Cancer and Potential Prevention with Lifestyle among Career Firefighters: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
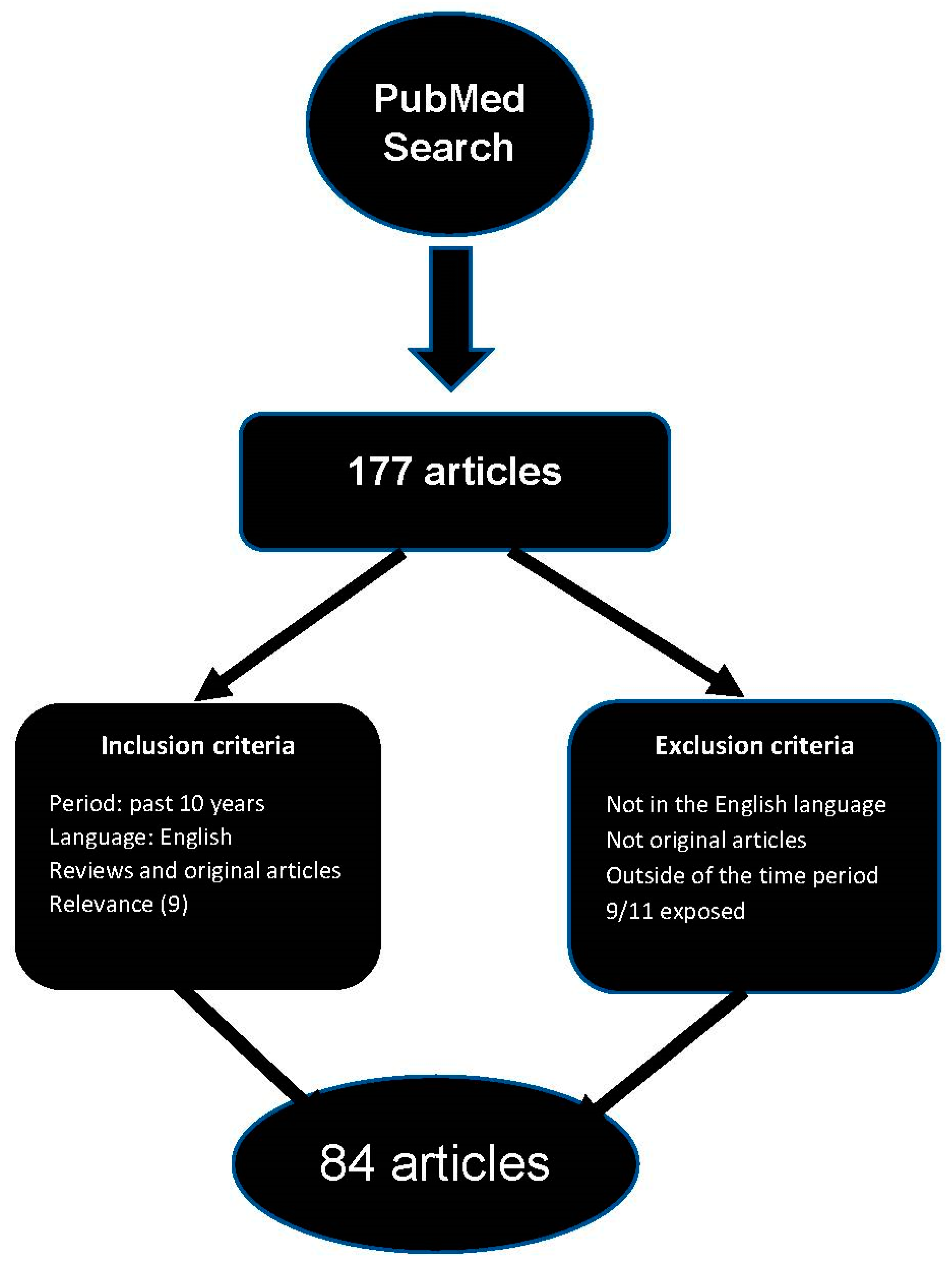
2. Methods
3. Results
3.1. Cancer Incidence
3.2. Overall Cancer Incidence
3.3. Types of Cancer
3.3.1. Skin Cancer
3.3.2. Urogenital and Reproductive Cancers
3.3.3. Other Cancers
3.4. Cancer Mortality
| Author/Year | Mortality Rate (95% CI) | Study Size | Study Location |
|---|---|---|---|
| North America | |||
| Muegge 2018 [32] | OR = 1.19 (1.08–1.30) | 2818 firefighters and 11,272 matched comparison death records | USA |
| Pinkerton 2020 [33] | MR = 1.12 (1.08–1.16) | 29,992 career firefighters | USA |
| Europe | |||
| Petersen 2018 [36] | SMR = 1.12 (1.00–1.26) | 4659 full-time firefighters | Denmark |
| Amadeo 2015 [34] | SMR = 0.95 (0.88–1.02) | 10,829 professional male firefighters | France |
| Ide 2014 [15] | Mortality rate/105/year [SD] = 20.4 [27.4] | ~2200 serving firefighters | Scotland |
| Zhao 2020 [37] | MRR = 1.00 (0.89–1.12) | 27,365 firefighters | Spain |
| Oceania | |||
| Glass 2016 [1] | SMR = 0.29 (0.01–1.64) (Low risk) SMR = 0.87 (0.40–1.65) (medium risk) a SMR = 1.47 (0.54–3.19) (High risk) | 611 firefighters | Australia |
| Glass 2016 [10] | SMR = 0.81 (0.74 to 0.89) | 17,394 full-time and 12,663 part-time male firefighters | Australia |
| Glass 2019 [17] | SMR = 0.83 (0.44–1.54) | 1682 paid female firefighters | Australia |
| Asia | |||
| Ahn 2015 [12] | SMR = 0.58 (0.50–0.68) | 29,453 firefighters | Korea |
3.5. Causes
3.5.1. Diesel Exhaust
3.5.2. Polycyclic Aromatic Hydrocarbons (PAHs)
3.5.3. Perfluoroalkyl Substances (PFAS)
3.5.4. Polybrominated Diphenyl Ethers (PBDEs)
3.5.5. Formaldehyde
3.6. Other Causes
3.6.1. Lifestyle Factors
3.6.2. Obesity
3.6.3. Sedentary Behavior
3.6.4. Shift Work and Poor Sleep Quality
3.6.5. Alcohol Consumption
3.6.6. Nutrition
4. Discussion
4.1. Prevention/Perception
4.2. Attitude towards Cleaning and Decontamination Processes
4.3. Prevention Methods
4.4. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Glass, D.C.; Del Monaco, A.; Pircher, S.; Hoorn, S.V.; Sim, M. Mortality and cancer incidence at a fire training college. Occup. Med. 2016, 66, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Demers, P.; DeMarini, D.M.; Fent, K.W.; Glass, D.C.; Hansen, J.; Adetona, O.; Andersen, M.H.; Freeman, L.E.B.; Caban-Martinez, A.J.; Daniels, R.D.; et al. Carcinogenicity of occupational exposure as a firefighter. Lancet Oncol. 2022, 23, 985–986. [Google Scholar] [CrossRef]
- Harrison, T.R.; Yang, F.; Morgan, S.E.; Muhamad, J.W.; Talavera, E.; Eaton, S.A.; Niemczyk, N.; Sheppard, V.; Kobetz, E. The Invisible Danger of Transferring Toxins with Bunker Gear: A Theory-Based Intervention to Increase Postfire Decontamination to Reduce Cancer Risk in Firefighters. J. Health Commun. 2018, 23, 999–1007. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Jin, Q.; Rainey, D.; Coyle, M.; Kales, S.N. Barriers and solutions to improving nutrition among fire academy recruits: A qualitative assessment. Int. J. Food Sci. Nutr. 2019, 70, 771–779. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Rexrode, K.M.; Spiegelman, D.; Logroscino, G.; Manson, J.E.; Rimm, E.B. Primary Prevention of Stroke by Healthy Lifestyle. Circulation 2008, 118, 947–954. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rexrode, K.; Spiegelman, D.; Manson, J.E.; Stampfer, M.J.; Albert, C. Adherence to a Low-Risk, Healthy Lifestyle and Risk of Sudden Cardiac Death Among Women. JAMA 2011, 306, 62–69. [Google Scholar] [CrossRef]
- Jones, S.; Agud, K.; McSweeney, J. Barriers and Facilitators to Seeking Mental Health Care among First Responders: “Removing the Darkness”. J. Am. Psychiatr. Nurses Assoc. 2020, 26, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Martinsen, J.I.; Weiderpass, E.; Kjaerheim, K.; Lynge, E.; Tryggvadottir, L.; Sparén, P.; Demers, P. Cancer incidence among firefighters: 45 years of follow-up in five Nordic countries. Occup. Environ. Med. 2014, 71, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.D.; Kubale, T.L.; Yiin, J.H.; Dahm, M.M.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; Pinkerton, L.E. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2014, 71, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.C.; Pircher, S.; Del Monaco, A.; Hoorn, S.V.; Sim, M.R. Mortality and cancer incidence in a cohort of male paid Australian firefighters. Occup. Environ. Med. 2016, 73, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, C.; Andersson, T.; Gustavsson, P.; Selander, J.; Tornling, G.; Gustavsson, A.; Bigert, C. Cancer incidence in Stockholm firefighters 1958–2012: An updated cohort study. Int. Arch. Occup. Environ. Health 2018, 91, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Jeong, K.S. Mortality due to malignant and non-malignant diseases in Korean professional emergency responders. PLoS ONE 2015, 10, e0120305. [Google Scholar] [CrossRef]
- Ahn, Y.-S.; Jeong, K.S.; Kim, K.-S. Cancer morbidity of professional emergency responders in Korea. Am. J. Ind. Med. 2012, 55, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.K.U.; Pedersen, J.E.; Bonde, J.P.; Ebbehoej, N.E.; Hansen, J. Long-term follow-up for cancer incidence in a cohort of Danish firefighters. Occup. Environ. Med. 2018, 75, 263–269. [Google Scholar] [CrossRef]
- Ide, C.W. Cancer incidence and mortality in serving whole-time Scottish firefighters 1984–2005. Occup. Med. 2014, 64, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bigert, C.; Martinsen, J.I.; Gustavsson, P.; Sparén, P. Cancer incidence among Swedish firefighters: An extended follow-up of the NOCCA study. Int. Arch. Occup. Environ. Health 2020, 93, 197–204. [Google Scholar] [CrossRef]
- Glass, D.C.; Del Monaco, A.; Pircher, S.; Hoorn, S.V.; Sim, M.R. Mortality and cancer incidence among female Australian firefighters. Occup. Environ. Med. 2019, 76, 215–221. [Google Scholar] [CrossRef]
- Daniels, R.D.; Bertke, S.; Dahm, M.M.; Yiin, J.H.; Kubale, T.L.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; et al. Exposure–response relationships for select cancer and non-cancer health outcomes in a cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2015, 72, 699–706. [Google Scholar] [CrossRef]
- Jalilian, H.; Ziaei, M.; Weiderpass, E.; Rueegg, C.S.; Khosravi, Y.; Kjaerheim, K. Cancer incidence and mortality among firefighters. Int. J. Cancer 2019, 145, 2639–2646. [Google Scholar] [CrossRef]
- Laroche, E.; L’Espérance, S. Cancer incidence and mortality among firefighters: An overview of epidemiologic systematic reviews. Int. J. Environ. Res. Public Health 2021, 18, 2519. [Google Scholar] [CrossRef]
- Lee, D.J.; Koru-Sengul, T.; Hernandez, M.N.; Caban-Martinez, A.J.; McClure, L.A.; Mackinnon, J.A.; Kobetz, E.N. Cancer risk among career male and female Florida firefighters: Evidence from the Florida Firefighter Cancer Registry (1981–2014). Am. J. Ind. Med. 2020, 63, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.J.; Luckhaupt, S.E.; Schumacher, P.; Cress, R.D.; Deapen, D.M.; Calvert, G.M. Risk of cancer among firefighters in California, 1988–2007. Am. J. Ind. Med. 2015, 58, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Kirkham, T.L.; MacLeod, J.S.; Tjepkema, M.; Peters, P.A.; Demers, P.A. Surveillance of cancer risks for firefighters, police, and armed forces among men in a Canadian census cohort. Am. J. Ind. Med. 2018, 61, 815–823. [Google Scholar] [CrossRef]
- Pukkala, E.; Engholm, G.; Schmidt, L.K.H.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Ólafsdóttir, E.; Tryggvadóttir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2018, 57, 440–455. [Google Scholar] [CrossRef]
- Sritharan, J.; MacLeod, J.S.; McLeod, C.B.; Peter, A.; Demers, P.A. Original quantitative research prostate cancer risk by occupation in the occupational disease surveillance system (ODSS) in Ontario, Canada. Health Promot. Chronic Dis. Prev. Can. Res. Policy Pract. 2019, 39, 178. [Google Scholar]
- Sritharan, J.; Pahwa, M.; Demers, P.A.; Harris, S.A.; Cole, D.C.; Parent, M.-E. Prostate cancer in firefighting and police work: A systematic review and meta-analysis of epidemiologic studies. Environ. Health 2017, 16, 124. [Google Scholar] [CrossRef]
- Stec, A.A.; Robinson, A.; Wolffe, T.A.; Bagkeris, E. Scottish firefighters occupational cancer and disease mortality rates: 2000–2020. Occup. Med. 2023, 73, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hadkhale, K.; Martinsen, J.I.; Weiderpass, E.; Kjærheim, K.; Sparén, P.; Tryggvadóttir, L.; Lynge, E.; Pukkala, E. Occupational variation in bladder cancer in Nordic males adjusted with approximated smoking prevalence. Acta Oncol. 2019, 58, 29–37. [Google Scholar] [CrossRef]
- Soteriades, E.S.; Kim, J.; Christophi, C.A.; Kales, S.N. Cancer incidence and mortality in firefighters: A state-of-the-art review and meta-analysis. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3221. [Google Scholar] [CrossRef]
- Casjens, S.; Brüning, T.; Taeger, D. Cancer risks of firefighters: A systematic review and meta-analysis of secular trends and region-specific differences. Int. Arch. Occup. Environ. Health 2020, 93, 839–852. [Google Scholar] [CrossRef]
- Sritharan, J.; Kirkham, T.L.; MacLeod, J.; Marjerrison, N.; Lau, A.; Dakouo, M.; Logar-Henderson, C.; Norzin, T.; DeBono, N.L.; Demers, P. Cancer risk among firefighters and police in the Ontario workforce. Occup. Environ. Med. 2022, 79, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Muegge, C.M.; Zollinger, T.W.; Song, Y.; Wessel, J.; Monahan, P.O.; Moffatt, S.M. Excess mortality among Indiana firefighters, 1985–2013. Am. J. Ind. Med. 2018, 61, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, L.; Bertke, S.J.; Yiin, J.; Dahm, M.; Kubale, T.; Hales, T.; Purdue, M.; Beaumont, J.J.; Daniels, R. Mortality in a cohort of US firefighters from San Francisco, Chicago and Philadelphia: An update. Occup. Environ. Med. 2020, 77, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, B.; Marchand, J.-L.; Moisan, F.; Donnadieu, S.; Coureau, G.; Mathoulin-Pélissier, S.; Lembeye, C.; Imbernon, E.; Brochard, P. French firefighter mortality: Analysis over a 30-year period. Am. J. Ind. Med. 2015, 58, 437–443. [Google Scholar] [CrossRef]
- Haldorsen, T.; Martinsen, J.I.; Kjærheim, K.; Grimsrud, T.K. Adjustment for tobacco smoking and alcohol consumption by simul-taneous analysis of several types of cancer. Cancer Causes Control 2017, 28, 155–165. [Google Scholar] [CrossRef]
- Petersen, K.U.; Pedersen, J.E.; Bonde, J.P.; Ebbehøj, N.E.; Hansen, J. Mortality in a cohort of Danish firefighters; 1970–2014. Int. Arch. Occup. Environ. Health 2018, 91, 759–766. [Google Scholar] [CrossRef]
- Zhao, G.; Erazo, B.; Ronda, E.; Brocal, F.; Regidor, E. Mortality Among Firefighters in Spain: 10 Years of Follow-Up. Ann. Work. Expo. Health 2020, 64, 614–621. [Google Scholar] [CrossRef]
- Bott, R.C.; Kirk, K.M.; Logan, M.B.; Reid, D.A. Diesel particulate matter and polycyclic aromatic hydrocarbons in fire stations. Environ. Sci. Process. Impacts 2017, 19, 1320–1326. [Google Scholar] [CrossRef]
- Silverman, D.T. Diesel Exhaust and Lung Cancer—Aftermath of Becoming an IARC Group 1 Carcinogen. Am. J. Epidemiol. 2018, 187, 1149–1152. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; Pereira, M.D.C.; Morais, S. Occupational exposure of firefighters to polycyclic aromatic hydrocarbons in non-fire work environments. Sci. Total. Environ. 2017, 592, 277–287. [Google Scholar] [CrossRef]
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.P.; Houldsworth, P.E.; Martin, F.L. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, J.; Gerona, R.R.; Lin, T.; Rudel, R.A.; Bessonneau, V.; Buren, H.; Morello-Frosch, R. Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco. Environ. Sci. Technol. 2020, 54, 3363–3374. [Google Scholar] [CrossRef] [PubMed]
- Clarity, C.; Trowbridge, J.; Gerona, R.; Ona, K.; McMaster, M.; Bessonneau, V.; Rudel, R.; Buren, H.; Morello-Frosch, R. Associations between polyfluoroalkyl substance and organophosphate flame retardant exposures and telomere length in a cohort of women firefighters and office workers in San Francisco. Environ. Health 2021, 20, 97. [Google Scholar] [CrossRef]
- Lucas, K.; Gaines, L.G.; Paris-Davila, T.; Nylander-French, L.A. Occupational exposure and serum levels of per- and polyfluoroalkyl substances (PFAS): A review. Am. J. Ind. Med. 2022, 66, 379–392. [Google Scholar] [CrossRef]
- Leary, D.B.; Takazawa, M.; Kannan, K.; Khalil, N. Perfluoroalkyl substances and metabolic syndrome in firefighters: A pilot study. J. Occup. Environ. Med. 2020, 62, 52–57. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Islam, A.; Qian, Y.; Ducatman, A. Perfluoroalkyl Acids Including Perfluorooctane Sulfonate and Perfluorohexane Sulfonate in Firefighters. J. Occup. Environ. Med. 2011, 53, 324–328. [Google Scholar] [CrossRef]
- Barton, K.E.; Starling, A.P.; Higgins, C.P.; McDonough, C.A.; Calafat, A.M.; Adgate, J.L. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int. J. Hyg. Environ. Health 2020, 223, 256–266. [Google Scholar] [CrossRef]
- Paris-Davila, T.; Gaines, L.G.T.; Lucas, K.; Nylander-French, L.A. Occupational exposures to airborne per- and polyfluoroalkyl substances (PFAS)—A review. Am. J. Ind. Med. 2023, 66, 393–410. [Google Scholar] [CrossRef]
- Alexander, B.M.; Baxter, C.S. Flame-retardant contamination of firefighter personal protective clothing—A potential health risk for firefighters. J. Occup. Environ. Hyg. 2016, 13, D148–D155. [Google Scholar] [CrossRef]
- Schecter, A.; Paepke, O.; Tung, K.C.; Joseph, J.; Harris, T.R.; Dahlgren, J. Polybrominated diphenyl ether flame retardants in the US population: Current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005, 47, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.R.; Carey, R.N.; Peters, S.; Glass, D.C.; Benke, G.; Reid, A.; Fritschi, L. The Australian Work Exposures Study: Prevalence of Occupational Exposure to Formaldehyde. Ann. Occup. Hyg. 2016, 60, 132–138. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 88: Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxypropan-2-ol. 2004. Available online: http://monographs.iarc.fr/ENG/Monographs/vol88/index.php (accessed on 21 December 2022).
- Stevenson, M.; Alexander, B.; Baxter, C.S.; Leung, Y.-K. Evaluating Endocrine Disruption Activity of Deposits on Firefighting Gear Using a Sensitive and High Throughput Screening Method. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2015, 57, e153. [Google Scholar] [CrossRef]
- Gainey, S.J.; Horn, G.P.; Towers, A.E.; Oelschlager, M.L.; Tir, V.L.; Drnevich, J.; Fent, K.W.; Kerber, S.; Smith, D.L.; Freund, G.G. Exposure to a firefighting overhaul environment without respiratory protection increases immune dysregulation and lung disease risk. PLoS ONE 2018, 13, e0201830. [Google Scholar] [CrossRef]
- Jeong, K.S.; Zhou, J.; Griffin, S.C.; Jacobs, E.T.; Dearmon-Moore, D.; Zhai, J.; Littau, S.R.; Gulotta, J.; Moore, P.; Peate, W.F.; et al. MicroRNA changes in firefighters. J. Occup. Environ. Med. 2018, 60, 469. [Google Scholar] [CrossRef]
- Burris, J.C.; Werner, C.M.; Woolf, K. The Relationship Between Dietary Intake and Dietary-Focused Lifestyle Interventions on Risk Factors Associated with Cardiovascular Disease in Firefighters. Curr. Nutr. Rep. 2022, 11, 206–224. [Google Scholar] [CrossRef]
- Yang, J.; Farioli, A.; Korre, M.; Kales, S.N. Dietary Preferences and Nutritional Information Needs among Career Firefighters in the United States. Glob. Adv. Health Med. 2015, 4, 16–23. [Google Scholar] [CrossRef]
- Korre, M.; Smith, D.; Kales, S.N. Obesity and health in the North American Fire Service: Research points the way to positive culture change. Occup. Med. 2018, 68, 160–162. [Google Scholar] [CrossRef]
- Lidoriki, I.; Sotos-Prieto, M.; Smith, D.L.; Kales, S.N. Firefighting-Associated Cancers: Can Healthier Body Weight and Eating be Potential Countermeasures? JOEM 2019, 61, e169–e171. [Google Scholar]
- Joe, M.J.; Hatsu, I.E.; Tefft, A.; Mok, S.; Adetona, O. Dietary behavior and diet interventions among structural firefighters: A nar-rative review. Nutrients 2022, 14, 4662. [Google Scholar] [CrossRef]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Cook, M.B.; Trabert, B.; Håkansson, N.; Larsson, S.C.; Wolk, A.; Gapstur, S.M.; Lynch, B.M.; et al. Amount and Intensity of Leisure-Time Physical Activity and Lower Cancer Risk. J. Clin. Oncol. 2020, 38, 686. [Google Scholar] [CrossRef]
- Song, C.; Zhang, R.; Wang, C.; Fu, R.; Song, W.; Dou, K.; Wang, S. Sleep quality and risk of cancer: Findings from the English longitudinal study of aging. Sleep 2020, 44, zsaa192. [Google Scholar] [CrossRef]
- Haddock, C.K.; Jahnke, S.A.; Poston, W.S.C.; Jitnarin, N.; Kaipust, C.M.; Tuley, B.; Hyder, M.L. Alcohol use among firefighters in the Central United States. Occup. Med. 2012, 62, 661–664. [Google Scholar] [CrossRef]
- Poston, W.S.C.; Haddock, C.K.; Jahnke, S.A.; Jitnarin, N.; Tuley, B.C.; Kales, S.N. The Prevalence of Overweight, Obesity, and Substandard Fitness in a Population-Based Firefighter Cohort. J. Occup. Environ. Med. 2011, 53, 266–273. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Jahnke, S.; Kaipust, C.; Jitnarin, N.; Hollerbach, B.S.; Koeppel, M.D.H.; Haddock, C.K.; Poston, W.S.C. Prevalence and predictors of obesity among women in the fire service. Occup. Environ. Med. 2022, 79, 289–294. [Google Scholar] [CrossRef]
- Dobson, M.; Choi, B.; Schnall, P.L.; Wigger, E.; Garcia-Rivas, J.; Israel, L.; Baker, D.B. Exploring Occupational and Health Behavioral Causes of Firefighter Obesity: A Qualitative Study. Am. J. Ind. Med. 2013, 56, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, S.C.; Howard, V.J.; Akinyemiju, T.; Judd, S.E.; Cushman, M.; Hooker, S.P.; Diaz, K.M. Association of Sedentary Behavior with Cancer Mortality in Middle-aged and Older US Adults. JAMA Oncol. 2020, 6, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Piazza-Gardner, A.K.; Barry, A.; Chaney, E.; Dodd, V.; Weiler, R.; Delisle, A. Covariates of alcohol consumption among career firefighters. Occup. Med. 2014, 64, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.B.; Wild, P.; Dorribo, V.; Amati, F.; Danuser, B. Eating habits of professional firefighters: Comparison with national guidelines and impact healthy eating promotion program. J. Occup. Environ. Med. 2019, 61, e183–e190. [Google Scholar] [CrossRef]
- Soteriades, E.S.; Smith, D.L.; Tsismenakis, A.J.; Baur, D.M.; Kales, S.N. Cardiovascular disease in US firefighters: A systematic review. Cardiol. Rev. 2011, 19, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Teehan, D.; Farioli, A.; Baur, D.M.; Smith, D.; Kales, S.N. Sudden Cardiac Death among Firefighters ≤ 45 Years of Age in the United States. Am. J. Cardiol. 2013, 112, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, A.C.; Knipper, B.S.; He, X.; Alexander, B.M.; Davis, K.G. Lifestyle and safety practices of firefighters and their relation to cardiovascular risk factors. Work 2015, 50, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.H.; Larivière, C.; Leduc, C.R.; McGillis, Z.; Eger, T.; Godwin, A.; Lariviere, M.; Dorman, S.C. Novel Tools in Determining the Physiological Demands and Nutritional Practices of Ontario FireRangers during Fire Deployments. PLoS ONE 2017, 12, e0169390. [Google Scholar] [CrossRef]
- Kadiwar, P.; Shah, N.; Black, T.; Caban-Martinez, A.J.; Steinberg, M.; Black, K.; Sackey, J.; Graber, J. Dietary Intake among Members of a Volunteer Fire Department Compared with US Daily Dietary Recommendations. J. Occup. Environ. Med. 2021, 63, 147–150. [Google Scholar] [CrossRef]
- Jitnarin, N.; Poston, W.S.; Jahnke, S.A.; Haddock, C.K.; Kelley, H.N. Cancer Perceptions among Smokeless Tobacco Users: A Qualitative Study of US Firefighters. Saf. Health Work 2020, 11, 284–290. [Google Scholar] [CrossRef]
- Anderson, D.A.; Harrison, T.R.; Yang, F.; Wendorf Muhamad, J.; Morgan, S.E. Firefighter perceptions of cancer risk: Results of a qualitative study. Am. J. Ind. Med. 2017, 60, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Schaefer Solle, N.; Caban-Martinez, A.J.; Levy, R.A.; Young, B.; Lee, D.; Harrison, T.; Kobetz, E. Perceptions of health and cancer risk among newly recruited firefighters in south Florida. Am. J. Ind. Med. 2018, 61, 77–84. [Google Scholar] [CrossRef]
- Macy, G.B.; Hwang, J.; Taylor, R.; Golla, V.; Cann, C.; Gates, B. Examining Behaviors Related to Retirement, Cleaning, and Storage of Turnout Gear Among Rural Firefighters. Work. Health Saf. 2020, 68, 129–138. [Google Scholar] [CrossRef]
- Watkins, E.R.; Walker, A.; Mol, E.; Jahnke, S.; Richardson, A.J. Women Firefighters’ Health and Well-Being: An International Survey. Women’s Health Issues 2019, 29, 424–431. [Google Scholar] [CrossRef]
- Harrison, T.R.; Muhamad, J.W.; Yang, F.; Morgan, S.E.; Talavera, E.; Caban-Martinez, A.; Kobetz, E. Firefighter attitudes, norms, beliefs, barriers, and behaviors toward post-fire decontamination processes in an era of increased cancer risk. J. Occup. Environ. Hyg. 2018, 15, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Caban-Martinez, A.J.; Hughes, J.; Bator, C. A Total Worker Health Approach to Skin Exposure Assessment: Experiences from the Firefighter Cancer Initiative. Ann. Work. Expo. Health 2021, 65, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Louzado-Feliciano, P.; Griffin, K.A.; Santiago, K.M.; Solle, N.S.; Koru-Sengul, T.; Grant, C.; Niemczyk, N.; Lee, D.J.; Kobetz, E.N.; Caban-Martinez, A.J. Fire service organizational-level characteristics are associated with adherence to contamination control practices in Florida fire departments: Evidence from the firefighter cancer initiative. J. Occup. Environ. Med. 2020, 62, e508–e514. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Cancer Incidence (95% CI) | Study Size | Study Location |
|---|---|---|---|
| North America | |||
| Daniels 2014 [9] | SIR = 1.09 (1.06–1.12) SIR = 1.09 (1.06–1.12) (non-Caucasian male firefighters) SIR = 1.24 (0.89–1.69) (female firefighters) | 29,993 career firefighters | USA |
| Europe | |||
| Bigert 2020 a [16] | SIR = 1.03 (0.97–1.09) | 8136 male firefighters | Sweden |
| Kullberg 2018 [11] | SIR = 0.81 (0.71–0.91) | 1080 firefighters | Sweden |
| Petersen 2018 [14] | SIR = 1.02 (0.96–1.09) | 9061 male Danish firefighters | Denmark |
| Ide 2014 [15] | Incidence rate/105/year [SD] = 86.5 [64.2] (vs. general population 123.7 [7.9], p < 0.001) | ~2200 serving firefighters | Scotland |
| Pukkala 2014 [8] | SIR = 1.06 (1.02–1.11) | 16,422 male firefighters | Nordic countries |
| Oceania | |||
| Glass 2019 [17] | SIR = 1.15 (0.80 to 1.67) | 1682 paid female firefighters | Australia |
| Glass 2016 [10] | SIR = 1.09 (1.03–1.14) | 30,057 male firefighters | Australia |
| Glass 2016 [1] | SIR = 1.85 (1.20–2.73) (High risk of chronic exposure group) SIR = 1.13 (0.80–1.55) (Medium risk of chronic exposure group) b SIR = 0.40 (0.15–0.87) (Low risk of chronic exposure group) | 611 male firefighters | Australia |
| Asia | |||
| Ahn 2012 [13] | SIR = 0.97 (0.88–1.06) | 29,438 firefighters | Korea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidossis, A.; Lan, F.-Y.; Hershey, M.S.; Hadkhale, K.; Kales, S.N. Cancer and Potential Prevention with Lifestyle among Career Firefighters: A Narrative Review. Cancers 2023, 15, 2442. https://doi.org/10.3390/cancers15092442
Sidossis A, Lan F-Y, Hershey MS, Hadkhale K, Kales SN. Cancer and Potential Prevention with Lifestyle among Career Firefighters: A Narrative Review. Cancers. 2023; 15(9):2442. https://doi.org/10.3390/cancers15092442
Chicago/Turabian StyleSidossis, Amalia, Fan-Yun Lan, Maria S. Hershey, Kishor Hadkhale, and Stefanos N. Kales. 2023. "Cancer and Potential Prevention with Lifestyle among Career Firefighters: A Narrative Review" Cancers 15, no. 9: 2442. https://doi.org/10.3390/cancers15092442
APA StyleSidossis, A., Lan, F.-Y., Hershey, M. S., Hadkhale, K., & Kales, S. N. (2023). Cancer and Potential Prevention with Lifestyle among Career Firefighters: A Narrative Review. Cancers, 15(9), 2442. https://doi.org/10.3390/cancers15092442





