Management of Gastro-Intestinal Toxicity of the Pi3 Kinase Inhibitor: Optimizing Future Dosing Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Literature Review
2.2. Review of the WHO Global Safety Database
2.3. Single-Center Experience
2.4. Review of the French Pharmacovigilance Database
2.5. Ethics Approval
2.6. Statistics
3. Results
3.1. History of Pi3 Kinase Inhibitor Development
3.2. New Pi3K Inhibitors and the Risk of Colitis
3.2.1. Literature Review
3.2.2. Pharmacovigilance Database
3.3. French Experience of Idelalisib-Induced Colitis
3.3.1. Single-Center Experience
3.3.2. Analysis of the French Pharmacovigilance Database
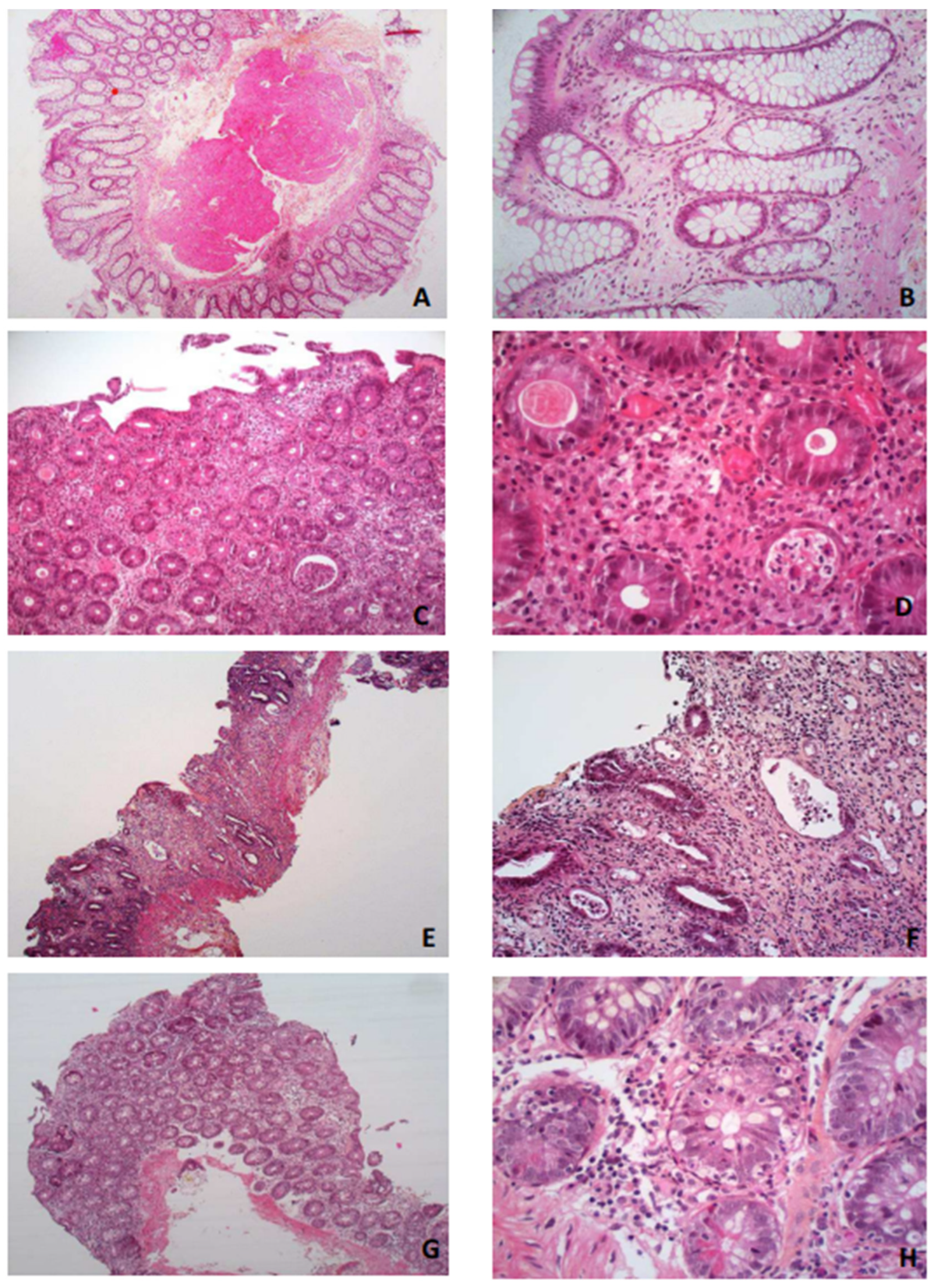
3.3.3. Histological Description
- -
- Increased lamina propria inflammation with lymphocytes, plasma cells, neutrophils and eosinophils;
- -
- Acute or chronic ischemic-like lesions with an atrophic or withered crypt appearance, including hyalinization of the lamina propria;
- -
- Apoptosis at the bases of the crypts with lymphocyte infiltration of the crypt epithelium, similar to what is seen in a graft vs. host disease but surrounded by more inflammatory cells.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Kahl, B.S.; de Vos, S.; Wagner-Johnston, N.D.; Schuster, S.J.; Jurczak, W.J.; Flinn, I.W.; Flowers, C.R.; Martin, P.; Viardot, A.; et al. PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N. Engl. J. Med. 2014, 370, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Matasar, M.J.; Capra, M.; Özcan, M.; Lv, F.; Li, W.; Yañez, E.; Sapunarova, K.; Lin, T.; Jin, J.; Jurczak, W.; et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 678–689. [Google Scholar] [CrossRef]
- Flinn, I.W.; Hillmen, P.; Montillo, M.; Nagy, Z.; Illés, A.; Etienne, G.; Delgado, J.; Kuss, B.J.; Tam, C.S.; Gasztonyi, Z.; et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 2018, 132, 2446–2455. [Google Scholar] [CrossRef]
- Fowler, N.H.; Samaniego, F.; Jurczak, W.; Ghosh, N.; Derenzini, E.; Reeves, J.A.; Zinzani, P.L. Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor in Patients With Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2021, 39, 1609–1618. [Google Scholar] [CrossRef]
- Curigliano, G.; Shah, R.R. Safety and Tolerability of Phosphatidylinositol-3-Kinase (PI3K) Inhibitors in Oncology. Drug Saf. 2019, 42, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Coutré, S.E.; Barrientos, J.C.; Brown, J.R.; de Vos, S.; Furman, R.R.; Keating, M.J.; Li, D.; O’Brien, S.M.; Pagel, J.M.; Poleski, M.H.; et al. Management of adverse events associated with idelalisib treatment: Expert panel opinion. Leuk. Lymphoma 2015, 56, 2779–2786. [Google Scholar] [CrossRef]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M.; et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet Lond. Engl. 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Miremont-Salamé, G.; Théophile, H.; Haramburu, F.; Bégaud, B. Causality assessment in pharmacovigilance: The French method and its successive updates. Therapies 2016, 71, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Faillie, J.-L. Case-non-case studies: Principle, methods, bias and interpretation. Therapie 2019, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Barrientos, J.C.; Brown, J.R.; Coiffier, B.; Delgado, J.; Egyed, M.; Ghia, P.; Illés, Á.; Jurczak, W.; Marlton, P.; et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017, 18, 297–311. [Google Scholar] [CrossRef]
- Romero, D. Haematological cancer: Idelalisib for CLL-risky benefit. Nat. Rev. Clin. Oncol. 2017, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.; Kater, A.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 32, 23–33. [Google Scholar] [CrossRef]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppä, S.; Follows, G.A.; Lenz, G.; Kim, W.S.; Nagler, A.; Panayiotidis, P.; Demeter, J.; et al. Phosphatidylinositol 3-Kinase Inhibition by Copanlisib in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2017, 35, 3898–3905. [Google Scholar] [CrossRef]
- Flinn, I.W.; Miller, C.B.; Ardeshna, K.M.; Tetreault, S.; Assouline, S.E.; Mayer, J.; Merli, M.; Lunin, S.D.; Pettitt, A.R.; Nagy, Z.; et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in Patients With Refractory Indolent Non-Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 912–922. [Google Scholar] [CrossRef]
- Lunning, M.; Vose, J.; Nastoupil, L.; Fowler, N.; Burger, J.A.; Wierda, W.G.; Schreeder, M.T.; Siddiqi, T.; Flowers, C.R.; Cohen, J.B.; et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2019, 134, 1811–1820. [Google Scholar] [CrossRef]
- Skanland, S.S.; Brown, J.R. PI3K inhibitors in chronic lymphocytic leukemia: Where do we go from here? Haematologica 2022, 108, 9–21. [Google Scholar] [CrossRef]
- Richardson, N.C.; Kasamon, Y.; Pazdur, R.; Gormley, N. The saga of PI3K inhibitors in haematological malignancies: Survival is the ultimate safety endpoint. Lancet Oncol. 2022, 23, 563–566. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppä, S.; Follows, G.; Lenz, G.; Kim, W.S.; Nagler, A.; Dimou, M.; Demeter, J.; et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am. J. Hematol. 2020, 95, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kuss, B.J.; Hillmen, P.; Montillo, M.; Moreno, C.; Essell, J.; Lamanna, N.; Nagy, Z.; Tam, C.S.; Stilgenbauer, S.; et al. Efficacy and Safety of Duvelisib Following Disease Progression on Ofatumumab in Patients with Relapsed/Refractory CLL or SLL in the DUO Crossover Extension Study. Clin. Cancer Res. 2020, 26, 2096–2103. [Google Scholar] [CrossRef]
- Davids, M.S.; O’Connor, O.A.; Jurczak, W.; Samaniego, F.; Fenske, T.S.; Zinzani, P.L.; Patel, M.R.; Ghosh, N.; Cheson, B.D.; Derenzini, E.; et al. Integrated safety analysis of umbralisib, a dual PI3Kδ/CK1ε inhibitor, in relapsed/refractory lymphoid malignancies. Blood Adv. 2021, 5, 5332–5343. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Barosi, G.; Danesi, R.; Fagiuoli, S.; Ghia, P.; Marzano, A.; Montillo, M.; Poletti, V.; Viale, P.; Zinzani, P.L. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper. Hematol. Oncol. 2018, 37, 3–14. [Google Scholar] [CrossRef]
- Phillips, T.J.; Michot, J.-M.; Ribrag, V. Can Next-Generation PI3K Inhibitors Unlock the Full Potential of the Class in Patients With B-Cell Lymphoma? Clin. Lymphoma Myeloma Leuk. 2021, 21, 8–20. [Google Scholar] [CrossRef]
- Ma, S.; Chan, R.J.; Gu, L.; Xing, G.; Rajakumaraswamy, N.; Ruzicka, B.B.; Wagner-Johnston, N.D. Retrospective Analysis of the Impact of Adverse Event–Triggered Idelalisib Interruption and Dose Reduction on Clinical Outcomes in Patients With Relapsed/Refractory B-Cell Malignancies. Clin. Lymphoma Myeloma Leuk. 2020, 21, e432–e448. [Google Scholar] [CrossRef]
- Mato, A.R.; Nabhan, C.; Barr, P.M.; Ujjani, C.S.; Hill, B.T.; Lamanna, N.; Skarbnik, A.P.; Howlett, C.; Pu, J.J.; Sehgal, A.R.; et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: A real world experience. Blood 2016, 128, 2199–2205. [Google Scholar] [CrossRef]
- Mato, A.R.; Hill, B.T.; Lamanna, N.; Barr, P.M.; Ujjani, C.S.; Brander, D.M.; Howlett, C.; Skarbnik, A.P.; Cheson, B.D.; Zent, C.S.; et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: Results from a multicenter study of 683 patients. Ann. Oncol. 2017, 28, 1050–1056. [Google Scholar] [CrossRef]
- LLC-Les Recommandations du FILO 2023. Available online: https://www.filo-leucemie.org/actualites/traitements/llc-les-recommandations-du-filo-2023/ (accessed on 6 April 2023).
- Weidner, A.S.; Panarelli, N.C.; Geyer, J.T.; Bhavsar, E.B.; Furman, R.R.; Leonard, J.P.; Jessurun, J.; Yantiss, R.K. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am. J. Surg. Pathol. 2015, 39, 1661–1667. [Google Scholar] [CrossRef]
- Tarantelli, C.; Argnani, L.; Zinzani, P.L.; Bertoni, F. PI3Kδ Inhibitors as Immunomodulatory Agents for the Treatment of Lymphoma Patients. Cancers 2021, 13, 5535. [Google Scholar] [CrossRef]
- Chellappa, S.; Kushekhar, K.; Munthe, L.A.; Tjønnfjord, G.E.; Aandahl, E.M.; Okkenhaug, K.; Taskén, K. The PI3K p110δ Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J. Immunol. 2019, 202, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Hanna, B.S.; Roessner, P.M.; Scheffold, A.; Jebaraj, B.M.C.; Demerdash, Y.; Öztürk, S.; Lichter, P.; Stilgenbauer, S.; Seiffert, M. PI3Kδ inhibition modulates regulatory and effector T-cell differentiation and function in chronic lymphocytic leukemia. Leukemia 2018, 33, 1427–1438. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; et al. Impaired B and T Cell Antigen Receptor Signaling in p110delta PI 3-Kinase Mutant Mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Mesenteric, B. Cells Centrally Inhibit CD4+ T Cell Colitis through Interaction with Regulatory T Cell Subsets. Available online: https://pubmed.ncbi.nlm.nih.gov/15684084/ (accessed on 10 March 2023).
- Louie, C.Y.; DiMaio, M.A.; Matsukuma, K.E.; Coutre, S.E.; Berry, G.J.; Longacre, T.A. Idelalisib-associated Enterocolitis: Clinicopathologic Features and Distinction From Other Enterocolitides. Am. J. Surg. Pathol. 2015, 39, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.C.; Hockenbery, D.M.; Westerhoff, M.; Coutre, S.E.; Sedlak, R.H.; Dubowy, R.L.; Munugalavadla, V.; Taylor, K.; Bosch, F. Pathological assessment of gastrointestinal biopsies from patients with idelalisib-associated diarrhea and colitis. Future Oncol. 2018, 14, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Koyasu, S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003, 24, 358–363. [Google Scholar] [CrossRef]
- Thompson, M.C.; Roeker, L.E.; Coombs, C.C.; Jensen, J.L.; Kamdar, M.; Skarbnik, A.; Pagel, J.M.; Bailey, N.; Pu, J.J.; Jacobs, R.; et al. Addressing a New Challenge in Chronic Lymphocytic Leukemia: Outcomes of Therapies after Exposure to Both a Covalent Bruton’s Tyrosine Kinase Inhibitor and Venetoclax. Blood 2021, 138, 2628. [Google Scholar] [CrossRef]
- Aronson, J.H.; Skånland, S.S.; Roeker, L.E.; Thompson, M.C.; Mato, A.R. Approach to a patient with ‘double refractory’ chronic lymphocytic leukemia: ‘Double, double toil and trouble’ (Shakespeare). Am. J. Hematol. 2022, 97 (Suppl. S2), S19–S25. [Google Scholar] [CrossRef]
| Pi3Kinhibitor | Target | Colitis Cases | Non-Cases | Reporting OR (95% CI) |
|---|---|---|---|---|
| - | 342 | 10,801 | 9.5 (8.6–10.6) | |
| Idelalisib | PI3Kδ | 296 | 6093 | 14.6 (13.0–16.4) |
| Alpelisib | PI3Kα | 27 | 4092 | 2.0 (1.4–2.9) |
| Duvelisib | PI3Kγ/PI3Kδ | 16 | 365 | 13.2 (8.0–21.7) |
| Copanlisib | All PI3K | 3 | 172 | 5.2 (1.7–16.4) |
| Umbralisib | PI3Kδ | 1 | 82 | - |
| Patient Characteristics | Idelalisib-Induced Colitis Characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age (Years) | Sex | Disease | Prior Tts | Concomitant Therapy | Grade | Intensive Care Admission | Delay (Months) | Colonoscopy | Treatment Stopped | Management | Recovery | Rechallenge | Death Occurred |
| 1 | 76 | M | WM | 6 | ofatumumab | 4 | Yes (hypokalemia) | 3 | Normal colonic mucosa | Yes | Symptomatic treatment | Yes | No | Yes (disease) |
| 2 | 71 | M | WM | 4 | ⁄ | 3 | No | 3 | Erythema and absence of vascular pattern in the colon | Yes | Prednisone 0.8 mg/kg | Yes | Yes | / |
| 3 | 76 | M | CLL | 6 | ofatumumab | 4 | Yes (hypovolemic shock) | 4 | Erythema, absence of vascular pattern and superficial ulcerations in the rectum and colon | Yes | Prednisone 1 mg/kg | Yes | No | Yes (disease) |
| 4 | 66 | M | WM | 1 | ofatumumab | 4 | Yes (hypovolemic shock) | 4 | Erythema and absence of vascular pattern in the rectum and colon | Yes | Prednisone 0.8 mg/kg | Yes | No | Yes (disease) |
| 5 | 84 | F | WM | 2 | ⁄ | 3 | No | 6 | Erythema and absence of vascular pattern in the colon | Yes | Enteric budesonide | Yes | Yes (fatal colitis) | Yes (colitis) |
| 6 | 89 | F | CLL | 2 | rituximab | 3 | No | 3 | Absence of vascular pattern in the sigmoid segment | Yes | Symptomatic treatment | Yes | Yes (no colitis occurrence) | / |
| Patient Characteristics | n (%) |
|---|---|
| Age—years mediane (range) | 71 (65–78) |
| Sex—male: female | 32:14 |
| Type of hemopathy | |
| 26 (56.5%) 20 (43.5%) |
| Anticancer drug treatments | |
| 27 (58.7%) 19 (41.3%) |
| Time to first digestive symptoms—days | 122 (74–212) |
| Time to specific management—days | 20 (6-30) |
| Colitis seriousness | |
| 43 (83.5%) 35 (76.1%) 4 (8.7%) |
| Colonoscopy findings | |
| 30 9 4 3 3 |
| Idelalisib management | |
| 43 2 1 |
| Therapeutic management | |
| 23 14 5 3 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breal, C.; Beuvon, F.; de Witasse-Thezy, T.; Dermine, S.; Franchi-Rezgui, P.; Deau-Fisher, B.; Willems, L.; Grignano, E.; Contejean, A.; Bouscary, D.; et al. Management of Gastro-Intestinal Toxicity of the Pi3 Kinase Inhibitor: Optimizing Future Dosing Strategies. Cancers 2023, 15, 2279. https://doi.org/10.3390/cancers15082279
Breal C, Beuvon F, de Witasse-Thezy T, Dermine S, Franchi-Rezgui P, Deau-Fisher B, Willems L, Grignano E, Contejean A, Bouscary D, et al. Management of Gastro-Intestinal Toxicity of the Pi3 Kinase Inhibitor: Optimizing Future Dosing Strategies. Cancers. 2023; 15(8):2279. https://doi.org/10.3390/cancers15082279
Chicago/Turabian StyleBreal, Claire, Frederic Beuvon, Thibault de Witasse-Thezy, Solene Dermine, Patricia Franchi-Rezgui, Benedicte Deau-Fisher, Lise Willems, Eric Grignano, Adrien Contejean, Didier Bouscary, and et al. 2023. "Management of Gastro-Intestinal Toxicity of the Pi3 Kinase Inhibitor: Optimizing Future Dosing Strategies" Cancers 15, no. 8: 2279. https://doi.org/10.3390/cancers15082279
APA StyleBreal, C., Beuvon, F., de Witasse-Thezy, T., Dermine, S., Franchi-Rezgui, P., Deau-Fisher, B., Willems, L., Grignano, E., Contejean, A., Bouscary, D., Faillie, J. L., Treluyer, J.-M., Guerin, C., Chouchana, L., & Vignon, M. (2023). Management of Gastro-Intestinal Toxicity of the Pi3 Kinase Inhibitor: Optimizing Future Dosing Strategies. Cancers, 15(8), 2279. https://doi.org/10.3390/cancers15082279





