Comparative In Silico Analysis of Ultra-Hypofractionated Intensity-Modulated Photon Radiotherapy (IMRT) Versus Intensity-Modulated Proton Therapy (IMPT) in the Pre-Operative Treatment of Retroperitoneal Sarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulation
2.2. Volume Delineation
2.3. Treatment Planning
2.4. Statistical Analysis
3. Results
3.1. Target Coverage
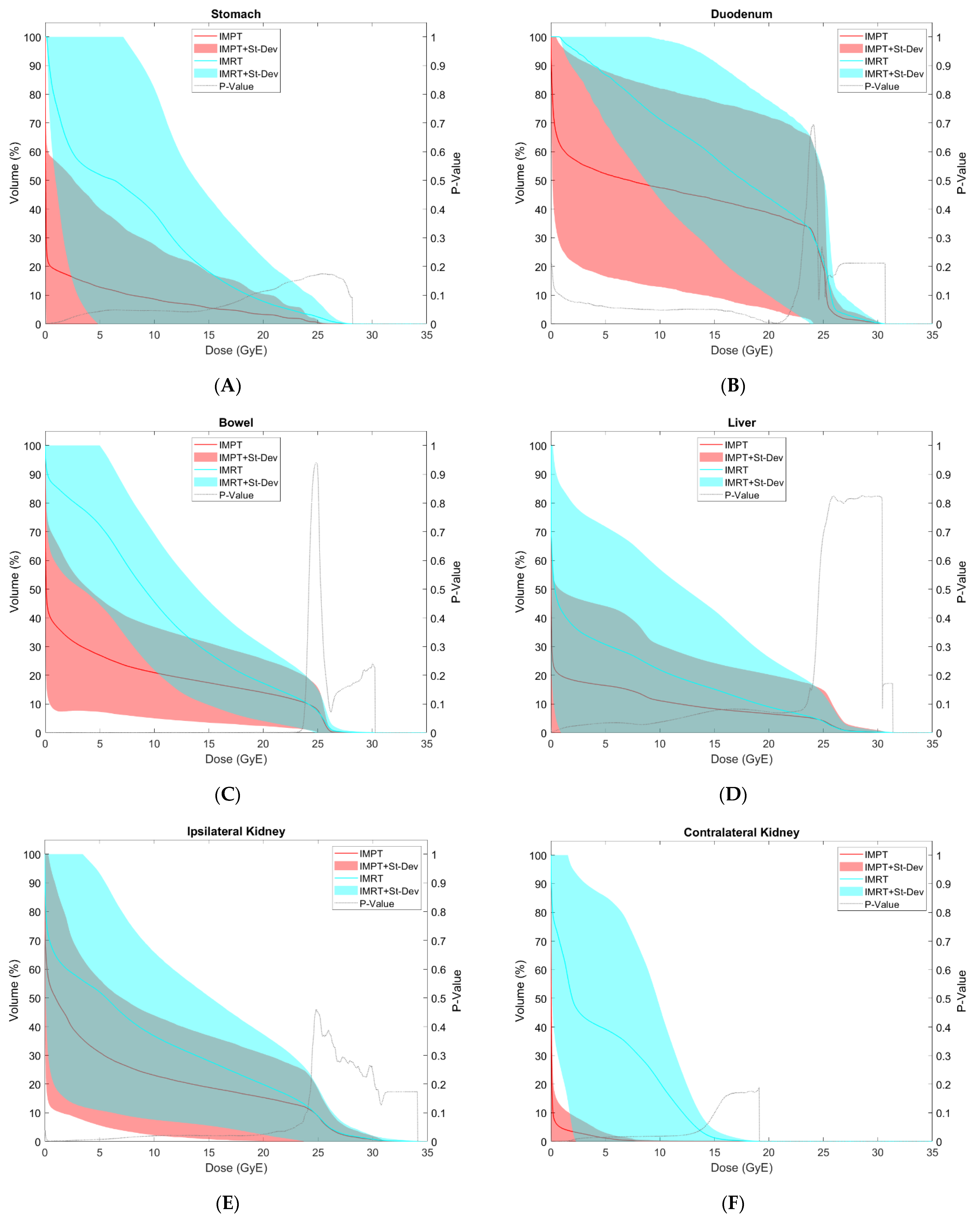
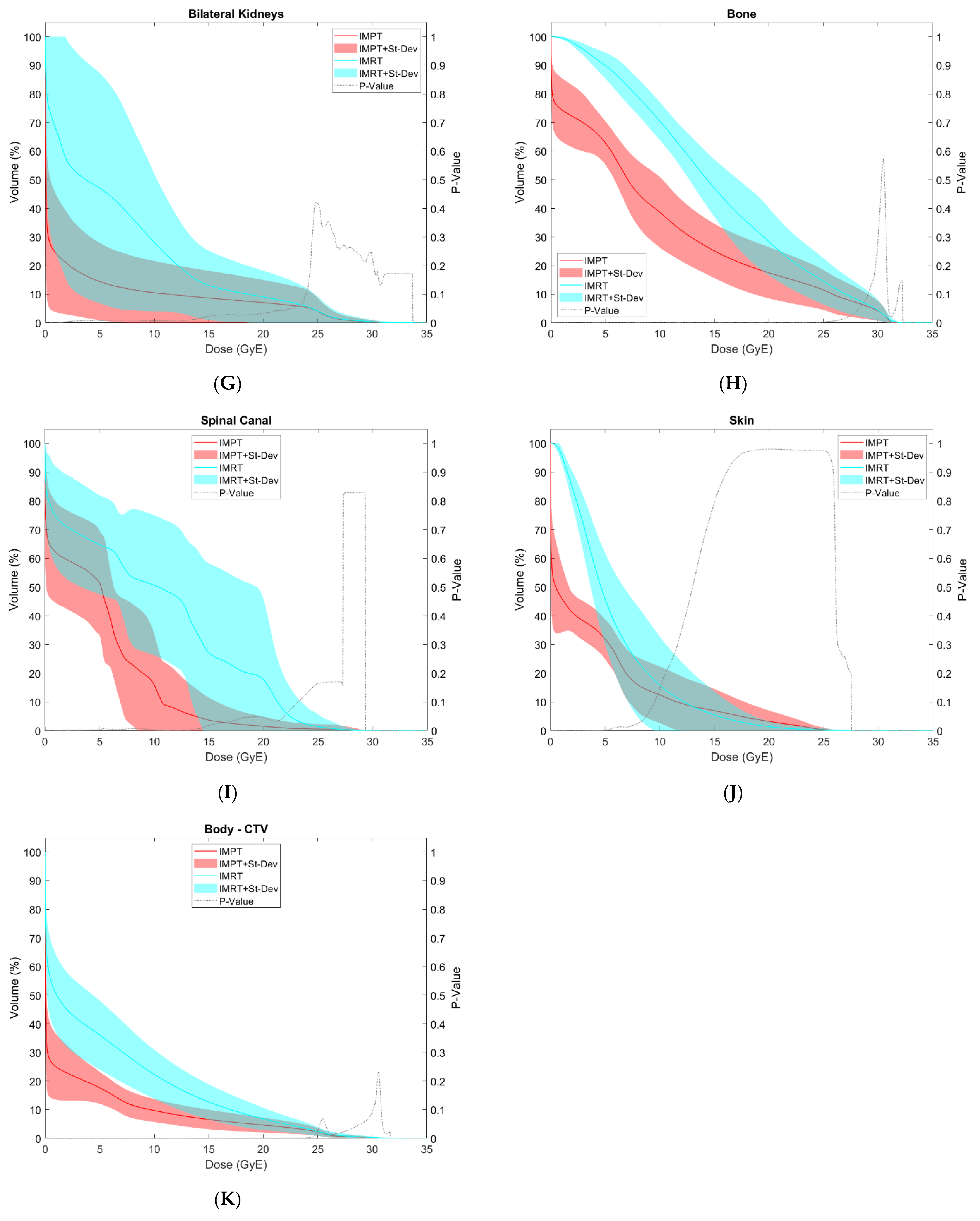
3.2. OAR Comparison
4. Discussion
4.1. Correlation between Dosimetry and Toxicity
4.2. Dosimetric Correlates for Toxicity with Ultra-Hypofractionation
4.3. Literature Reporting Outcomes with Proton Therapy for RPS
4.4. Secondary Malignancy
4.5. Limitations and Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulita, F.; Verras, G.-I.; Liolis, E.; Tchabashvili, L.; Kehagias, D.; Kaplanis, C.; Perdikaris, I.; Kehagias, I. Recurrent Retroperitoneal Liposarcoma: A Case Report and Literature Review. Clin. Case Rep. 2021, 9, e04717. [Google Scholar] [CrossRef]
- Vijay, A.; Ram, L. Retroperitoneal Liposarcoma: A Comprehensive Review. Am. J. Clin. Oncol. 2015, 38, 213–219. [Google Scholar] [CrossRef]
- Gilbeau, L.; Kantor, G.; Stoeckle, E.; Lagarde, P.; Thomas, L.; Kind, M.; Richaud, P.; Coindre, J.M.; Bonichon, F.; Bui, B.N. Surgical Resection and Radiotherapy for Primary Retroperitoneal Soft Tissue Sarcoma. Radiother. Oncol. 2002, 65, 137–143. [Google Scholar] [CrossRef]
- Nathan, H.; Raut, C.P.; Thornton, K.; Herman, J.M.; Ahuja, N.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. Predictors of Survival after Resection of Retroperitoneal Sarcoma: A Population-Based Analysis and Critical Appraisal of the AJCC Staging System. Ann. Surg. 2009, 250, 970–976. [Google Scholar] [CrossRef]
- Strauss, D.C.; Hayes, A.J.; Thomas, J.M. Retroperitoneal Tumours: Review of Management. Ann. R Coll Surg. Engl. 2011, 93, 275–280. [Google Scholar] [CrossRef]
- Van De Voorde, L.; Delrue, L.; van Eijkeren, M.; De Meerleer, G. Radiotherapy and Surgery-an Indispensable Duo in the Treatment of Retroperitoneal Sarcoma. Cancer 2011, 117, 4355–4364. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative Radiotherapy plus Surgery versus Surgery Alone for Patients with Primary Retroperitoneal Sarcoma (EORTC-62092: STRASS): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rivoire, M.; Castaing, M.; Stoeckle, E.; Le Cesne, A.; Blay, J.Y.; Laplanche, A. Primary Retroperitoneal Sarcomas: A Multivariate Analysis of Surgical Factors Associated with Local Control. J. Clin. Oncol. 2009, 27, 31–37. [Google Scholar] [CrossRef]
- Gieschen, H.L.; Spiro, I.J.; Suit, H.D.; Ott, M.J.; Rattner, D.W.; Ancukiewicz, M.; Willett, C.G. Long-Term Results of Intraoperative Electron Beam Radiotherapy for Primary and Recurrent Retroperitoneal Soft Tissue Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 127–131. [Google Scholar] [CrossRef]
- Youssef, E.; Fontanesi, J.; Mott, M.; Kraut, M.; Lucas, D.; Mekhael, H.; Ben-Josef, E. Long-Term Outcome of Combined Modality Therapy in Retroperitoneal and Deep-Trunk Soft-Tissue Sarcoma: Analysis of Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 514–519. [Google Scholar] [CrossRef]
- Zlotecki, R.A.; Katz, T.S.; Morris, C.G.; Lind, D.S.; Hochwald, S.N. Adjuvant Radiation Therapy for Resectable Retroperitoneal Soft Tissue Sarcoma: The University of Florida Experience. Am. J. Clin. Oncol. 2005, 28, 310–316. [Google Scholar] [CrossRef]
- Krempien, R.; Roeder, F.; Oertel, S.; Weitz, J.; Hensley, F.W.; Timke, C.; Funk, A.; Lindel, K.; Harms, W.; Buchler, M.W.; et al. Intraoperative Electron-Beam Therapy for Primary and Recurrent Retroperitoneal Soft-Tissue Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 773–779. [Google Scholar] [CrossRef]
- Gronchi, A.; Lo Vullo, S.; Fiore, M.; Mussi, C.; Stacchiotti, S.; Collini, P.; Lozza, L.; Pennacchioli, E.; Mariani, L.; Casali, P.G. Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2009, 27, 24–30. [Google Scholar] [CrossRef]
- McBride, S.M.; Raut, C.P.; Lapidus, M.; Devlin, P.M.; Marcus, K.J.; Bertagnolli, M.; George, S.; Baldini, E.H. Locoregional Recurrence after Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Adverse Impact of Multifocal Disease and Potential Implications of Dose Escalation. Ann. Surg. Oncol. 2013, 20, 2140–2147. [Google Scholar] [CrossRef]
- Alford, S.; Choong, P.; Chander, S.; Henderson, M.; Powell, G.; Ngan, S. Outcomes of Preoperative Radiotherapy and Resection of Retroperitoneal Sarcoma. ANZ J. Surg. 2013, 83, 336–341. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, S.Y.; Kwon, T.-W.; Yook, J.H.; Kim, S.-C.; Han, D.-J.; Kim, C.-S.; Ahn, H.; Chang, H.M.; Ahn, J.-H.; et al. Treatment Outcome of Postoperative Radiotherapy for Retroperitoneal Sarcoma. Radiat. Oncol. J. 2011, 29, 260–268. [Google Scholar] [CrossRef]
- Fuks, D.; Verhaeghe, J.-L.; Marchal, F.; Guillemin, F.; Beckendorf, V.; Peiffert, D.; Leroux, A.; Rios, M.; Troufléau, P.; Marchal, C. Surgery and Postoperative Radiation Therapy in Primary Retroperitoneal Sarcomas: Experience of the Cancer Centre Alexis-Vautrin. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2012, 16, 194–200. [Google Scholar] [CrossRef]
- Paryani, N.N.; Zlotecki, R.A.; Swanson, E.L.; Morris, C.G.; Grobmyer, S.R.; Hochwald, S.N.; Marcus, R.B.; Indelicato, D.J. Multimodality Local Therapy for Retroperitoneal Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1128–1134. [Google Scholar] [CrossRef]
- Lewis, J.J.; Leung, D.; Woodruff, J.M.; Brennan, M.F. Retroperitoneal Soft-Tissue Sarcoma: Analysis of 500 Patients Treated and Followed at a Single Institution. Ann. Surg. 1998, 228, 355–365. [Google Scholar] [CrossRef]
- Cosper, P.F.; Olsen, J.; DeWees, T.; Van Tine, B.A.; Hawkins, W.; Michalski, J.; Zoberi, I. Intensity Modulated Radiation Therapy and Surgery for Management of Retroperitoneal Sarcomas: A Single-Institution Experience. Radiat. Oncol. 2017, 12, 198. [Google Scholar] [CrossRef]
- Roeder, F.; Ulrich, A.; Habl, G.; Uhl, M.; Saleh-Ebrahimi, L.; Huber, P.E.; Schulz-Ertner, D.; Nikoghosyan, A.V.; Alldinger, I.; Krempien, R.; et al. Clinical Phase I/II Trial to Investigate Preoperative Dose-Escalated Intensity-Modulated Radiation Therapy (IMRT) and Intraoperative Radiation Therapy (IORT) in Patients with Retroperitoneal Soft Tissue Sarcoma: Interim Analysis. BMC Cancer 2014, 14, 617. [Google Scholar] [CrossRef]
- Stuschke, M.; Budach, V.; Klaes, W.; Sack, H. Radiosensitivity, Repair Capacity, and Stem Cell Fraction in Human Soft Tissue Tumors: An in Vitro Study Using Multicellular Spheroids and the Colony Assay. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 69–80. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The Alfa and Beta of Tumours: A Review of Parameters of the Linear-Quadratic Model, Derived from Clinical Radiotherapy Studies. Radiat. Oncol. 2018, 13, 96. [Google Scholar] [CrossRef]
- Meyer, J.M.; Perlewitz, K.S.; Hayden, J.B.; Doung, Y.C.; Hung, A.Y.; Vetto, J.T.; Pommier, R.F.; Mansoor, A.; Beckett, B.R.; Tudorica, A.; et al. Phase I Trial of Preoperative Chemoradiation plus Sorafenib for High-Risk Extremity Soft Tissue Sarcomas with Dynamic Contrast-Enhanced MRI Correlates. Clin. Cancer Res. 2013, 19, 6902–6911. [Google Scholar] [CrossRef]
- Koseła-Paterczyk, H.; Szacht, M.; Morysiński, T.; Ługowska, I.; Dziewirski, W.; Falkowski, S.; Zdzienicki, M.; Pieńkowski, A.; Szamotulska, K.; Switaj, T.; et al. Preoperative Hypofractionated Radiotherapy in the Treatment of Localized Soft Tissue Sarcomas. Eur. J. Surg. Oncol. 2014, 40, 1641–1647. [Google Scholar] [CrossRef]
- Kubicek, G.J.; Kim, T.W.; Gutowski, C.J.; Kaden, M.; Eastwick, G.; Khrizman, P.; Xu, Q.; Lackman, R. Preoperative Stereotactic Body Radiation Therapy for Soft-Tissue Sarcoma: Results of Phase 2 Study. Adv. Radiat. Oncol. 2022, 7, 100855. [Google Scholar] [CrossRef]
- Gobo Silva, M.L.; Lopes de Mello, C.A.; Aguiar Junior, S.; D’Almeida Costa, F.; Stevanato Filho, P.R.; Santoro Bezerra, T.; Nakagawa, S.A.; Nascimento, A.G.; Werneck da Cunha, I.; Spencer Sobreira Batista, R.M.; et al. Neoadjuvant Hypofractionated Radiotherapy and Chemotherapy for Extremity Soft Tissue Sarcomas: Safety, Feasibility, and Early Oncologic Outcomes of a Phase 2 Trial. Radiother. Oncol. 2021, 159, 161–167. [Google Scholar] [CrossRef]
- Kalbasi, A.; Kamrava, M.; Chu, F.-I.; Telesca, D.; Van Dams, R.; Yang, Y.; Ruan, D.; Nelson, S.D.; Dry, S.M.; Hernandez, J.; et al. A Phase 2 Trial of Five-Day Neoadjuvant Radiation Therapy for Patients with High-Risk Primary Soft Tissue Sarcoma. Clin. Cancer Res. 2020, 26, 1829–1836. [Google Scholar] [CrossRef]
- Bedi, M.; Singh, R.; Charlson, J.; Kelly, T.; Johnstone, C.; Wooldridge, A.; Hackbarth, D.; Moore, N.; Neilson, J.; King, D. Is 5 the New 25? Long-Term Oncologic Outcomes from a Phase II, Prospective, 5-Fraction Preoperative Radiation Therapy Trial in Patients with Localized Soft Tissue Sarcoma. Adv. Radiat. Oncol. 2022, 7, 100850. [Google Scholar] [CrossRef]
- Montero, A.; Nuñez, M.; Hernando, O.; Vicente, E.; Ciervide, R.; Zucca, D.; Sanchez, E.; López, M.; Quijano, Y.; Garcia-Aranda, M.; et al. Retroperitoneal Soft-Tissue Sarcomas: Radiotherapy Experience from a Tertiary Cancer Center and Review of Current Evidence. Rep. Pract. Oncol. Radiother. 2020, 25, 643–655. [Google Scholar] [CrossRef]
- Baldini, E.H.; Wang, D.; Haas, R.L.M.; Catton, C.N.; Indelicato, D.J.; Kirsch, D.G.; Roberge, D.; Salerno, K.; Deville, C.; Guadagnolo, B.A.; et al. Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 602–612. [Google Scholar] [CrossRef]
- Salerno, K.E.; Alektiar, K.M.; Baldini, E.H.; Bedi, M.; Bishop, A.J.; Bradfield, L.; Chung, P.; DeLaney, T.F.; Folpe, A.; Kane, J.M.; et al. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 339–351. [Google Scholar] [CrossRef]
- WILSON, R.R. Radiological Use of Fast Protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Chen, Y.-L.; Baldini, E.H.; Wang, D.; Adams, J.; Hickey, S.B.; Yeap, B.Y.; Hahn, S.M.; De Amorim Bernstein, K.; Nielsen, G.P.; et al. Phase 1 Trial of Preoperative Image Guided Intensity Modulated Proton Radiation Therapy with Simultaneously Integrated Boost to the High Risk Margin for Retroperitoneal Sarcomas. Adv. Radiat. Oncol. 2017, 2, 85–93. [Google Scholar] [CrossRef]
- Baldini, E.H.; Bosch, W.; Kane, J.M.; Abrams, R.A.; Salerno, K.E.; Deville, C.; Raut, C.P.; Petersen, I.A.; Chen, Y.-L.; Mullen, J.T.; et al. Retroperitoneal Sarcoma (RPS) High Risk Gross Tumor Volume Boost (HR GTV Boost) Contour Delineation Agreement Among NRG Sarcoma Radiation and Surgical Oncologists. Ann. Surg. Oncol. 2015, 22, 2846–2852. [Google Scholar] [CrossRef]
- Ming, X.; Wang, W.; Shahnazi, K.; Sun, J.; Zhang, Q.; Li, P.; Hong, Z.; Sheng, Y. Dosimetric Comparison between Carbon, Proton and Photon Radiation for Renal Retroperitoneal Soft Tissue Sarcoma Recurrence or Metastasis after Radical Nephrectomy. Int. J. Radiat. Biol. 2022, 98, 183–190. [Google Scholar] [CrossRef]
- Swanson, E.L.; Indelicato, D.J.; Louis, D.; Flampouri, S.; Li, Z.; Morris, C.G.; Paryani, N.; Slopsema, R. Comparison of Three-Dimensional (3D) Conformal Proton Radiotherapy (RT), 3D Conformal Photon RT, and Intensity-Modulated RT for Retroperitoneal and Intra-Abdominal Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1549–1557. [Google Scholar] [CrossRef]
- Chung, C.; Trofimov, A.; Adams, J.; Kung, J.; Kirsch, D.G.; Yoon, S.; Doppke, K.; Bortfeld, T.; Delaney, T.F. Comparison of 3D Conformal Proton Therapy, Intensity-Modulated Proton Therapy, and Intensity-Modulated Photon Therapy for Retroperitoneal Sarcoma. Sarcoma 2022, 2022, 5540615. [Google Scholar] [CrossRef]
- Mak, K.S.; Phillips, J.G.; Barysauskas, C.M.; Lee, L.K.; Mannarino, E.G.; Van Benthuysen, L.; Raut, C.P.; Mullen, J.T.; Fairweather, M.; DeLaney, T.F.; et al. Acute Gastrointestinal Toxicity and Bowel Bag Dose-Volume Parameters for Preoperative Radiation Therapy for Retroperitoneal Sarcoma. Pract. Radiat. Oncol. 2016, 6, 360–366. [Google Scholar] [CrossRef]
- Banerjee, R.; Chakraborty, S.; Nygren, I.; Sinha, R. Small Bowel Dose Parameters Predicting Grade ≥3 Acute Toxicity in Rectal Cancer Patients Treated With Neoadjuvant Chemoradiation: An Independent Validation Study Comparing Peritoneal Space Versus Small Bowel Loop Contouring Techniques. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1225–1231. [Google Scholar] [CrossRef]
- Rasmusson, E.; Nilsson, P.; Kjellén, E.; Gunnlaugsson, A. Long-Term Risk of Hip Complications After Radiation Therapy for Prostate Cancer: A Dose-Response Study. Adv. Radiat. Oncol. 2021, 6, 100571. [Google Scholar] [CrossRef]
- Bae, S.H.; Kim, M.-S.; Kim, S.Y.; Jang, W.I.; Cho, C.K.; Yoo, H.J.; Kim, K.B.; Lee, D.H.; Han, C.J.; Yang, K.Y.; et al. Severe Intestinal Toxicity after Stereotactic Ablative Radiotherapy for Abdominopelvic Malignancies. Int. J. Colorectal. Dis. 2013, 28, 1707–1713. [Google Scholar] [CrossRef]
- Tseng, Y.D.; Wo, J.Y.; Ancukiewicz, M.; Adams, J.; Depauw, N.; Mamon, H.J.; Hong, T.S. Dosimetric Predictors of Nausea and Vomiting: An Exploratory Analysis of a Prospective Phase I/II Trial with Neoadjuvant Accelerated Short-Course Radiotherapy and Capecitabine for Resectable Pancreatic Cancer. J. Radiat. Oncol. 2013, 2, 427–434. [Google Scholar] [CrossRef]
- ISRCTN—ISRCTN10639376: A Trial Looking at Whether Stereotactic Radiotherapy Together with Chemotherapy Is a Useful Treatment for People with Locally Advanced Bile Duct Cancer (ABC-07). Available online: https://www.isrctn.com/ISRCTN10639376?q=%22rare%20diseases%22&filters=&sort=&offset=9&totalResults=21&page=1&pageSize=10&searchType=basic-search (accessed on 26 October 2022).
- Holyoake, D.L.P.; Robinson, M.; Silva, M.; Grose, D.; McIntosh, D.; Sebag-Montefiore, D.; Radhakrishna, G.; Mukherjee, S.; Hawkins, M.A. SPARC, a Phase-I Trial of Pre-operative, Margin Intensified, Stereotactic Body Radiation Therapy for Pancreatic Cancer. Radiother. Oncol. 2021, 155, 278–284. [Google Scholar] [CrossRef]
- Apisarnthanarax, S.; Barry, A.; Cao, M.; Czito, B.; DeMatteo, R.; Drinane, M.; Hallemeier, C.L.; Koay, E.J.; Lasley, F.; Meyer, J.; et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 28–51. [Google Scholar] [CrossRef]
- Koong, A.C.; Le, Q.T.; Ho, A.; Fong, B.; Fisher, G.; Cho, C.; Ford, J.; Poen, J.; Gibbs, I.C.; Mehta, V.K.; et al. Phase I Study of Stereotactic Radiosurgery in Patients with Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1017–1021. [Google Scholar] [CrossRef]
- Colbert, L.E.; Rebueno, N.; Moningi, S.; Beddar, S.; Sawakuchi, G.O.; Herman, J.M.; Koong, A.C.; Das, P.; Holliday, E.B.; Koay, E.J.; et al. Dose Escalation for Locally Advanced Pancreatic Cancer: How High Can We Go? Adv. Radiat. Oncol. 2018, 3, 693–700. [Google Scholar] [CrossRef]
- Schneider, R.A.; Vitolo, V.; Albertini, F.; Koch, T.; Ares, C.; Lomax, A.; Goitein, G.; Hug, E.B. Small Bowel Toxicity after High Dose Spot Scanning-Based Proton Beam Therapy for Paraspinal/Retroperitoneal Neoplasms. Strahlenther. Onkol. 2013, 189, 1020–1025. [Google Scholar] [CrossRef]
- Yoon, S.S.; Chen, Y.-L.; Kirsch, D.G.; Maduekwe, U.N.; Rosenberg, A.E.; Nielsen, G.P.; Sahani, D.V.; Choy, E.; Harmon, D.C.; DeLaney, T.F. Proton-Beam, Intensity-Modulated, and/or Intraoperative Electron Radiation Therapy Combined with Aggressive Anterior Surgical Resection for Retroperitoneal Sarcomas. Ann. Surg. Oncol. 2010, 17, 1515–1529. [Google Scholar] [CrossRef]
- Lee, A.; Kang, J.J.; Bernstein, H.; Marqueen, K.E.; Neal, B.; Kelly, C.M.; Dickson, M.A.; Jillian Tsai, C.; Tap, W.; Singer, S.; et al. Proton Radiotherapy for Recurrent or Metastatic Sarcoma with Palliative Quad Shot. Cancer Med. 2021, 10, 4221–4227. [Google Scholar] [CrossRef]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second Cancer Risk after Primary Cancer Treatment with Three-Dimensional Conformal, Intensity-Modulated, or Proton Beam Radiation Therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]

| Target | Goal | Hard Constraint | Dose (GyE) | Max Point Dose (%) |
|---|---|---|---|---|
| GTV | 100% of the volume to 100% of the dose | 98% of the volume to 100% of the dose | 25 | 108 |
| CTV | 98% of the volume to 100% of the dose | 95% of the volume to 100% of the dose | 25 | 108 |
| CTV Boost | 95% of the volume to 100% of the dose | 90% of the volume to 100% of the dose | 30 | 108 |
| Tissue | Constraint |
|---|---|
| Stomach | Max Dose < 30 GyE |
| Bowel | Max Dose < 30 GyE |
| Liver | Spare at least 700 cc < 15 GyE |
| Kidneys | V12 GyE < 33% |
| Spinal Canal | Max Dose < 25 GyE |
| Tissue | Published Dose Constraint | 5 Fraction BED Equivalent |
|---|---|---|
| Stomach | V45 ≤ 100% | V25.55 ≤ 100% |
| V50 ≤ 50% | V27.90 ≤ 50% | |
| Max Dose < 56 Gy | Max Dose < 30.65 Gy | |
| Duodenum | V45 ≤ 100% | V25.55 ≤ 100% |
| V50 ≤ 50% | V27.90 ≤ 50% | |
| Max Dose < 56 Gy | Max Dose < 30.65 Gy | |
| Bowel | V15 < 830 cc | V10.45 < 830 cc |
| V45 ≤ 195 cc | V25.55 ≤ 195 cc | |
| Liver | Mean Dose < 26 Gy | Mean Dose < 16.30 Gy |
| Kidney, if Both Remain | Mean Dose < 15 Gy | Mean Dose < 10.45 Gy |
| V18 < 50% | V12.10 < 50% | |
| Kidney, if 1 Resected | V18 < 15% | V12.10 < 15% |
| Femoral Head | V40 < 64% | V23.20 < 64% |
| Max Dose < 50 Gy | Max Dose < 27.90 Gy | |
| Mean Dose < 37 Gy | Mean Dose < 21.75 Gy | |
| Spinal Canal | Max Dose < 50 Gy | Max Dose < 27.90 Gy |
| Target/OAR | Dosimetric Endpoint | IMRT | IMPT | p-Value | ||
|---|---|---|---|---|---|---|
| Mean | St-Dev | Mean | St-Dev | |||
| GTV | V25 GyE (%) | 100 | 0.0 | 100 | 0.0 | N/A |
| CTV | V25 GyE (%) | 99.7 | 0.2 | 99.5 | 0.3 | 0.080 |
| CTV Boost | V30 GyE (%) | 100 | 0.0 | 100 | 0.0 | N/A |
| PTV | V25 GyE (%) | 96.5 | 0.7 | 97.6 | 1.2 | 0.003 * |
| PTV Boost | V30 GyE (%) | 96.1 | 0.9 | 96.2 | 0.7 | 0.413 |
| Stomach | V5 GyE (cc) | 144.6 | 156.5 | 26.1 | 50.8 | 0.056 |
| V10 GyE (cc) | 103.3 | 116.1 | 17.4 | 35.2 | 0.055 | |
| V15 GyE (cc) | 43.4 | 52.0 | 11.2 | 22.7 | 0.039 * | |
| V20 GyE (cc) | 16.8 | 28.2 | 6.7 | 13.3 | 0.082 | |
| V25 GyE (cc) | 4.9 | 9.9 | 1.1 | 1.7 | 0.163 | |
| D50% (GyE) | 7.4 | 7.3 | 1.6 | 3.8 | 0.022 * | |
| Max Dose (GyE) | 17.9 | 10.3 | 11.6 | 12.8 | 0.024 * | |
| Mean Dose (GyE) | 7.8 | 7.2 | 2.1 | 4.3 | 0.019 * | |
| Duodenum | V5 GyE (cc) | 45.3 | 27.8 | 31.8 | 27.1 | 0.056 |
| V10 GyE (cc) | 39.7 | 27.9 | 29.1 | 25.6 | 0.083 | |
| V15 GyE (cc) | 34.2 | 27.0 | 27.0 | 24.6 | 0.093 | |
| V20 GyE (cc) | 27.2 | 24.8 | 24.5 | 23.4 | 0.051 | |
| V25 GyE (cc) | 14.4 | 20.4 | 13.3 | 21.4 | 0.235 | |
| D50% (GyE) | 17.6 | 8.8 | 13.2 | 12.5 | 0.095 | |
| Max Dose (GyE) | 27.3 | 3.1 | 26.8 | 3.1 | 0.105 | |
| Mean Dose (GyE) | 16.5 | 6.5 | 11.6 | 8.7 | 0.040 * | |
| Bowel | V5 GyE (cc) | 1552.5 | 1127.2 | 503.8 | 412.5 | 0.002 * |
| V10 GyE (cc) | 916.3 | 642.8 | 414.8 | 346.1 | 0.000 * | |
| V15 GyE (cc) | 554.9 | 444.1 | 347.0 | 294.1 | 0.001 * | |
| V20 GyE (cc) | 351.3 | 299.5 | 280.0 | 240.4 | 0.007 * | |
| V25 GyE (cc) | 138.6 | 135.6 | 157.3 | 149.9 | 0.095 | |
| D50% (GyE) | 8.9 | 5.5 | 1.3 | 3.4 | 0.000 * | |
| Max Dose (GyE) | 27.3 | 4.0 | 25.6 | 8.9 | 0.161 | |
| Mean Dose (GyE) | 10.6 | 4.8 | 5.0 | 3.8 | 0.000 * | |
| Liver | V5 GyE (cc) | 570.9 | 846.4 | 302.5 | 603.4 | 0.048 * |
| V10 GyE (cc) | 393.0 | 689.2 | 184.6 | 329.6 | 0.056 | |
| V15 GyE (cc) | 266.7 | 534.2 | 125.5 | 212.3 | 0.112 | |
| V20 GyE (cc) | 141.5 | 258.0 | 93.0 | 159.5 | 0.107 | |
| V25 GyE (cc) | 48.8 | 94.9 | 48.1 | 101.5 | 0.460 | |
| D50% (GyE) | 4.7 | 7.8 | 2.3 | 5.0 | 0.029 * | |
| Mean Dose (GyE) | 5.1 | 7.2 | 2.9 | 4.9 | 0.016 * | |
| Ipsilateral Kidney | V10 GyE (%) | 36.8 | 29.2 | 23.1 | 20.8 | 0.020 * |
| V12 GyE (%) | 33.0 | 26.2 | 21.2 | 19.6 | 0.021 * | |
| D50% (GyE) | 8.2 | 6.9 | 3.8 | 4.2 | 0.006 * | |
| Mean Dose (GyE) | 8.9 | 6.8 | 6.1 | 5.2 | 0.006 * | |
| Contralateral Kidney | V10 GyE (%) | 20.6 | 26.4 | 0.1 | 0.3 | 0.018 * |
| V12 GyE (%) | 10.5 | 14.0 | 0.1 | 0.2 | 0.021 * | |
| D50% (GyE) | 4.6 | 4.6 | 0.0 | 0.0 | 0.006 * | |
| Mean Dose (GyE) | 4.7 | 4.5 | 0.2 | 0.3 | 0.006 * | |
| Bilateral Kidneys | V10 GyE (%) | 28.1 | 24.1 | 10.5 | 11.1 | 0.007 * |
| V12 GyE (%) | 20.7 | 17.1 | 9.7 | 10.4 | 0.007 * | |
| D50% (GyE) | 5.1 | 4.8 | 0.2 | 0.2 | 0.005 * | |
| Mean Dose (GyE) | 6.8 | 5.1 | 2.9 | 2.8 | 0.003 * | |
| Ipsilateral Femoral Head | V23.2 GyE (%) | 13.0 | 17.5 | 4.4 | 9.3 | 0.041 * |
| V30 GyE (cc) | 0.9 | 2.2 | 1.7 | 4.1 | 0.182 | |
| Max Dose (GyE) | 15.0 | 16.1 | 13.4 | 14.9 | 0.092 | |
| Mean Dose (GyE) | 8.2 | 9.7 | 4.1 | 5.8 | 0.058 | |
| Contralateral Femoral Head | V23.2 GyE (%) | 0.0 | 0.0 | 0.0 | 0.0 | N/A |
| V30 GyE (cc) | 0.0 | 0.0 | 0.0 | 0.0 | N/A | |
| Max Dose (GyE) | 5.8 | 7.1 | 0.1 | 0.1 | 0.050 | |
| Mean Dose (GyE) | 3.2 | 4.5 | 0.0 | 0.0 | 0.070 | |
| Bone | V5 GyE (cc) | 1131.4 | 521.9 | 690.1 | 296.3 | 0.005 * |
| V10 GyE (cc) | 897.2 | 443.7 | 446.5 | 213.0 | 0.001 * | |
| V15 GyE (cc) | 610.6 | 364.5 | 303.2 | 193.7 | 0.001 * | |
| V20 GyE (cc) | 378.1 | 299.4 | 219.4 | 173.9 | 0.003 * | |
| V25 GyE (cc) | 191.4 | 170.7 | 144.2 | 118.0 | 0.016 * | |
| Mean Dose (GyE) | 15.2 | 1.9 | 9.3 | 2.6 | 0.000 * | |
| Spinal Canal | Max Dose (GyE) | 19.4 | 6.5 | 13.9 | 8.9 | 0.004 * |
| Skin | V12 GyE (%) | 10.5 | 13.5 | 9.4 | 9.4 | 0.346 |
| Body | Max Dose (GyE) | 32.6 | 0.8 | 31.6 | 0.2 | 0.000 * |
| Body—CTV | Integral Dose (J) | 22.0 | 9.3 | 10.2 | 4.3 | 0.000 * |
| Phase | Identifier | Sponsor | Date of Initiation | Estimated Study Completion Date | Last Update Posted | Recruitment Status | Estimated Enrollment | Arm(s) | Primary Endpoint | Trial Design |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypofractionated photon trials | ||||||||||
| II | NCT03972930 | University of Wisconsin (USA) | June 2019 | September 2027 | September 2022 | Recruiting | 48 | IMRT—60 Gy3–8 fx (most commonly 6 fx) (QOD) | 2-year local control as determined by RECIST | Single arm trial enrolling soft tissue sarcomas deemed unresectable of any location |
| II | NCT05224934 | Chinese Academy of Medical Sciences (China) | January 2022 | December 2024 | February 2022 | Recruiting | 28 | SBRT—25–50 Gy5 fx | Perioperative complications within 1 wk post-op | Single arm trial investigating feasibility and perioperative complications of pre-op SBRT followed by surgery 1–2 months later |
| Conventionally fractionated particle therapy trials | ||||||||||
| I/II | NCT01659203 | Massachusetts General Hospital (USA) | December 2012 | August 2025 | September 2020 | Recruiting | Phase I: 11 | Phase I: IMRT/IMPT—50.4 GyE (SIB: 60.2–63.0 GyE)28 fx | Phase I: maximum tolerated dose | Separate cohorts of patients receiving pre-op IMRT and IMPT. Phase I portion of each cohort utilized dose escalation for the SIB from 60.2 to 63.0 GyE showing no dose limiting toxicities, after which enrollment began on Phase II portion for each cohort |
| Phase II: 60 | Phase II: IMRT/IMPT—50.4 GyE (SIB: 63.0 GyE)28 fx | Phase II: local control | ||||||||
| III | NCT02838602 | Hospices Civils de Lyon (France) | December 2017 | December 2026 | September 2021 | Recruiting | 250 | Arm 1: Photon and/or proton RT—64.0–70.0 GyE * 32–35 fx | 5-year progression free survival | Randomized trial comparing carbon vs photon and/or proton RT for radioresistant unresectable or resected with gross residual tumors, including chordomas, adenoid cystic head/neck cancers, and sarcomas |
| Arm 2: Carbon—70.4–73.6 GyE * 16 fx (4 fxwk) | ||||||||||
| Hypofractionated particle therapy trials | ||||||||||
| II | NCT04219202 | University Hospital Heidelberg (Germany) | May 2019 | May 2024 | June 2021 | Recruiting | 64 | Arm 1: IMPT—39 GyE13 fx (6 fxwk) | Grade 3–5 toxicity | Randomized trial investigating safety and feasibility of hypofractionated, accelerated, pre-op RT based on grade 3-5 NCI-CTCAE toxicity and/or termination of planned therapy |
| Arm 2: Carbon—39 GyE13 fx (6 fxwk) | ||||||||||
| II | NCT05302570 | Johns Hopkins University (USA) | December 2022 † | December 2027 | July 2022 | Not yet recruiting | 45 | IMPT—25 GyE (SIB: 30 GyE)5 daily fx | Grade 3–5 toxicity | Single arm trial evaluating safety and efficacy of hypofractionated pre-op proton therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogineni, E.; Chen, H.; Istl, A.C.; Johnston, F.M.; Narang, A.; Deville, C., Jr. Comparative In Silico Analysis of Ultra-Hypofractionated Intensity-Modulated Photon Radiotherapy (IMRT) Versus Intensity-Modulated Proton Therapy (IMPT) in the Pre-Operative Treatment of Retroperitoneal Sarcoma. Cancers 2023, 15, 3482. https://doi.org/10.3390/cancers15133482
Gogineni E, Chen H, Istl AC, Johnston FM, Narang A, Deville C Jr. Comparative In Silico Analysis of Ultra-Hypofractionated Intensity-Modulated Photon Radiotherapy (IMRT) Versus Intensity-Modulated Proton Therapy (IMPT) in the Pre-Operative Treatment of Retroperitoneal Sarcoma. Cancers. 2023; 15(13):3482. https://doi.org/10.3390/cancers15133482
Chicago/Turabian StyleGogineni, Emile, Hao Chen, Alexandra C. Istl, Fabian M. Johnston, Amol Narang, and Curtiland Deville, Jr. 2023. "Comparative In Silico Analysis of Ultra-Hypofractionated Intensity-Modulated Photon Radiotherapy (IMRT) Versus Intensity-Modulated Proton Therapy (IMPT) in the Pre-Operative Treatment of Retroperitoneal Sarcoma" Cancers 15, no. 13: 3482. https://doi.org/10.3390/cancers15133482
APA StyleGogineni, E., Chen, H., Istl, A. C., Johnston, F. M., Narang, A., & Deville, C., Jr. (2023). Comparative In Silico Analysis of Ultra-Hypofractionated Intensity-Modulated Photon Radiotherapy (IMRT) Versus Intensity-Modulated Proton Therapy (IMPT) in the Pre-Operative Treatment of Retroperitoneal Sarcoma. Cancers, 15(13), 3482. https://doi.org/10.3390/cancers15133482






