Yes | 10 (7) b |
| No | 12 (8) |
| Missing | 6 (4) |
| BRAF status, n (%) | |
| Mutant (positive) | 58 (38) |
| Wild-type (negative) | 65(43) |
| Unknown | 2 (1) |
| Missing | 27 (18) |
| ECOG performance status, n (%) | |
| 0 | 97 (64) |
| 1 | 14 (9) |
| 2 | 1 (1) |
| Missing | 40 (26) |
| Previous enrollment in an interventional study, n (%) | 2 (1) |
| Median time from surgical resection to index date, months (range) | 1.2 (0.1–14.2) |
| Adjuvant Nivolumab (n = 152) | ||
|---|---|---|
| Any Grade, n (%) | Grade 3 or 4, n (%) | |
| Any AE | 146 (96) | 49 (32) |
| TRAE | 131 (86) | 21 (14) |
| Any AE leading to treatment discontinuation | 35 (23) | 15 (10) |
| TRAE leading to treatment discontinuation | 29 (19) | 12 (8) |
| Adjuvant Nivolumab (n = 152) | ||
|---|---|---|
| Any Grade, n (%) | Grade 3 or 4, n (%) b | |
| TRAE | 131 (86) | 21 (14) |
| Fatigue | 75 (49) | 1 (1) |
| Pruritus | 36 (24) | 0 |
| Diarrhea | 23 (15) | 1 (1) |
| Hypothyroidism | 22 (14) | 0 |
| Nausea | 16 (11) | 0 |
| Hyperthyroidism | 14 (9) | 0 |
| Dry mouth | 12 (8) | 0 |
| Rash | 12 (8) | 0 |
| Dry skin | 9 (6) | 0 |
| Arthralgia | 9 (6) | 0 |
| Colitis | 8 (5) | 2 (1) |
| Myalgia | 8 (5) | 1 (1) |
| Headache | 8 (5) | 0 |
| Hepatitis | 7 (5) | 2 (1) |
| Total (n = 152) | ||
|---|---|---|
| Any Grade, n (%) | Grade 3 or 4, n (%) | |
| Hepatitis | 7 (5) | 2 (1) a |
| Pneumonitis | 6 (4) | 3 (2) b |
| Adrenal insufficiency | 4 (3) | 1 (1) c |
| Renal disorders | 3 (2) | 0 |
| Meningitis aseptic | 1 (1) | 1 (1) d |
| Myasthenia gravis | 1 (1) | 0 |
| Myocarditis | 1 (1) | 1 (1) d |
| Subscale | Mean Score (SD) | Difference | |
|---|---|---|---|
| PRESERV MEL (n = 117) a | General Population | ||
| EORTC QLQ-C30 | |||
| Global health status/QoL | 73.8 (19.8) | 66.1 (21.7) b | 7.7 (4.1 to 11.4) |
| Physical functioning | 88.5 (17.4) | 85.1 (18.9) b | 3.4 (0.2 to 6.6) |
| Role functioning | 80.3 (27.8) | 84.3 (24.6) b | −4.0 (−9.0 to 1.1) |
| Emotional functioning | 78.3 (22.5) | 74.2 (24.7) b | 4.1 (0.1 to 8.2) |
| Cognitive functioning | 89.6 (19.7) | 84.8 (21.3) b | 4.8 (1.2 to 8.4) |
| Social functioning | 87.9 (20.8) | 86.2 (24.1) b | 1.7 (−2.1 to 5.5) |
| Fatigue | 24.9 (24.4) | 29.5 (25.5) b | −4.6 (−9.1 to −0.2) |
| Nausea/vomiting | 1.7 (7.7) | 5.9 (16.0) b | −4.2 (−5.6 to −2.8) |
| Pain | 19.8 (24.9) | 23.5 (27.1) b | −3.7 (−8.2 to 0.8) |
| Dyspnea | 7.4 (18.6) | 15.9 (24.6) b | −8.5 (−11.9 to −5.1) |
| Insomnia | 26.5 (30.8) | 26.6 (30.3) b | −0.1 (−5.7 to 5.5) |
| Appetite loss | 10.3 (21.6) | 10.0 (21.6) b | 0.3 (−3.7 to 4.1) |
| Constipation | 10.1 (21.2) | 12.5 (23.3) b | −2.4 (−6.3 to 1.4) |
| Diarrhea | 5.7 (16.0) | 9.5 (20.9) b | −3.8 (−6.7 to −0.9) |
| Financial difficulties | 4.8 (17.1) | 10.6 (23.6) b | −5.8 (−8.9 to −2.6) |
| EQ-5D-3L | |||
| VAS | 79.5 (17.3) | Female: 76.7 (0.8) c; Male: 78.6 (0.8) c | NA |
| Utility index | 0.82 (0.18) | 0.79 (95% CI: 0.786–0.799) d | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogiers, A.; Willemot, L.; McDonald, L.; Van Campenhout, H.; Berchem, G.; Jacobs, C.; Blockx, N.; Rorive, A.; Neyns, B. Real-World Effectiveness, Safety, and Health-Related Quality of Life in Patients Receiving Adjuvant Nivolumab for Melanoma in Belgium and Luxembourg: Results of PRESERV MEL. Cancers 2023, 15, 4823. https://doi.org/10.3390/cancers15194823
Rogiers A, Willemot L, McDonald L, Van Campenhout H, Berchem G, Jacobs C, Blockx N, Rorive A, Neyns B. Real-World Effectiveness, Safety, and Health-Related Quality of Life in Patients Receiving Adjuvant Nivolumab for Melanoma in Belgium and Luxembourg: Results of PRESERV MEL. Cancers. 2023; 15(19):4823. https://doi.org/10.3390/cancers15194823
Chicago/Turabian StyleRogiers, Anne, Laurence Willemot, Laura McDonald, Hilde Van Campenhout, Guy Berchem, Celine Jacobs, Nathalie Blockx, Andrée Rorive, and Bart Neyns. 2023. "Real-World Effectiveness, Safety, and Health-Related Quality of Life in Patients Receiving Adjuvant Nivolumab for Melanoma in Belgium and Luxembourg: Results of PRESERV MEL" Cancers 15, no. 19: 4823. https://doi.org/10.3390/cancers15194823
APA StyleRogiers, A., Willemot, L., McDonald, L., Van Campenhout, H., Berchem, G., Jacobs, C., Blockx, N., Rorive, A., & Neyns, B. (2023). Real-World Effectiveness, Safety, and Health-Related Quality of Life in Patients Receiving Adjuvant Nivolumab for Melanoma in Belgium and Luxembourg: Results of PRESERV MEL. Cancers, 15(19), 4823. https://doi.org/10.3390/cancers15194823






