Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review Protocol and Eligibility Criteria
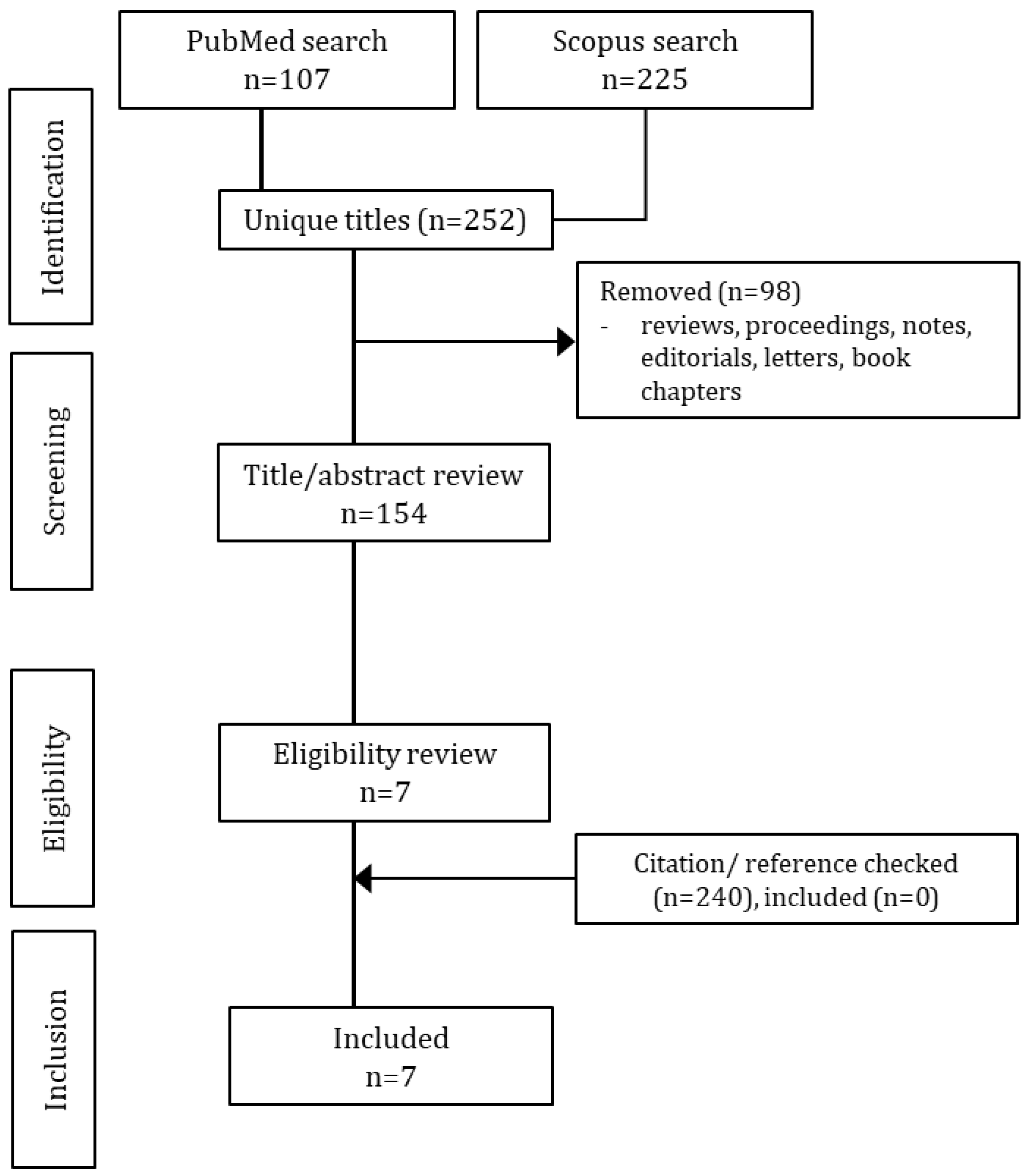
2.2. Search Strategy and Selection Process
2.3. Quality Assessment
2.4. Data Review, Extraction, and Synthesis
2.5. Meta-Analyses
3. Results
3.1. Study Selection and Quality Assessment
3.2. Characteristics of Included Studies
3.3. QOL and PRO Measurements
3.4. Studies Comparing Proton Therapy and Photon Radiotherapy
3.5. Effect of Time
3.6. Effect of Dose Factors
3.7. Effect of Clinical Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoxbroe Michaelsen, S.; Gronhoj, C.; Hoxbroe Michaelsen, J.; Friborg, J.; von Buchwald, C. Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur. J. Cancer 2017, 78, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.; Ramasamy, S.; Cardale, K.; Dyker, K.; Garcez, K.; Lee, L.W.; McPartlin, A.; Murray, P.; Sen, M.; Slevin, N.; et al. Long term patient reported swallowing function following chemoradiotherapy for oropharyngeal carcinoma. Radiother. Oncol. 2018, 128, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Sapir, E.; Tao, Y.; Feng, F.; Samuels, S.; El Naqa, I.; Murdoch-Kinch, C.A.; Feng, M.; Schipper, M.; Eisbruch, A. Predictors of Dysgeusia in Patients With Oropharyngeal Cancer Treated With Chemotherapy and Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Windon, M.J.; Fakhry, C.; Faraji, F.; Troy, T.; Gourin, C.G.; Kiess, A.P.; Koch, W.; Eisele, D.W.; D’Souza, G. Priorities of human papillomavirus-associated oropharyngeal cancer patients at diagnosis and after treatment. Oral Oncol. 2019, 95, 11–15. [Google Scholar] [CrossRef]
- Gabrys, H.S.; Buettner, F.; Sterzing, F.; Hauswald, H.; Bangert, M. Parotid gland mean dose as a xerostomia predictor in low-dose domains. Acta Oncol. 2017, 56, 1197–1203. [Google Scholar] [CrossRef]
- Han, P.; Lakshminarayanan, P.; Jiang, W.; Shpitser, I.; Hui, X.; Lee, S.H.; Cheng, Z.; Guo, Y.; Taylor, R.H.; Siddiqui, S.A.; et al. Dose/Volume histogram patterns in Salivary Gland subvolumes influence xerostomia injury and recovery. Sci. Rep. 2019, 9, 3616. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nihei, K.; Nakajima, Y.; Kito, S.; Sasai, K.; Karasawa, K. Comparison of xerostomia incidence after three-dimensional conformal radiation therapy and contralateral superficial lobe parotid-sparing intensity-modulated radiotherapy for oropharyngeal and hypopharyngeal cancer. Auris Nasus Larynx 2018, 45, 1073–1079. [Google Scholar] [CrossRef]
- Mogadas, S.; Busch, C.-J.; Pflug, C.; Hanken, H.; Krüll, A.; Petersen, C.; Tribius, S. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020, 196, 522–529. [Google Scholar] [CrossRef]
- Cui, T.; Ward, M.C.; Joshi, N.P.; Woody, N.M.; Murray, E.J.; Potter, J.; Dorfmeyer, A.A.; Greskovich, J.F.; Koyfman, S.A.; Xia, P. Correlation between plan quality improvements and reduced acute dysphagia and xerostomia in the definitive treatment of oropharyngeal squamous cell carcinoma. Head Neck 2019, 41, 1096–1103. [Google Scholar] [CrossRef]
- Beddok, A.; Vela, A.; Calugaru, V.; Tessonnier, T.; Kubes, J.; Dutheil, P.; Gérard, A.; Vidal, M.; Goudjil, F.; Florescu, C.; et al. Proton therapy for head and neck squamous cell carcinomas: From physics to clinic. Cancer Radiother. 2019, 23, 439–448. [Google Scholar] [CrossRef]
- Simone, C.B.; Ly, D.; Dan, T.D.; Ondos, J.; Ning, H.; Belard, A.; O’Connell, J.; Miller, R.W.; Simone, N.L. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother. Oncol. 2011, 101, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kandula, S.; Zhu, X.; Garden, A.S.; Gillin, M.; Rosenthal, D.I.; Ang, K.-K.; Mohan, R.; Amin, M.V.; Garcia, J.A.; Wu, R.; et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: A treatment planning comparison. Med. Dosim. 2013, 38, 390–394. [Google Scholar] [CrossRef]
- Yahya, N.; Mohamad Salleh, S.A.; Mohd Nasir, N.F.; Abdul Manan, H. Toxicity profile of patients treated with proton and carbon-ion therapy for primary nasopharyngeal carcinoma: A systematic review and meta-analysis. Asia Pac. J. Clin. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Apinorasethkul, O.; Kirk, M.; Teo, K.; Swisher-McClure, S.; Lukens, J.N.; Lin, A. Pencil beam scanning proton therapy vs rotational arc radiation therapy: A treatment planning comparison for postoperative oropharyngeal cancer. Med. Dosim. 2017, 42, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, K.; Smulders, B.; Specht, L.; Zhu, M.; Friborg, J.; Rasmussen, J.H.; Bentzen, S.M.; Vogelius, I.R. Radiation dose-painting with protons vs. photons for head-and-neck cancer. Acta Oncol. 2020, 59, 525–533. [Google Scholar] [CrossRef]
- Moreno, A.C.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; Reddy, J.P.; Morrison, W.H.; Williamson, T.D.; Holliday, E.B.; et al. Intensity modulated proton therapy (IMPT)—The future of IMRT for head and neck cancer. Oral Oncol. 2019, 88, 66–74, PMID: 30616799; PMCID: PMCPMC6615027. [Google Scholar] [CrossRef]
- Poulsen, P.R.; Eley, J.; Langner, U.; Simone, C.B.; Langen, K. Efficient Interplay Effect Mitigation for Proton Pencil Beam Scanning by Spot-Adapted Layered Repainting Evenly Spread out Over the Full Breathing Cycle. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 226–234. [Google Scholar] [CrossRef]
- Rana, S.; Rosenfeld, A.B. Investigating volumetric repainting to mitigate interplay effect on 4D robustly optimized lung cancer plans in pencil beam scanning proton therapy. J. Appl. Clin. Med. Phys. 2021, 22, 107–118. [Google Scholar] [CrossRef]
- Engwall, E.; Fredriksson, A.; Glimelius, L. 4D robust optimization including uncertainties in time structures can reduce the interplay effect in proton pencil beam scanning radiation therapy. Med. Phys. 2018, 45, 4020–4029. [Google Scholar] [CrossRef]
- Meijers, A.; Knopf, A.-C.; Crijns, A.P.; Ubbels, J.F.; Niezink, A.G.; Langendijk, J.A.; Wijsman, R.; Both, S. Evaluation of interplay and organ motion effects by means of 4D dose reconstruction and accumulation. Radiother. Oncol. 2020, 150, 268–274. [Google Scholar] [CrossRef]
- Beddok, A.; Vela, A.; Calugaru, V.; Tessonnier, T.; Kubes, J.; Dutheil, P.; Gerard, A.; Vidal, M.; Goudjil, F.; Florescu, C.; et al. Proton therapy for head and neck squamous cell carcinomas: A review of the physical and clinical challenges. Radiother. Oncol. 2020, 147, 30–39. [Google Scholar] [CrossRef]
- Unkelbach, J.; Chan, T.C.Y.; Bortfeld, T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Phys. Med. Biol. 2007, 52, 2755–2773. [Google Scholar] [CrossRef] [PubMed]
- Botas, P.; Kim, J.; Winey, B.; Paganetti, H. Online adaption approaches for intensity modulated proton therapy for head and neck patients based on cone beam CTs and Monte Carlo simulations. Phys. Med. Biol. 2018, 64, 015004. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Linge, A.; Leger, K.; Maile, T.; Kemper, M.; Haim, D.; Jöhrens, K.; Troost, E.G.C.; Krause, M.; Löck, S. Assessment of gene expressions from squamous cell carcinoma of the head and neck to predict radiochemotherapy-related xerostomia and dysphagia. Acta Oncol. 2022, 61, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Charters, E.K.; Bogaardt, H.; Freeman-Sanderson, A.L.; Ballard, K.J. Systematic review and meta-analysis of the impact of dosimetry to dysphagia and aspiration related structures. Head Neck 2019, 41, 1984–1998. [Google Scholar] [CrossRef]
- Paganetti, H.; Botas, P.; Sharp, G.C.; Winey, B. Adaptive proton therapy. Phys. Med. Biol. 2021, 66, 22TR01. [Google Scholar] [CrossRef]
- Verma, V.; Simone, C.B., 2nd; Mishra, M.V. Quality of Life and Patient-Reported Outcomes Following Proton Radiation Therapy: A Systematic Review. J. Nat. Cancer Inst. 2018, 110, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- National Heart Lung, and Blood Institute. Study Quality Assessment Tools; National Institutes of Health: Bethesda, MD, USA, 2018.
- Yahya, N.; Manan, H.A. Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur. J. Cancer Care 2021, 30, e13329. [Google Scholar] [CrossRef]
- Voon, N.; Lau, F.; Zakaria, R.; Rani, S.M.; Ismail, F.; Manan, H.; Yahya, N. MRI-based brain structural changes following radiotherapy of Nasopharyngeal Carcinoma: A systematic review. Cancer Radiother. 2021, 25, 62–71. [Google Scholar] [CrossRef]
- Blanchard, P.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Morrison, W.H.; Hernandez, M.; Crutison, J.; Lee, J.J.; Ye, R.; Fuller, C.D.; et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—A case matched analysis. Radiother. Oncol. 2016, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Manzar, G.S.; Lester, S.C.; Routman, D.M.; Harmsen, W.S.; Petersen, M.M.; Sloan, J.A.; Mundy, D.W.; Hunzeker, A.E.; Amundson, A.C.; Anderson, J.L.; et al. Comparative analysis of acute toxicities and patient reported outcomes between intensity-modulated proton therapy (IMPT) and volumetric modulated arc therapy (VMAT) for the treatment of oropharyngeal cancer. Radiother. Oncol. 2020, 147, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhou, O.; Thompson, R.; Gabriel, P.; Chalian, A.; Rassekh, C.; Weinstein, G.S.; O’Malley, B.W.; Aggarwal, C.; Bauml, J.; et al. Quality of Life of Postoperative Photon versus Proton Radiation Therapy for Oropharynx Cancer. Int. J. Part Ther. 2018, 5, 11–17. [Google Scholar] [CrossRef]
- Sio, T.T.; Lin, H.-K.; Shi, Q.; Gunn, G.B.; Cleeland, C.S.; Lee, J.J.; Hernandez, M.; Blanchard, P.; Thaker, N.G.; Phan, J.; et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Ye, R.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Fuller, C.; Morrison, W.H.; Phan, J.; Sturgis, E.M.; Ferrarotto, R.; et al. Xerostomia-related quality of life for patients with oropharyngeal carcinoma treated with proton therapy. Radiother. Oncol. 2020, 142, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.R.; Hutcheson, K.A.; Ye, R.; Garden, A.S.; Morrison, W.H.; Rosenthal, D.I.; Gunn, G.B.; Fuller, C.D.; Phan, J.; Reddy, J.P.; et al. Prospective longitudinal patient-reported outcomes of swallowing following intensity modulated proton therapy for oropharyngeal cancer. Radiother. Oncol. 2020, 148, 133–139. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Jiang, B.; Chen, J.; Wang, X.; Wang, L.; Sahoo, N.; Zhu, X.R.; Ye, R.; Blanchard, P.; et al. Intensity-modulated proton therapy for oropharyngeal cancer reduces rates of late xerostomia. Radiother. Oncol. 2021, 160, 32–39. [Google Scholar] [CrossRef]
- Singer, S.; Arraras, J.I.; Baumann, I.; Boehm, A.; Chie, W.-C.; Galalae, R.; Langendijk, J.A.; Guntinas-Lichius, O.; Hammerlid, E.; Pinto, M.; et al. Quality of life in patients with head and neck cancer receiving targeted or multimodal therapy—Update of the EORTC QLQ-H&N35, Phase, I. Head Neck 2013, 35, 1331–1338. [Google Scholar] [CrossRef]
- Henson, B.S.; Inglehart, M.R.; Eisbruch, A.; Ship, J.A. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncol. 2001, 37, 84–93. [Google Scholar] [CrossRef]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Beetz, I.; Burlage, F.R.; Bijl, H.P.; Hoegen-Chouvalova, O.; Christianen, M.E.; Vissink, A.; van der Laan, B.; de Bock, G.H.; Langendijk, J.A. The Groningen Radiotherapy-Induced Xerostomia questionnaire: Development and validation of a new questionnaire. Radiother. Oncol. 2010, 97, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Holliday, E.B.; Kocak-Uzel, E.; Feng, L.; Thaker, N.G.; Blanchard, P.; Rosenthal, D.; Gunn, G.B.; Garden, A.S.; Frank, S.J. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med. Dosim. 2016, 41, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tambas, M.; van der Laan, H.P.; Steenbakkers, R.J.; Doyen, J.; Timmermann, B.; Orlandi, E.; Hoyer, M.; Haustermans, K.; Georg, P.; Burnet, N.G.; et al. Current practice in proton therapy delivery in adult cancer patients across Europe. Radiother. Oncol. 2022, 167, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, J.M.; Langendijk, J.A.; Paul van der Laan, H.; Lukens, J.N.; Swisher-McClure, S.D.; Lin, A. A Model-Based Approach to Predict Short-Term Toxicity Benefits With Proton Therapy for Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Yang, P.; Blanchard, P.; Garden, A.S.; Gunn, B.; Fuller, C.D.; Chambers, M.; Hutcheson, K.A.; Ye, R.; et al. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother. Oncol. 2017, 123, 401–405. [Google Scholar] [CrossRef]
- Gunn, G.B.; Blanchard, P.; Garden, A.S.; Zhu, X.R.; Fuller, C.D.; Mohamed, A.S.; Morrison, W.H.; Phan, J.; Beadle, B.M.; Skinner, H.D.; et al. Clinical Outcomes and Patterns of Disease Recurrence After Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 360–367. [Google Scholar] [CrossRef]
- Van De Water, T.A.; Lomax, A.J.; Bijl, H.P.; De Jong, M.E.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1216–1224. [Google Scholar] [CrossRef]
- Rades, D.; Warwas, B.; Gerull, K.; Pries, R.; Leichtle, A.; Bruchhage, K.L.; Hakim, S.G.; Schild, S.E.; Cremers, F. Prognostic Factors for Complete Recovery From Xerostomia After Radiotherapy of Head-and-Neck Cancers. In Vivo 2022, 36, 1795–1800. [Google Scholar] [CrossRef]
- Megee, F.; Gough, K.; Frowen, J.; Dixon, B.; Magarey, M.; Wiesenfeld, D.; Ramakrishnan, A. Predictors of distress associated with altered appearance and function in people treated surgically for oral cancers: A cross-sectional study. Int. J. Oral Maxillofac. Surg. 2023. [Google Scholar] [CrossRef]
- Lysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef]
- Jones, D.A.; Smith, J.; Mei, X.W.; Hawkins, M.A.; Maughan, T.; Heuvel, F.V.D.; Mee, T.; Kirkby, K.; Kirkby, N.; Gray, A. A systematic review of health economic evaluations of proton beam therapy for adult cancer: Appraising methodology and quality. Clin. Transl. Radiat. Oncol. 2020, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J.; Cruickshank, C.; Baines, H.; Banner, R.; Beasley, M.; Betts, G.; Bulbeck, H.; Charlwood, F.; Christian, J.; Clarke, M.; et al. TORPEdO: A phase III trial of intensity-modulated proton beam therapy versus intensity-modulated radiotherapy for multi-toxicity reduction in oropharyngeal cancer. Clin. Transl. Radiat. Oncol. 2023, 38, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.J.; Tishler, R.B.; Pham, N.L.; Punglia, R.S. Cost-Effectiveness Analysis of Intensity Modulated Radiation Therapy Versus Proton Therapy for Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, A.; Bandurska-Luque, A.; Stützer, K.; Haase, R.; Löck, S.; Wack, L.-J.; Mönnich, D.; Thorwarth, D.; Perez, D.; Lühr, A.; et al. Identification of Patient Benefit From Proton Therapy for Advanced Head and Neck Cancer Patients Based on Individual and Subgroup Normal Tissue Complication Probability Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1165–1174. [Google Scholar] [CrossRef]
- Bijman, R. (.G.; Breedveld, S.; Arts, T.; Astreinidou, E.; De Jong, M.A.; Granton, P.V.; Petit, S.F.; Hoogeman, M.S. Impact of model and dose uncertainty on model-based selection of oropharyngeal cancer patients for proton therapy. Acta Oncol. 2017, 56, 1444–1450. [Google Scholar] [CrossRef]
- Yahya, N.; Sukiman, N.K.; Suhaimi, N.A.; Azmi, N.A.; Manan, H.A. How many roads must a Malaysian walk down? Mapping the accessibility of radiotherapy facilities in Malaysia. PLoS ONE 2019, 14, e0213583. [Google Scholar] [CrossRef]
- Yahya, N.; Roslan, N. Estimating radiotherapy demands in South East Asia countries in 2025 and 2035 using evidence-based optimal radiotherapy fractions. Asia Pac. J. Clin. Oncol. 2018, 14, e543–e547. [Google Scholar] [CrossRef]
- Atun, R.; A Jaffray, D.; Barton, M.B.; Bray, F.; Baumann, M.; Vikram, B.; Hanna, T.P.; Knaul, F.M.; Lievens, Y.; Lui, T.Y.M.; et al. Expanding global access to radiotherapy. Lancet Oncol. 2015, 16, 1153–1186. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Neurocognitive impairment following proton therapy for paediatric brain tumour: A systematic review of post-therapy assessments. Support. Care Cancer 2020, 29, 3035–3047. [Google Scholar] [CrossRef]
- Schippers, J.M.; Lomax, A.J. Emerging technologies in proton therapy. Acta Oncol. 2011, 50, 838–850. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Janssens, G.; De Wilde, O.; Bossier, V.; Lerot, X.; Pouppez, A.; Yan, D.; Stevens, C.; Kabolizadeh, P.; et al. The first prototype of spot-scanning proton arc treatment delivery. Radiother. Oncol. 2019, 137, 130–136. [Google Scholar] [CrossRef] [PubMed]
| Reference | No. | Stage III/IV (%) | Female (%) | Median Age (Range) | % HPV Positive (+), Unknown (?) | Type of Proton Therapy | Dose (Gy RBE) | Other Treatments | Therapy Comparison | Details of Therapy Comparison |
|---|---|---|---|---|---|---|---|---|---|---|
| Bagley 2020 [36] | 69 | AJCC7 stage III–IV, M0—100 | 13 | 64 (37–84) | +84 ? 14 | Spot-scanning IMPT | Median—69.3, range 60–70 | Induction—5, concurrent—38, Induction + concurrent—11 | - | - |
| Blanchard 2016 [32] | 50 | T3–T4—20 N2–N3—80 | 14.7 | IMRT—55.5 (34–78), IMPT—61 (37–84) | +88 ? 10 | Spot-scanning IMPT | Small volume disease—66, advanced disease—70 | Concurrent—64% | 100 IMRT | 2:1, matched laterality, site, HPV, T and N status, smoking, and chemotherapy |
| Cao 2021 [38] | 103 | T3–T4—IMRT (31.9), IMPT (35); N2–N3—IMRT (82.3), IMPT (77.1) | IMRT—14.2, IMPT—12.6 | IMRT—59 (32–84), IMPT—60 (33–85) | IMRT (+68.8 ? 18.1) IMPT (+76.7 ? 17.4) | spot-scanning IMPT | With concurrent chemo—70 Gy Without chemo—60 Gy | Neoadjuvant—35%, Concurrent—69% | 429 IMRT | No significant difference for demographic and treatment factors tested (age, sex, race, tumor site, location, clinical stage, human papillomavirus status, or chemotherapy received) |
| Grant 2020 [37] | 71 | AJCC 7th edition stage III/IV—100 | 12.7 | 63 (37–84) | +85.9 ? 7.0 | IMPT | Range 66–70 Gy | Induction—5, concurrent—41, induction + concurrent—10 | - | - |
| Manzar 2020 [33] | 46 | AJCC 7 th edition Stage III/IV—84.8 | 12.5 | VMAT—61 (42–88), IMPT—66 (40–79) | +76.1 ? 13.0 | spot-scanning IMPT | Adjuvant, range 60–66; definitive70 | Concurrent—36 | 259 VMAT | Significant difference: age (IMPT older) smoking status and pack-years (VMAT higher), dose category (more definitive RT in IMPT) |
| Sharma 2018 [34] | 31 | Stage I–III—13 IVA 87 | VMAT—18 Proton—13 | VMAT (mean)—58, Proton (mean)—60 | Not stated | spot-scanning, single-field uniform dose | Median 61.7 | Chemotherapy—12 | 33 VMAT | No significant difference |
| Sio 2016 [35] | 35 | Stage III–IV—94.3 | IMRT—8.7, IMPT—14.3 | IMRT (mean, SD)—58.2 (9.9) IMPT (mean, SD)—59.1 (10.2) | +74.3 ? 20 | Spot-scanning IMPT | Median 70.0, range 59.0–70.0 | Concurrent chemotherapy—all, induction—26 | 46 IMRT | Significant difference: location (more tonsil), T-stage (more T3–T4), lower induction chemotherapy, higher total radiation dose |
| Reference | QOL/PRO Measures | Endpoints | Type and Frequency of Assessment | Median Follow-Up |
|---|---|---|---|---|
| Bagley 2020 [36] | 15-item Xerostomia-Related QoL Scale (XeQoLS); range score—0–75 [40] | Mean XeQoLS scores and subdomain (physical, personal, pain, social) | Prospective—baseline, 6 weeks on-treatment, and follow-up visits at 10 weeks and at 6, 12, and 24 months | 64 weeks from the start of treatment |
| Blanchard 2016 [32] | Not specified; range 0–3 scale from none to severe | Grade 2–3 patient-rated fatigue and dry mouth | Prospective—during treatment, 3 months after, and 1 year after | 29 months (range 8–49)—IMPT, 33 months (range 2–55)—IMRT |
| Cao 2021 [38] | Eight-item self-reported xerostomia-specific questionnaire; range score—0–100 [41] | Moderate–severe score ≥ 50 and no–mild score < 50 | Prospective—every 3 months and clustered into 0–6, 6–9, 9–12, 12–18, 18–24, and 24–36 months | 36.2 months |
| Grant 2020 [37] | MD Anderson Dysphagia Inventory; 20 questions from which global, composite, functional, emotional, and physical scores were derived and normalized; score range 20 (extremely low functioning) to 100 (high functioning) | Score changes over time | Prospective—baseline, treatment week 6, follow-up week 10, month 6, year 1, and year 2 | More than 50% of patients evaluable at 24 months |
| Manzar 2020 [33] | EORTC QLQ-H&N35—35 questions; 35 questions covering aspects of QOL [39] | End-of-treatment scores for each question | Prospective—for QoL only end of treatment analyzed | 12 months (IMPT) and 30 months (VMAT) |
| Sharma 2018 [34] | QLQ-30 version 3, EORTC QLQ-H&N35, and the Groningen Xerostomia, Work Status, and Performance Status Scale—Head and Neck Cancer (GRIX) questionnaires, normalized a 0 to 100 scale; EORTC—general health domain, physical and role function, overall xerostomia, dental issues, head and neck pain, and fatigue scores; GRIX—day and night xerostomia and separate subscales for sticky saliva [39,42] | Score at 3, 6, and 12 months | Prospective—pretreatment and at 3, 6, and 12 months | Not mentioned |
| Sio 2016 [35] | MD Anderson Symptom Inventory-Head and Neck Cancer (MDASI-HN)—top 11 most severe burdens | During treatment (acute phase), within the first 3 months after treatment (subacute phase), and afterward (chronic phase) | Prospective—weekly during the 6- to 7-week radiotherapy period (the acute phase). Data in the subacute phase were obtained during the first 3 months after the end of radiotherapy | 7.7 (IQR 3.97–22.77) months—IMPT, 2.68 (0.30–10.27) months—IMRT |
| Reference | Endpoints | Statistically Significant Endpoints | Non-Statistically Significant Endpoints | Significant Clinical Factors | Dose Factors |
|---|---|---|---|---|---|
| Comparison to Baseline | |||||
| Bagley 2020 [36] | mean XeQoLS scores and subdomain (physical, personal, pain, social) | General xerostomia, including physical, personal, pain, and social domains Baseline: 0.24 ± 0.57 6 weeks: 2.00 ± 1.01 10 weeks: 1.03 ± 0.76 6 months: 0.97 ± 0.78 1 year: 0.82 ± 0.69 2 years: 0.70 ± 0.75 (all p < 0.001) | - | Time, baseline XeQoLS score, stage and N status | Univariate—oral cavity dose Multivariate—not significant |
| Grant 2020 [37] | MDADI score changes over time | Poor composite score for dysphagia Baseline: 5.6% 6 weeks: 61.2% 10 weeks: 19.1% 6 months: 13.3% 1 year: 13.5% 2 years: 11.1% | - | T-stage | Not studied |
| Comparison to photon-based therapy | |||||
| Blanchard 2016 [32] | Grade 2–3 patient-rated fatigue and dry mouth | Xerostomia at 3 months (favors IMPT 42% vs. 61.2%, p = 0.009) | Fatigue at 3 months (IMPT = 40.8% vs. IMRT = 36.2%); fatigue (IMPT = 14.6% vs. IMRT = 22.1%) and xerostomia (IMPT = 42% vs. IMRT = 47.2%) at 1 year | Not studied | Not studied |
| Cao 2021 [38] | Moderate–severe xerostomia score ≥ 50 | Moderate–severe at 18–24 months (favors IMPT 6% vs. 20%; p = 0.025) and at 24–36 months (favors IMPT 6% vs. 20%; p = 0.01) | Up until 18 months after treatment 0–6 months (IMPT 38% vs. IMRT 37%), 6–9 months (IMPT 25% vs. IMRT 28%), 9–12 months (IMPT 10% vs. IMRT 16%), 12–18 months (IMPT 7% vs. IMRT 7%) | 18–24 months—disease site (base of tongue vs. tonsil/other: OR = 0.320, p = 0.009) 24–36 months—gender (male vs. female, OR = 2.786, p = 0.023), concurrent CT (OR = 0.349, p = 0.024) | 0–6 months—higher Dmean, V5, V10, V15, V20, V25, V30, V35, V40, and V45 of the contralateral parotid gland for moderate–severe 24–36 months—higher V25, V30, V35, V40, V45, V50, V55, V60, V65, and V70 of the oral cavity |
| Manzar 2020 [33] | End-of-treatment scores for each question | Overall (mean difference to baseline): Cough (IMPT 6.7 vs. IMRT 29.3, p = 0.003), need for nutritional supplements (IMPT 26.5 vs. IMRT 48.1, p = 0.007), and dysgeusia (IMPT 3.7 vs. IMRT 6.9, p = 0.043) (all favor IMPT) | EORTC H&N QLQ-35 questions not stated in the previous cell | Not studied | Significant dose difference between PT and VMAT. Associations to endpoints not analyzed |
| Sharma 2018 [34] | Score at 3, 6, and 12 months. | 3 months—dental problem (IMPT 0% vs. IMRT 19.05%, p = 0.016) 6 months—moderate to severe dry mouth (IMPT 22.22% vs. IMRT 63.16%, p = 0.02), xerostomia day (IMPT 25.80% vs. IMRT 39.20%, p = 0.038), xerostomia night (IMPT 22.80% vs. IMRT 35.10%, p = 0.042), dental problems (IMPT 1.96% vs. IMRT 17.54%, p = 0.048), physical function (IMPT 97.04% vs. IMRT 89.47%, p = 0.006), role function (IMPT 96.30% vs. IMRT 76.32%, p = 0.0008) 12 months—H&N pain (IMPT 8.33% vs. IMRT 21.97%, p = 0.011), xerostomia (IMPT 23.53% vs. IMRT 54.55%, p = 0.003), moderate-severe dry mouth (IMPT 11.76% vs. IMRT 50.00%, p = 0.038), role function (IMPT 81.86% vs. IMRT 72.73%, p = 0.041) | Fatigue, sticky saliva (day general), and global health and time points not stated in the previous cell | Not studied | Significant dose difference between PT and IMRT (all favoring PT); associations to endpoints not analyzed |
| Sio 2016 [35] | Average symptom burden in the top 11 (most severe) items in the MDASI during treatment (acute phase) within the first 3 months after treatment (subacute phase), and afterward (chronic phase) | Burden in subacute phase—food taste (IMPT score (SD) 5.76 (3.60) vs. IMRT 7.70 (2.44), p = 0.010) and appetite (IMPT 4.68 (3.53) vs. IMRT 6.37 (3.21), p = 0.048). Burden in chronic phase—appetite (IMPT 2.12 (3.08) vs. IMRT 4.14 (3.01), p = 0.036) moderate to severe symptoms—subacute phase—food taste and mucus (favor IMPT, p < 0.039 for both) mean of top 5 MDASI higher during subacute phase for IMRT IMPT 8.7 (8.8) vs. IMRT 36.4 (22.4) | Other top 11 symptoms—Dry mouth, swallowing/chewing, fatigue, pain, sleep, mouth sores, drowsiness, and distress. | Not studied | Not studied |
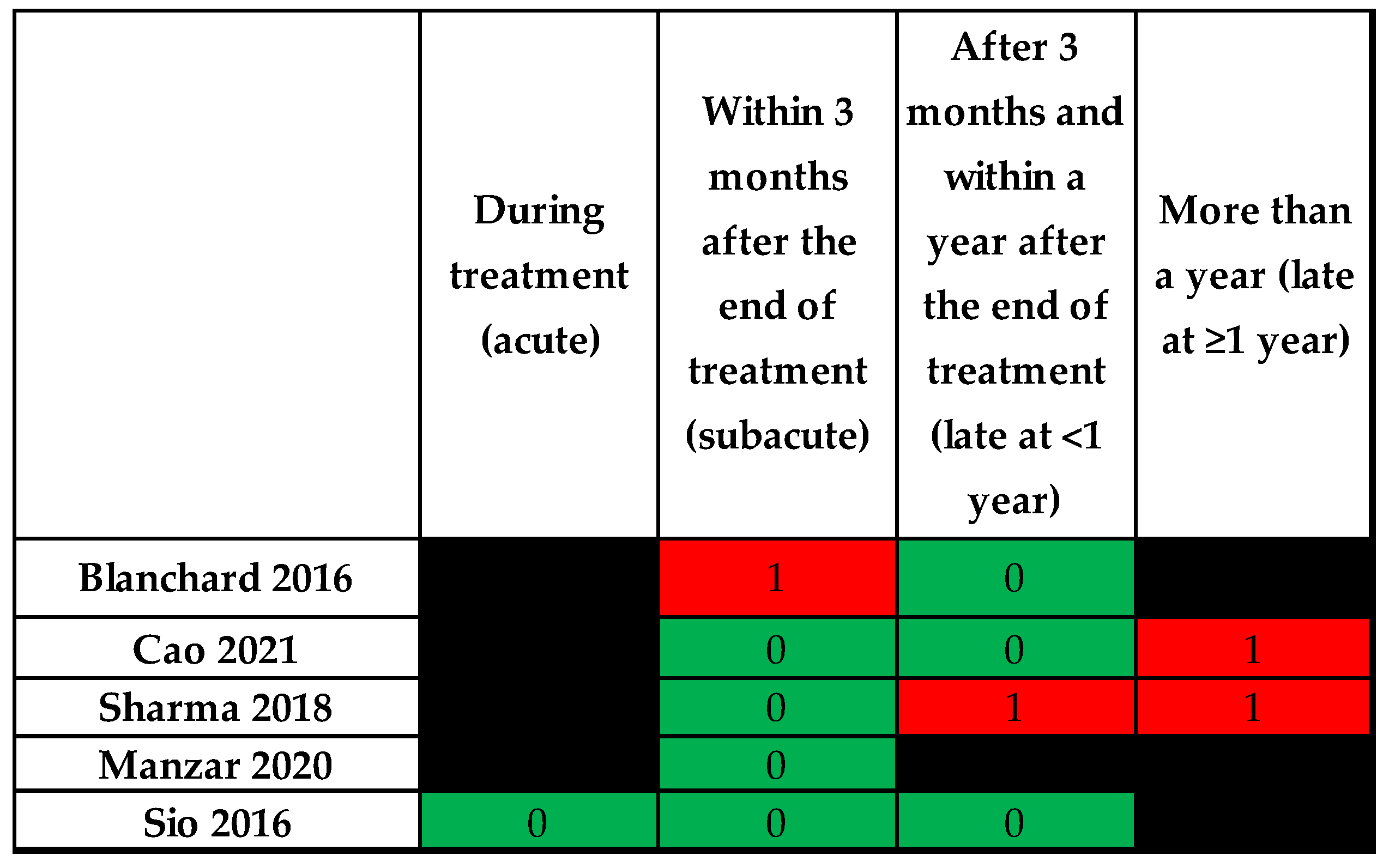
| During Treatment (Acute) | Within 3 Months after the End of Treatment (Subacute) | After 3 Months and Within a Year after the End of Treatment (Late at <1 Year) | More than a Year (Late at ≥1 Year) | |
|---|---|---|---|---|
| Comparison to Photon | ||||
| Blanchard 2016 [32] | - | Xerostomia score (favors IMPT), fatigue—no significant difference | Xerostomia and fatigue score—no significant difference | - |
| Cao 2021 [38] | Xerostomia—no significant difference | Xerostomia—no significant difference | Moderate–severe xerostomia (favors IMPT) | |
| Sharma 2018 [34] | - | Dental problem (favors IMPT) | Moderate to severe dry mouth, xerostomia day, xerostomia night, dental problems, physical function, role function (all favor IMPT) | H&N pain, xerostomia, moderate–severe dry mouth, role function (all favor IMPT) |
| Manzar 2020 [33] | - | Cough, need for nutritional supplements and dysgeusia (favors IMPT) | - | - |
| Sio 2016 [35] | No difference. | Mean symptom scores—food taste and appetite (favors IMPT); moderate to severe symptoms—food taste and mucus (favors IMPT) | Mean symptom scores—appetite (favors IMPT) | - |
| Comparison to baseline | ||||
| Bagley 2020 [36] | Worst xerostomia score | Significantly better xerostomia score than acute, significantly worse than baseline | Significantly better xerostomia than subacute, significantly worse than baseline | Not significantly different xerostomia than at 1 year, significantly worse than baseline |
| Grant 2020 [37] | Worst dysphagia score | Significantly better dysphagia score than acute, significantly worse than baseline | Significantly better dysphagia score than subacute, significantly worse than baseline | Not significantly different dysphagia score than at 1 year, significantly worse than baseline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, N.; Manan, H.A. Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review. Cancers 2023, 15, 2252. https://doi.org/10.3390/cancers15082252
Yahya N, Manan HA. Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review. Cancers. 2023; 15(8):2252. https://doi.org/10.3390/cancers15082252
Chicago/Turabian StyleYahya, Noorazrul, and Hanani Abdul Manan. 2023. "Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review" Cancers 15, no. 8: 2252. https://doi.org/10.3390/cancers15082252
APA StyleYahya, N., & Manan, H. A. (2023). Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Carcinoma: A Systematic Review. Cancers, 15(8), 2252. https://doi.org/10.3390/cancers15082252





