Single Ultra-High Dose Rate Proton Transmission Beam for Whole Breast FLASH-Irradiation: Quantification of FLASH-Dose and Relation with Beam Parameters
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Planning
- (1)
- The first scenario used a single TB and placed spots of equal intensity, e.g., equal monitor units (MUs), in a uniform square grid. The spot spacing distance of the grid was varied between 4 and 10 mm to determine the grid that achieved the best trade-off between plan quality and the amount of FLASH-dose. For this simple scenario, it was expected that typically a larger portion of the dose could be delivered at UHDRs for larger spot spacing, but that plan quality would be sacrificed;
- (2)
- In the second scenario, we used a TB with a similar uniform grid and the optimal spot spacing distance obtained from the first scenario, but allowing spots to have different intensities. It was expected that most spot MUs would be high and within a narrow range, and that lower MU spots would only be necessary at the target boundary. As previous research has shown that low MU spots can substantially decrease the dose rate [23,25], different minimum MU (minMU) thresholds were used (100–800 MU), meaning that spots with fewer MUs than this threshold were removed after optimization. Similar to the previous scenario, a higher minMU threshold will likely lead to higher dose rates, but at the expense of plan quality;
- (3)
- In order to increase the amount of FLASH-dose without affecting dosimetry, we included a third scenario, which uses the concept of beam splitting described earlier for head-and-neck plans [25]. The single TB was split into two beams: one so-called FLASH-beam delivering all spots with MUs above a certain splitMU threshold and another beam delivering the remaining spots. The FLASH-beam can be delivered at a higher current as it is not limited by lower MU spots, and this will presumably lead to a higher FLASH-dose. The second beam with low MU spots was not expected to meet the FLASH-requirements but was necessary to improve plan quality. Splitting was only considered for one plan in the second scenario, namely the plan with the lowest minMU threshold of 100. This plan was expected to achieve the best quality, as more freedom in spot intensity was allowed. SplitMU thresholds of 100–1000 were evaluated (with splitMU = 100 corresponding to a non-split plan).
2.2. Machine Parameters
3. Results
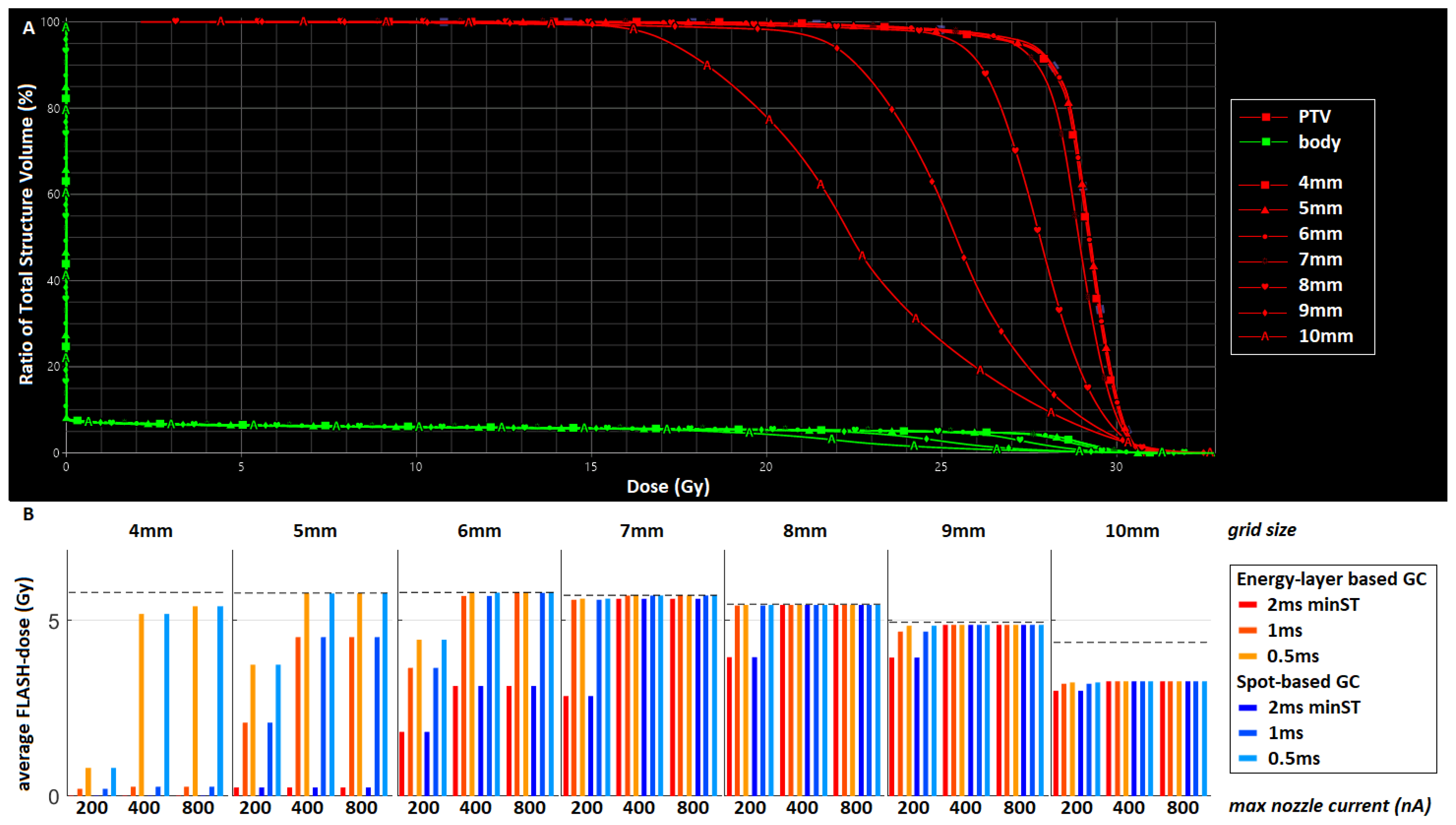
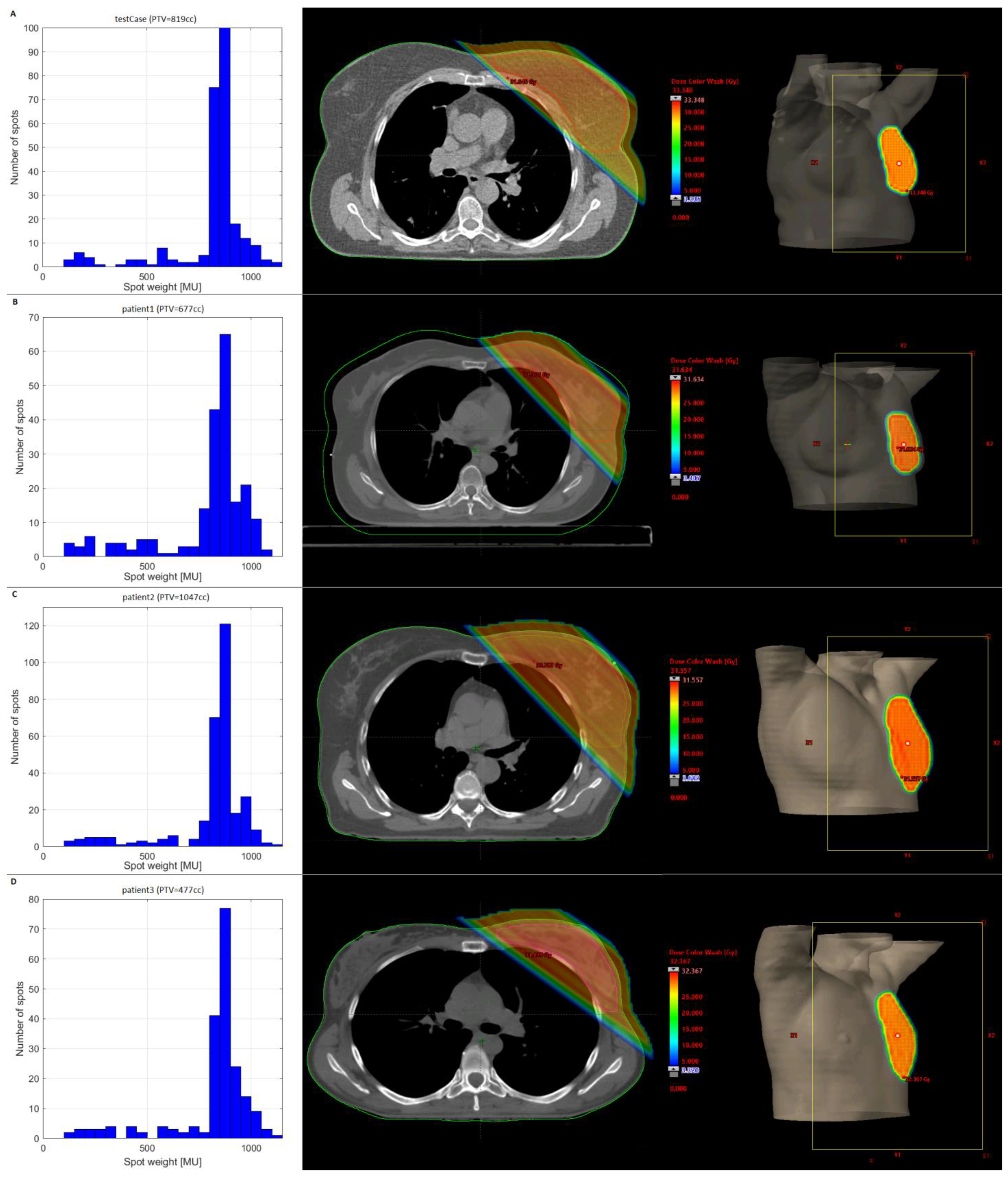
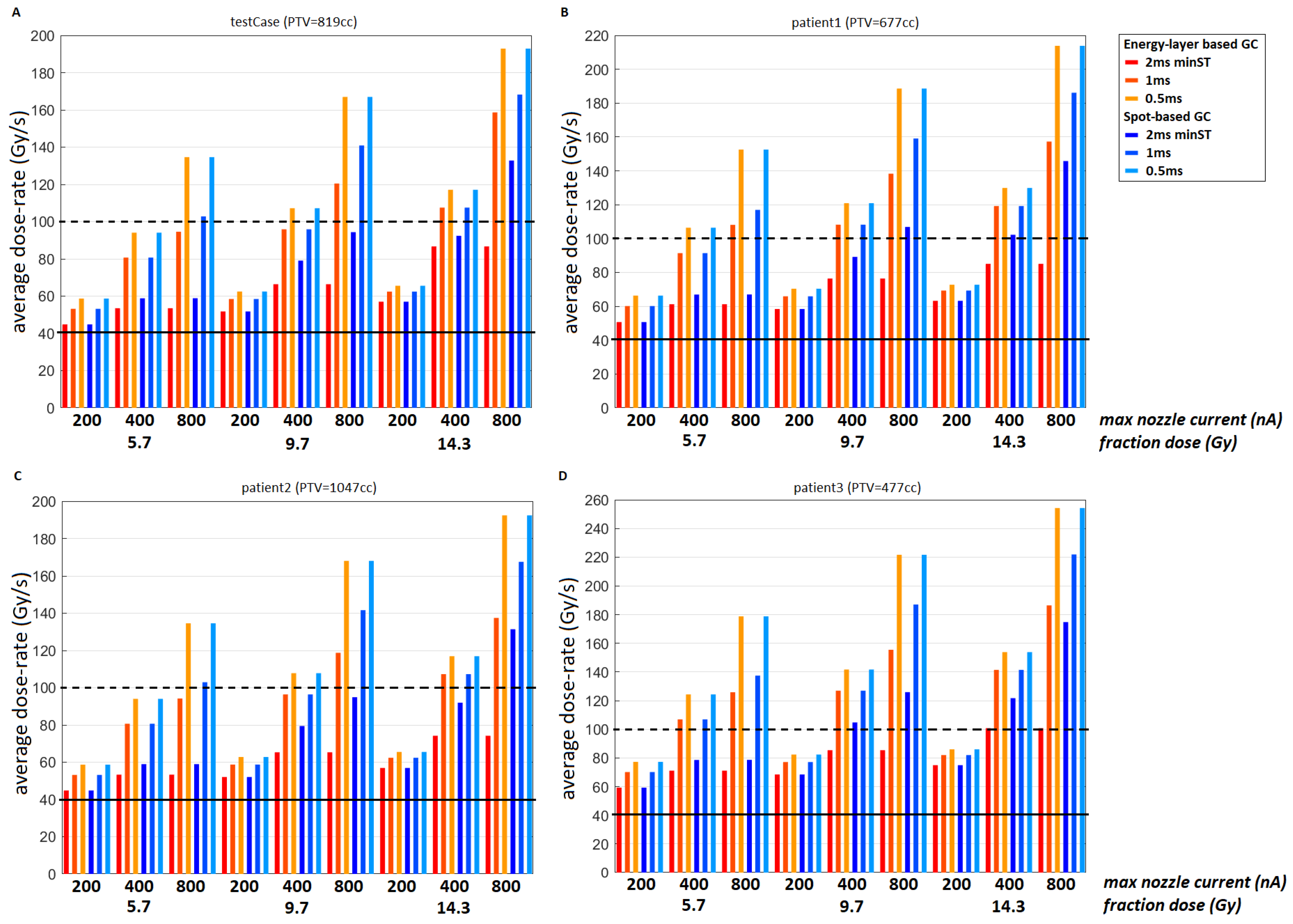
3.1. Test Case
3.2. Clinical Datasets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adrian, G.; Konradsson, E.; Beyer, S.; Wittrup, A.; Butterworth, K.T.; McMahon, S.J.; Ghita, M.; Petersson, K.; Ceberg, C. Cancer cells can exhibit a sparing FLASH effect at low doses under normoxic in vitro-conditions. Front. Oncol. 2021, 11, 686142. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Abel, E.; Katsis, A.; Rodriquez, A.; Senapati, S.; KuVillanueva, A.; Jackson, I.L.; Eley, J.; Vujaskovic, Z.; Parry, R. FLASH: A novel paradigm changing tumor irradiation platform that enhances therapeutic ratio by reducing normal tissue toxicity and activating immune pathways. Abstract AACR 2019. Cancer Res. 2019, 79, LB-280. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Zhu, H.; Wang, J.; Xiao, D.; Zhou, Z.; Dai, T.; Zhang, Y.; Feng, G.; Li, J.; et al. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother. Oncol. 2021, 166, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Jorge, P.G.; Petit, B.; Petridis, I.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Dokic, I.; Meister, S.; Bojcevski, J.; Tessonnier, T.; Walsh, D.; Knoll, M.; Mein, S.; Tang, Z.; Vogelbacher, L.; Rittmueller, C.; et al. Neuroprotective effects of ultra-high dose rate flash bragg peak proton irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 614–623. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Kim, M.M.; Verginadis, I.I.; Goia, D.; Haertter, A.; Shoniyozov, K.; Zou, W.; Maity, A.; Busch, T.M.; Metz, J.M.; Cengel, K.A.; et al. Comparison of FLASH proton entrance and the spread-out Bragg peak dose regions in the sparing of mouse intestinal crypts and in a pancreatic tumor model. Cancers 2021, 13, 4244. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.; Shoniyozov, K.; Chiango, J.; et al. FLASH proton radiotherapy spares normal epithelial and mesenchymal tissues while preserving sarcoma response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Sitarz, M.K.; Ankjærgaard, C.; Johansen, J.; Andersen, C.E.; Kanouta, E.; Overgaard, C.; Grau, C.; Poulsen, P. In vivo validation and tissue sparing factor for acute damage of pencil beam scanning proton FLASH. Radiother. Oncol. 2021, 167, 109–115. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The advantage of flash radiotherapy confirmed in mini-pig and cat-cancer patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef] [PubMed]
- FAST-01 Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04592887 (accessed on 1 May 2022).
- IMPulse Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02044471 (accessed on 1 May 2022).
- Berthelsen, A.K.; Dobbs, J.; Kjellén, E.; Landberg, T.; Möller, T.; Nilsson, P.; Specht, L.; Wambersie, A. What’s new in target volume definition for radiologists in ICRU Report 71? How can the ICRU volume definitions be integrated in clinical practice? Cancer Imaging 2007, 7, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Bellon, J.R.; Blitzblau, R.; Freedman, G.; Haffty, B.; Hahn, C.; Halberg, F.; Hoffman, K.; Horst, K.; Moran, J.; et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 2018, 8, 145–152. [Google Scholar] [CrossRef]
- Allali, S.; Kirova, Y. Radiodermatitis and Fibrosis in the Context of Breast Radiation Therapy: A Critical Review. Cancers 2021, 13, 5928. [Google Scholar] [CrossRef]
- Yarnold, J.R.; Brunt, A.M.; Chatterjee, S.; Somaiah, N.; Kirby, A.M. From 25 fractions to five: How hypofractionation has revolutionized adjuvant breast radiotherapy. Clin. Oncol. 2022, 34, 280–287. [Google Scholar] [CrossRef]
- van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W.F.A.R. Bringing FLASH to the clinic: Treatment planning considerations for ultrahigh dose-rate proton beams. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 621–629. [Google Scholar] [CrossRef]
- van Marlen, P.; Verbakel, W.F.; Slotman, B.J.; Dahele, M. Single-fraction 34 Gy lung stereotactic body radiation therapy using proton transmission beams: Flash-dose calculations and the influence of different dose-rate methods and dose/dose-rate thresholds. Adv. Radiat. Oncol. 2022, 7, 100954. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W.; Harris, J.R. Irradiation in early-stage breast cancer: Conventional whole-breast, accelerated partial-breast, and accelerated whole-breast strategies compared. Oncology 2012, 26, 820. [Google Scholar]
- van Marlen, P.; Dahele, M.; Folkerts, M.; Abel, E.; Slotman, B.J.; Verbakel, W.F.A.R. Ultra-high dose rate transmission beam proton therapy for conventionally fractionated head and neck cancer: Treatment planning and dose-rate distributions. Cancers 2021, 13, 1859. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-high dose rate (FLASH) radiotherapy: Silver bullet or fool’s gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [PubMed]
- Chabi, S.; Van To, T.H.; Leavitt, R.; Poglio, S.; Jorge, P.G.; Jaccard, M.; Petersson, K.; Petit, B.; Roméo, P.-H.; Pflumio, F.; et al. Ultra-high-dose-rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.M.; Hasan, Y.; Waller, J.; Saulsberry, L.; Huo, D. Has Hypofractionated Whole-Breast Radiation Therapy Become the Standard of Care in the United States? An Updated Report from National Cancer Database. Clin. Breast Cancer 2021, 22, e8–e20. [Google Scholar] [CrossRef] [PubMed]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Brunt, A.; Haviland, J.; Kirby, A.; Somaiah, N.; Wheatley, D.; Bliss, J.; Yarnold, J. Five-fraction Radiotherapy for Breast Cancer: FAST-Forward to Implementation. Clin. Oncol. 2021, 33, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.M.; Haviland, J.S.; Sydenham, M.; Agrawal, R.K.; Algurafi, H.; Alhasso, A.; Barrett-Lee, P.; Bliss, P.; Bloomfield, D.; Bowen, J.; et al. Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J. Clin. Oncol. 2020, 38, 3261–3272. [Google Scholar] [CrossRef]
- Lewis, P.; Brunt, A.M.; Coles, C.; Griffin, S.; Locke, I.; Roques, T. Moving Forward Fast with FAST-Forward. Clin. Oncol. 2021, 33, 427–429. [Google Scholar] [CrossRef]
- Spencer, K.; Jones, C.M.; Girdler, R.; Roe, C.; Sharpe, M.; Lawton, S.; Miller, L.; Lewis, P.; Evans, M.; Sebag-Montefiore, D.; et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: A population-based study. Lancet Oncol. 2021, 22, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, S.; Safai, S.; Schippers, J.M.; Weber, D.C.; Lomax, A.J. Towards FLASH proton therapy: The impact of treatment planning and machine characteristics on achievable dose rates. Acta Oncol. 2019, 58, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, M.M.; Abel, E.; Busold, S.; Perez, J.R.; Krishnamurthi, V.; Ling, C.C. A framework for defining FLASH dose rate for pencil beam scanning. Med. Phys. 2020, 47, 6396–6404. [Google Scholar] [CrossRef] [PubMed]
- Krieger, M.; van de Water, S.; Folkerts, M.M.; Mazal, A.; Fabiano, S.; Bizzocchi, N.; Weber, D.C.; Safai, S.; Lomax, A.J. A quantitative FLASH effectiveness model to reveal potentials and pitfalls of high dose rate proton therapy. Med. Phys. 2022, 49, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Traneus, E.; Safai, S.; Kolano, A.; van de Water, S. Treatment planning for Flash radiotherapy: General aspects and applications to proton beams. Med. Phys. 2022, 49, 2861–2874. [Google Scholar] [CrossRef]
- Safai, S.; Bula, C.; Meer, D.; Pedroni, E. Improving the precision and performance of proton pencil beam scanning. Transl. Cancer Res. 2012, 1, 196–206. [Google Scholar]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Bula, C.; Belosi, M.F.; Eichin, M.; Hrbacek, J.; Meer, D. Dynamic beam current control for improved dose accuracy in PBS proton therapy. Phys. Med. Biol. 2019, 64, 175003. [Google Scholar] [CrossRef]
- Pedroni, E. Proton Beam Delivery Technique and Commissioning Issues: Scanned Protons. Educational Meeting PTCOG 2008. Available online: https://www.ptcog.ch/archive/conference_p&t&v/PTCOG47/presentations/1_Education_Monday/EPedroni.pdf (accessed on 10 June 2022).
- Kang, M.; Wei, S.; Choi, J.I.; Lin, H.; Simone, C.B. A universal range shifter and range compensator can enable proton pencil beam scanning single-energy bragg peak flash-rt treatment using current commercially available proton systems. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 203–213. [Google Scholar] [CrossRef]
- Eckstein, J.; Taylor, P.; Zheng, R.; Lee, L.; Chen, W.; Potters, L.; Evans, C. Implementation of External Beam Five-Fraction Adjuvant Breast Irradiation in a US Center. Cancers 2022, 14, 1556. [Google Scholar] [CrossRef]
- Oliver, P.A.K.; Monajemi, T.T. Skin dose in chest wall radiotherapy with bolus: A Monte Carlo study. Phys. Med. Biol. 2020, 65, 155016. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Jiang, R.; Leung, M.K.K. Dosimetry of oblique tangential photon beams calculated by superposition/convolution algorithms: A Monte Carlo evaluation. J. Appl. Clin. Med. Phys. 2010, 12, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Banjade, D.P.; Shrestha, S.L.; Shukri, A.; Tajuddin, A.A.; Bhat, M. A simplified approach for exit dose in vivo measurements in radiotherapy and its clinical application. Australas. Phys. Eng. Sci. Med. 2002, 25, 110–118. [Google Scholar] [CrossRef]
- Kelleter, L.; Tham, B.Z.-H.; Saakyan, R.; Griffiths, J.; Amos, R.; Jolly, S.; Gibson, A. Technical Note: Simulation of dose buildup in proton pencil beams. Med. Phys. 2019, 46, 3734–3738. [Google Scholar] [CrossRef]
- Singletary, E.S.; Cristofanilli, M. Defining the clinical diagnosis of inflammatory breast cancer. Semin. Oncol. 2008, 35, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Bley, C.R.; Wolf, F.; Jorge, P.G.; Grilj, V.; Petridis, I.; Petit, B.; Böhlen, T.T.; Moeckli, R.; Limoli, C.; Bourhis, J.; et al. Dose- and volume-limiting late toxicity of flash radiotherapy in cats with squamous cell carcinoma of the nasal planum and in mini pigs. Clin. Cancer Res. 2022, 28, 3814–3823. [Google Scholar] [CrossRef]
- Rothwell, B.; Lowe, M.; Traneus, E.; Krieger, M.; Schuemann, J. Treatment planning considerations for the development of FLASH proton therapy. Radiother. Oncol. 2022, 175, 222–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Marlen, P.; van de Water, S.; Dahele, M.; Slotman, B.J.; Verbakel, W.F.A.R. Single Ultra-High Dose Rate Proton Transmission Beam for Whole Breast FLASH-Irradiation: Quantification of FLASH-Dose and Relation with Beam Parameters. Cancers 2023, 15, 2579. https://doi.org/10.3390/cancers15092579
van Marlen P, van de Water S, Dahele M, Slotman BJ, Verbakel WFAR. Single Ultra-High Dose Rate Proton Transmission Beam for Whole Breast FLASH-Irradiation: Quantification of FLASH-Dose and Relation with Beam Parameters. Cancers. 2023; 15(9):2579. https://doi.org/10.3390/cancers15092579
Chicago/Turabian Stylevan Marlen, Patricia, Steven van de Water, Max Dahele, Berend J. Slotman, and Wilko F. A. R. Verbakel. 2023. "Single Ultra-High Dose Rate Proton Transmission Beam for Whole Breast FLASH-Irradiation: Quantification of FLASH-Dose and Relation with Beam Parameters" Cancers 15, no. 9: 2579. https://doi.org/10.3390/cancers15092579
APA Stylevan Marlen, P., van de Water, S., Dahele, M., Slotman, B. J., & Verbakel, W. F. A. R. (2023). Single Ultra-High Dose Rate Proton Transmission Beam for Whole Breast FLASH-Irradiation: Quantification of FLASH-Dose and Relation with Beam Parameters. Cancers, 15(9), 2579. https://doi.org/10.3390/cancers15092579



