Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Endpoints
- (a)
- BM contains <5% blasts, including monoblasts and promonocytes in M5 leukemia;
- (b)
- BM cellularity of at least 20% with maturation of all cell lines;
- (c)
- Absence of Auer rods;
- (d)
- Absence of extramedullary leukemia;
- (e)
- Absence of peripheral leukemic blasts;
- (f)
- Hemoglobin levels ≥ 9 g/dL, absolute neutrophil count ≥ 1.5 × 109/L, and platelet count ≥ 100 × 109/L.
- (a)
- BM contains 5–25% blasts, or <5% blasts in the presence of Auer rods;
- (b)
- Absence of peripheral leukemic blasts.
2.4. Induction and Consolidation Chemotherapy
2.5. Post-Remission Therapy
2.6. Bone Marrow Assessment
2.7. Quality of Life Assessments
2.8. Statistical Analysis Plan
2.8.1. Disease-Free Survival
2.8.2. Number of Hospitalizations
2.8.3. Quality of Life
2.8.4. Overall Survival
3. Results
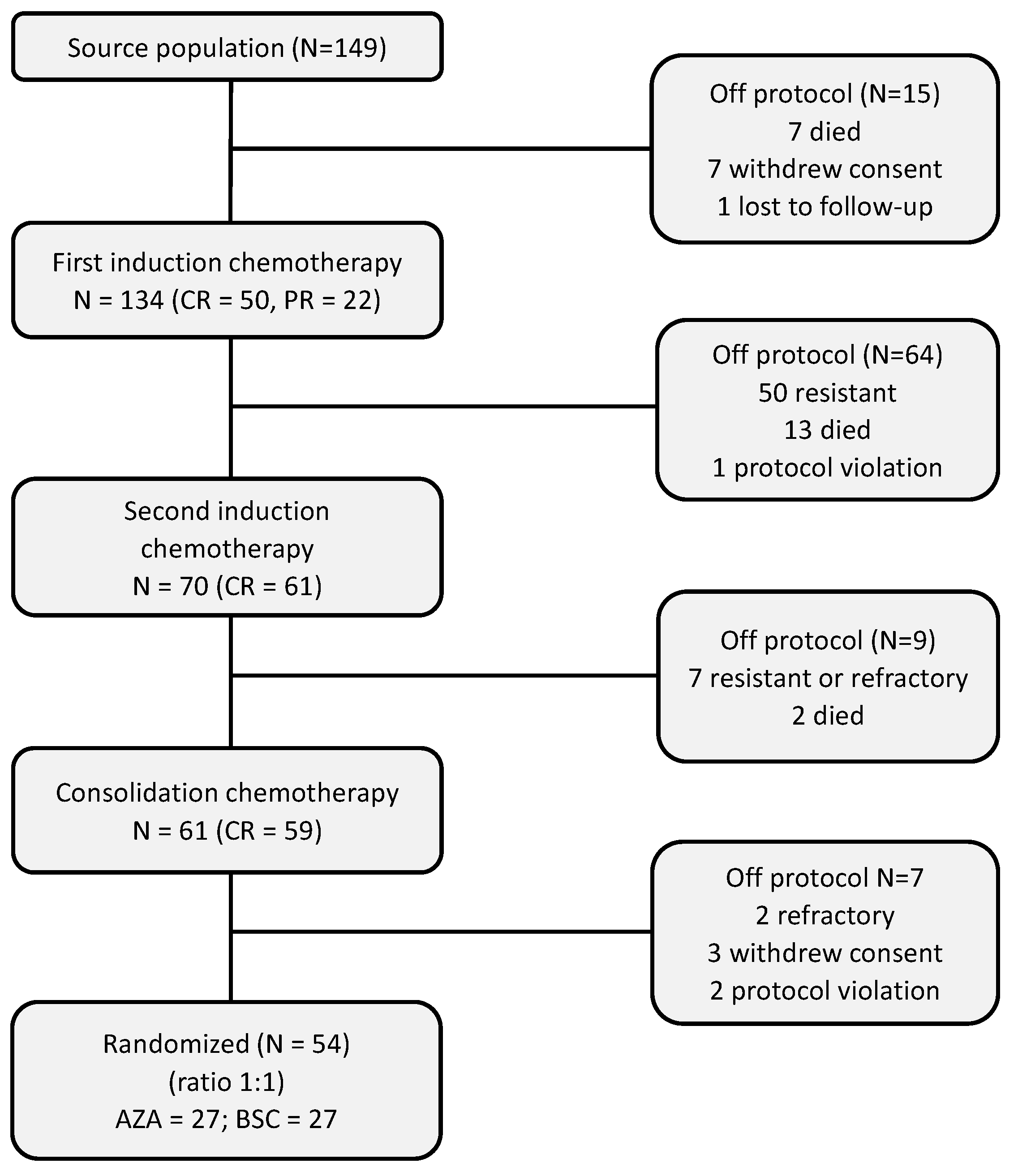
3.1. Patients
3.2. Pre-Randomization Phase
3.3. Post-Randomization Phase
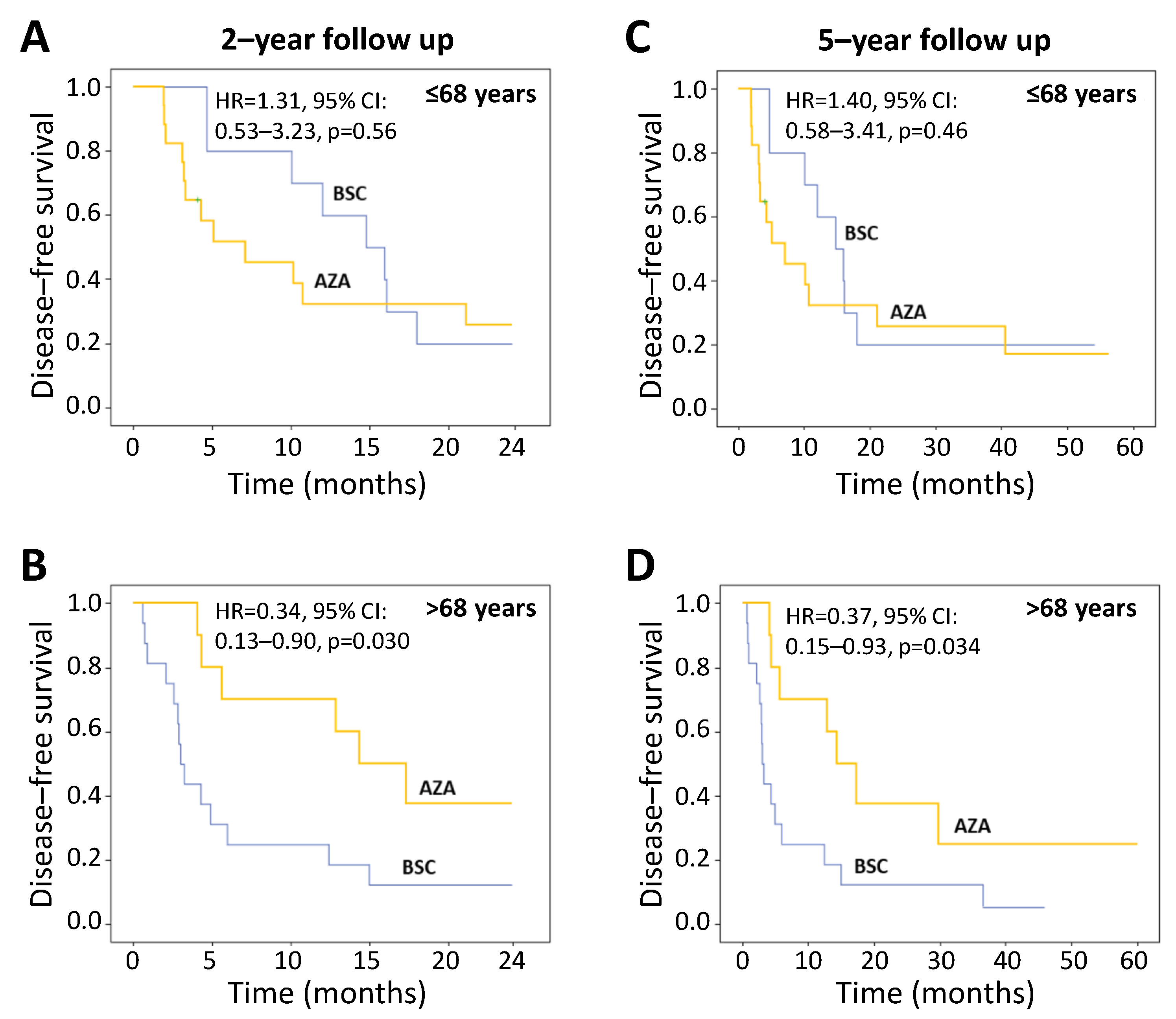
3.4. Primary Endpoint: Disease-Free Survival
3.5. Safety
3.6. Secondary Endpoints
3.6.1. Number and Duration of Hospitalizations during the Study
3.6.2. Quality of Life
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.; Büchner, T.; Dombret, H.; Ebert, B.; Fenaux, P.; Larson, R.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Hernandez-Valladares, M.; Aasebø, E.; Berven, F.; Selheim, F.; Bruserud, Ø. Biological Characteristics of Aging in Human Acute Myeloid Leukemia Cells: The Possible Importance of Aldehyde Dehydrogenase, the Cytoskeleton and Altered Transcriptional Regulation. Aging 2020, 12, 24734–24777. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F. Conventional Chemotherapy or Hypomethylating Agents for Older Patients with Acute Myeloid Leukaemia? Hematol. Oncol. 2014, 32, 1–9. [Google Scholar] [CrossRef]
- Webster, J.A.; Pratz, K.W. Acute Myeloid Leukemia in the Elderly: Therapeutic Options and Choice. Leuk. Lymphoma 2018, 59, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing Survival Outcomes with Post-Remission Therapy in Acute Myeloid Leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Breccia, M.; Foa, R.; Jabbour, E.; Kadia, T.M. Maintenance Therapy in AML: The Past, the Present and the Future. Am. J. Hematol. 2019, 94, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.; Roboz, G.J.; Platzbecker, U.; Craddock, C.; Ossenkoppele, G. AML and the Art of Remission Maintenance. Blood Rev. 2021, 49, 100829. [Google Scholar] [CrossRef]
- Reville, P.K.; Kadia, T.M. Maintenance Therapy in AML. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Ramos, F.; Thépot, S.; Pleyer, L.; Maurillo, L.; Itzykson, R.; Bargay, J.; Stauder, R.; Venditti, A.; Seegers, V.; Martínez-Robles, V.; et al. Azacitidine Frontline Therapy for Unfit Acute Myeloid Leukemia Patients: Clinical Use and Outcome Prediction. Leuk. Res. 2015, 39, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Mazzone, C.; Niscola, P.; Carmosino, I.; Di Veroli, A.; De Gregoris, C.; Bonanni, F.; Perrone, S.; Cenfra, N.; Fianchi, L.; et al. Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers 2022, 14, 4897. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Döhner, H.; Wei, A.H.; Torre, I.L.; Skikne, B.; Beach, C.L.; Santini, V. Oral Azacitidine (CC-486) for the Treatment of Myeloid Malignancies. Clin. Lymphoma Myeloma Leuk. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Huls, G.; Chitu, D.A.; Havelange, V.; Jongen-Lavrencic, M.; van de Loosdrecht, A.A.; Biemond, B.J.; Sinnige, H.; Hodossy, B.; Graux, C.; Kooy, R.V.M.; et al. Azacitidine Maintenance after Intensive Chemotherapy Improves DFS in Older AML Patients. Blood 2019, 133, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- Oliva, E.N.; Nobile, F.; Dimitrov, B.D. Development and Validation of QOL-E© Instrument for the Assessment of Health-Related Quality of Life in Myelodysplastic Syndromes. Cent. Eur. J. Med. 2013, 8, 835–844. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations from an International Expert Panel, on Behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Ringash, J.; O’Sullivan, B.; Bezjak, A.; Redelmeier, D.A. Interpreting Clinically Significant Changes in Patient-Reported Outcomes. Cancer 2007, 110, 196–202. [Google Scholar] [CrossRef]
- Gay, F.; Jackson, G.; Rosiñol, L.; Holstein, S.A.; Moreau, P.; Spada, S.; Davies, F.; Lahuerta, J.J.; Leleu, X.; Bringhen, S.; et al. Maintenance Treatment and Survival in Patients With Myeloma: A Systematic Review and Network Meta-Analysis. JAMA Oncol. 2018, 4, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Golfier, C.; Salles, G. Antibody Therapy Maintenance in Follicular Lymphoma. Hematol. Oncol. Clin. N. Am. 2020, 34, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Walter, R.B.; Tallman, M.S.; Appelbaum, F.R.; DiPersio, J.F. Maintenance Therapy in Acute Myeloid Leukemia: An Evidence-Based Review of Randomized Trials. Blood 2016, 128, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.M.; Wang, V.X.; Rowe, J.M.; Litzow, M.R.; Paietta, E.; Ketterling, R.P.; Lazarus, H.; Rybka, W.B.; Craig, M.D.; Karp, J.; et al. Tipifarnib as Maintenance Therapy Did Not Improve Disease-Free Survival in Patients with Acute Myelogenous Leukemia at High Risk of Relapse: Results of the Phase III Randomized E2902 Trial. Leuk. Res. 2021, 111, 106736. [Google Scholar] [CrossRef] [PubMed]
- Grövdal, M.; Karimi, M.; Khan, R.; Aggerholm, A.; Antunovic, P.; Astermark, J.; Bernell, P.; Engström, L.-M.; Kjeldsen, L.; Linder, O.; et al. Maintenance Treatment with Azacytidine for Patients with High-Risk Myelodysplastic Syndromes (MDS) or Acute Myeloid Leukaemia Following MDS in Complete Remission after Induction Chemotherapy. Br. J. Haematol. 2010, 150, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Yokoyama, A.; Yoneda, M.; Ohashi, K.; Hosoda, T.; Kagoo, T.; Boku, S.; Ueno, H.; Yano, T. Azacitidine as the Post-Remission Therapy for Elderly Patients with Acute Myeloid Leukemia. Ann. Hematol. 2014, 93, 2081–2082. [Google Scholar] [CrossRef]
- Griffin, P.T.; Komrokji, R.S.; De Castro, C.M.; Rizzieri, D.A.; Melchert, M.; List, A.F.; Lancet, J.E. A Multicenter, Phase II Study of Maintenance Azacitidine in Older Patients with Acute Myeloid Leukemia in Complete Remission after Induction Chemotherapy. Am. J. Hematol. 2015, 90, 796–799. [Google Scholar] [CrossRef]
- Kungwankiattichai, S.; Ponvilawan, B.; Roy, C.; Tunsing, P.; Kuchenbauer, F.; Owattanapanich, W. Maintenance With Hypomethylating Agents After Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 801632. [Google Scholar] [CrossRef]
- Blum, W.; Sanford, B.L.; Klisovic, R.; DeAngelo, D.J.; Uy, G.; Powell, B.L.; Stock, W.; Baer, M.R.; Kolitz, J.E.; Wang, E.S.; et al. Maintenance Therapy with Decitabine in Younger Adults with Acute Myeloid Leukemia in First Remission: A Phase 2 Cancer and Leukemia Group B Study (CALGB 10503). Leukemia 2017, 31, 34–39. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ravandi, F.; Wei, A.H.; Dombret, H.; Thol, F.; Voso, M.T.; Schuh, A.C.; Porkka, K.; La Torre, I.; Skikne, B.; et al. Oral Azacitidine Prolongs Survival of Patients with AML in Remission Independently of Measurable Residual Disease Status. Blood 2022, 139, 2145–2155. [Google Scholar] [CrossRef]
- Oliva, E.N.; Nobile, F.; Alimena, G.; Ronco, F.; Specchia, G.; Impera, S.; Breccia, M.; Vincelli, I.; Carmosino, I.; Guglielmo, P.; et al. Quality of Life in Elderly Patients with Acute Myeloid Leukemia: Patients May Be More Accurate than Physicians. Haematologica 2011, 96, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in First Complete Remission: Systematic Review and Meta-Analysis of Prospective Clinical Trials. JAMA 2009, 301, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Kohlschmidt, J.; Mrózek, K.; Eisfeld, A.-K.; Nicolet, D.; Sterling, L.J.; Becker, H.; Metzeler, K.H.; Papaioannou, D.; Powell, B.L.; et al. Ten-Year Outcome of Patients with Acute Myeloid Leukemia Not Treated with Allogeneic Transplantation in First Complete Remission. Blood Adv. 2018, 2, 1645–1650. [Google Scholar] [CrossRef]
- Barrett, A.J.; Battiwalla, M. Relapse after Allogeneic Stem Cell Transplantation. Expert. Rev. Hematol. 2010, 3, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Bejanyan, N.; Weisdorf, D.J.; Logan, B.R.; Wang, H.-L.; Devine, S.M.; de Lima, M.; Bunjes, D.W.; Zhang, M.-J. Survival of Patients with Acute Myeloid Leukemia Relapsing after Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol. Blood Marrow Transplant. 2015, 21, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.-K.; Lane, S.W. Better the Cure You Know: Why Patients with AML ≥60 Years of Age Should Be Offered Early Allogeneic Stem Cell Transplantation. Blood Adv. 2022, 6, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Sophie, S.; Yves, B.; Frédéric, B. Current Status and Perspectives of Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia. Stem Cells Transl. Med. 2022, 11, 461–477. [Google Scholar] [CrossRef]
- Deeg, H.J. Not All Patients with AML over 60 Years of Age Should Be Offered Early Allogeneic Stem Cell Transplantation. Blood Adv. 2022, 6, 1623–1627. [Google Scholar] [CrossRef]
- Muffly, L.; Pasquini, M.C.; Martens, M.; Brazauskas, R.; Zhu, X.; Adekola, K.; Aljurf, M.; Ballen, K.K.; Bajel, A.; Baron, F.; et al. Increasing Use of Allogeneic Hematopoietic Cell Transplantation in Patients Aged 70 Years and Older in the United States. Blood 2017, 130, 1156–1164. [Google Scholar] [CrossRef]
- Noguera, N.I.; Ammatuna, E.; Zangrilli, D.; Lavorgna, S.; Divona, M.; Buccisano, F.; Amadori, S.; Mecucci, C.; Falini, B.; Lo-Coco, F. Simultaneous detection of NPM1 and FLT3-ITD mutations by capillary electrophoresis in acute myeloid leukemia. Leukemia 2005, 19, 1479–1482. [Google Scholar] [CrossRef]


| Characteristics | All Patients (N = 149) |
|---|---|
| Age, median years (IQR) | 69 (65–74) |
| Male, N (%) | 78 (52%) |
| AML de novo, N (%) | 121 (81%) |
| Hemoglobin, mean g/dL (±SD) | 9.1 ± 1.4 |
| White blood cell × 103, median (IQR) | 7.9 (2.5–28.8) |
| Platelet × 103, median (IQR) | 52 (26–84) |
| WHO Classification, N (%) | |
| AML with minimal differentiation | 28 (18.8%) |
| Acute myelomonocytic leukemia | 28 (18.8%) |
| AML with myelodysplasia-related changes | 28 (18.8%) |
| AML with maturation | 24 (16.1%) |
| Acute monoblastic and monocytic leukemia | 17 (11.4%) |
| AML without maturation | 12 (8.1%) |
| AML with recurrent genetic abnormalities | 8 (5.3%) |
| Therapy-related myeloid neoplasms | 2 (1.3%) |
| Acute erythroid leukemia | 1 (0.7%) |
| Acute megakaryoblastic leukemia | 1 (0.7%) |
| Cytogenetic risk profile, N (%) | |
| Good | 1 (0.7%) |
| Intermediate | 104 (69.8%) |
| Poor | 26 (17.4%) |
| Not evaluable | 18 (12.1%) |
| Characteristics | AZA (N = 27) | BSC (N = 27) | All Patients (N = 54) | p Value |
|---|---|---|---|---|
| Age, mean years (±SD) | 67.7 ± 5.2 | 70.4 ± 5.5 | 69.1 ± 5.5 | 0.069 |
| Male, N (%) | 17 (63%) | 14 (52%) | 78 (57%) | 0.583 |
| AML de novo, N (%) | 21 (78%) | 26 (96%) | 47 (87%) | 0.100 |
| Hemoglobin, mean g/dL (±SD) | 8.9 ± 1.0 | 9.3 ± 1.3 | 9.1 ± 1.2 | 0.206 |
| White blood cell × 103, median (IQR) | 4.1 (2.1–23.8) | 17.0 (2.5–25.7) | 6.0 (2.2–24.4) | 0.382 |
| Platelet × 103, median (IQR) | 39 (26–63) | 54 (24–77) | 42 (26–74) | 0.574 |
| BM blasts (%), median (IQR) | 70 (49–80) | 70 (50–90) | 70 (50–89) | 0.696 |
| PB blasts (%), median (IQR) | 17 (3–70) | 40 (15–75) | 32 (8–70) | 0.158 |
| WHO Classification, N (%) | 0.259 | |||
| AML with minimal differentiation | 6 (22%) | 3 (11%) | 9 (17%) | |
| Acute myelomonocytic leukemia | 4 (15%) | 7 (26%) | 11 (20%) | |
| AML with myelodysplasia-related changes | 6 (22%) | 1 (4%) | 7 (13%) | |
| AML with maturation | 3 (11%) | 6 (22%) | 9 (17%) | |
| Acute monoblastic and monocytic leukemia | 3 (11%) | 5 (19%) | 8 (15%) | |
| AML without maturation | 1 (4%) | 1 (4%) | 2 (4%) | |
| AML with recurrent genetic abnormalities | 3 (11%) | 2 (7%) | 5 (9%) | |
| Therapy-related myeloid neoplasms | 0 | 2 (7%) | 2 (4%) | |
| Acute erythroid leukemia | 1 (4%) | 0 | 1 (2%) | |
| Baseline cytogenetic risk profile, N (%) | 0.375 | |||
| Good | 1 (4%) | 1 (2%) | ||
| Intermediate | 19 (70%) | 22 (81%) | 41 (76%) | |
| Poor | 4 (15%) | 4 (15%) | 8 (15%) | |
| Not evaluable | 3 (11%) | 1 (4%) | 4 (7%) | |
| MRD at random, N (%) | 0.861 | |||
| Present | 13 (48.1%) | 12 (44.4%) | 25 (46.3%) | |
| Absent | 12 (44.4%) | 10 (37.0%) | 22 (40.7%) | |
| Not evaluable | 2 (7.5%) | 5 (18.6%) | 7(13.0%) | |
| Mutation at random, N (%) | ||||
| FLT3 | 2 (7.4%) | 1 (3.7%) | 3 (5.6%) | 0.315 |
| NPM1 | 1 (3.7%) | 4 (14.8%) | 5 (9.3%) | 0.343 |
| IDH1 | 2 (7.4%) | 0 | 2 (3.7%) | 0.232 |
| IDH2 | 1 (3.7%) | 3 (11.1%) | 4 (7.4%) | 0.606 |
| TP53 at diagnosis | 10 (37.0%) | 9 (33.3%) | 19 (35.2%) | 0.752 |
| Adverse Event | AZA | BSC | ||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Neutropenia | 5 | 5 | 1 | 0 |
| Thrombocytopenia | 3 | 0 | 0 | 0 |
| Anemia | 2 | 0 | 0 | 0 |
| Pneumonia | 1 | 0 | 0 | 0 |
| CVC bacterial infection | 1 | 0 | 0 | 0 |
| Pericarditis | 1 | 0 | 0 | 0 |
| Abdominal pain | 0 | 1 | 0 | 0 |
| Urothelial bladder cancer | 1 | 0 | 0 | 0 |
| Total | 14 | 6 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, E.N.; Candoni, A.; Salutari, P.; Palumbo, G.A.; Reda, G.; Iannì, G.; Tripepi, G.; Cuzzola, M.; Capelli, D.; Mammì, C.; et al. Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE). Cancers 2023, 15, 2441. https://doi.org/10.3390/cancers15092441
Oliva EN, Candoni A, Salutari P, Palumbo GA, Reda G, Iannì G, Tripepi G, Cuzzola M, Capelli D, Mammì C, et al. Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE). Cancers. 2023; 15(9):2441. https://doi.org/10.3390/cancers15092441
Chicago/Turabian StyleOliva, Esther Natalie, Anna Candoni, Prassede Salutari, Giuseppe A. Palumbo, Gianluigi Reda, Giuseppe Iannì, Giovanni Tripepi, Maria Cuzzola, Debora Capelli, Corrado Mammì, and et al. 2023. "Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE)" Cancers 15, no. 9: 2441. https://doi.org/10.3390/cancers15092441
APA StyleOliva, E. N., Candoni, A., Salutari, P., Palumbo, G. A., Reda, G., Iannì, G., Tripepi, G., Cuzzola, M., Capelli, D., Mammì, C., Alati, C., Cannatà, M. C., Niscola, P., Serio, B., Musto, P., Vigna, E., Volpe, A., Melillo, L. M. A., Arcadi, M. T., ... Latagliata, R. (2023). Azacitidine Post-Remission Therapy for Elderly Patients with AML: A Randomized Phase-3 Trial (QoLESS AZA-AMLE). Cancers, 15(9), 2441. https://doi.org/10.3390/cancers15092441







