CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Cell Lines Culture and Transfections
2.2. Quantitive Real-Time PCR (qPCR) and Sanger Sequencing
2.3. Western Blot
2.4. Cell Proliferation Assay
2.5. Transwell Assay
2.6. Wound Healing Assay
2.7. Fluorescence In Situ Hybridization (FISH)
2.8. RNA Immunoprecipitation (RIP)
2.9. Luciferase Reporter Assay
2.10. Immunohistochemistry (IHC)
2.11. Animal Models
2.12. Statistical Analysis
3. Results
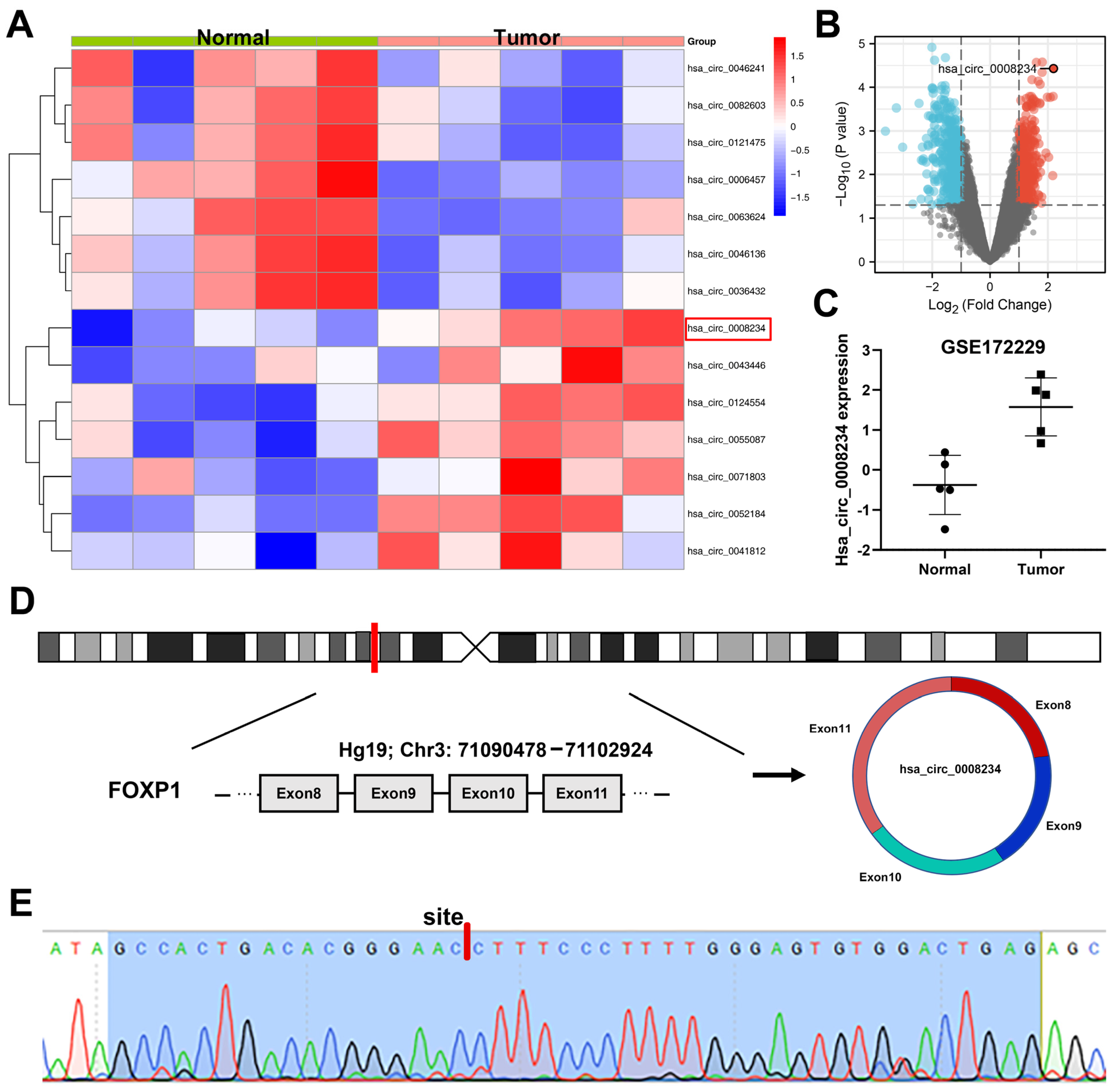
3.1. CircRNA hsa_circ_0008234 Is Overexpressed in Colon Cancer Tissue and Cell Lines
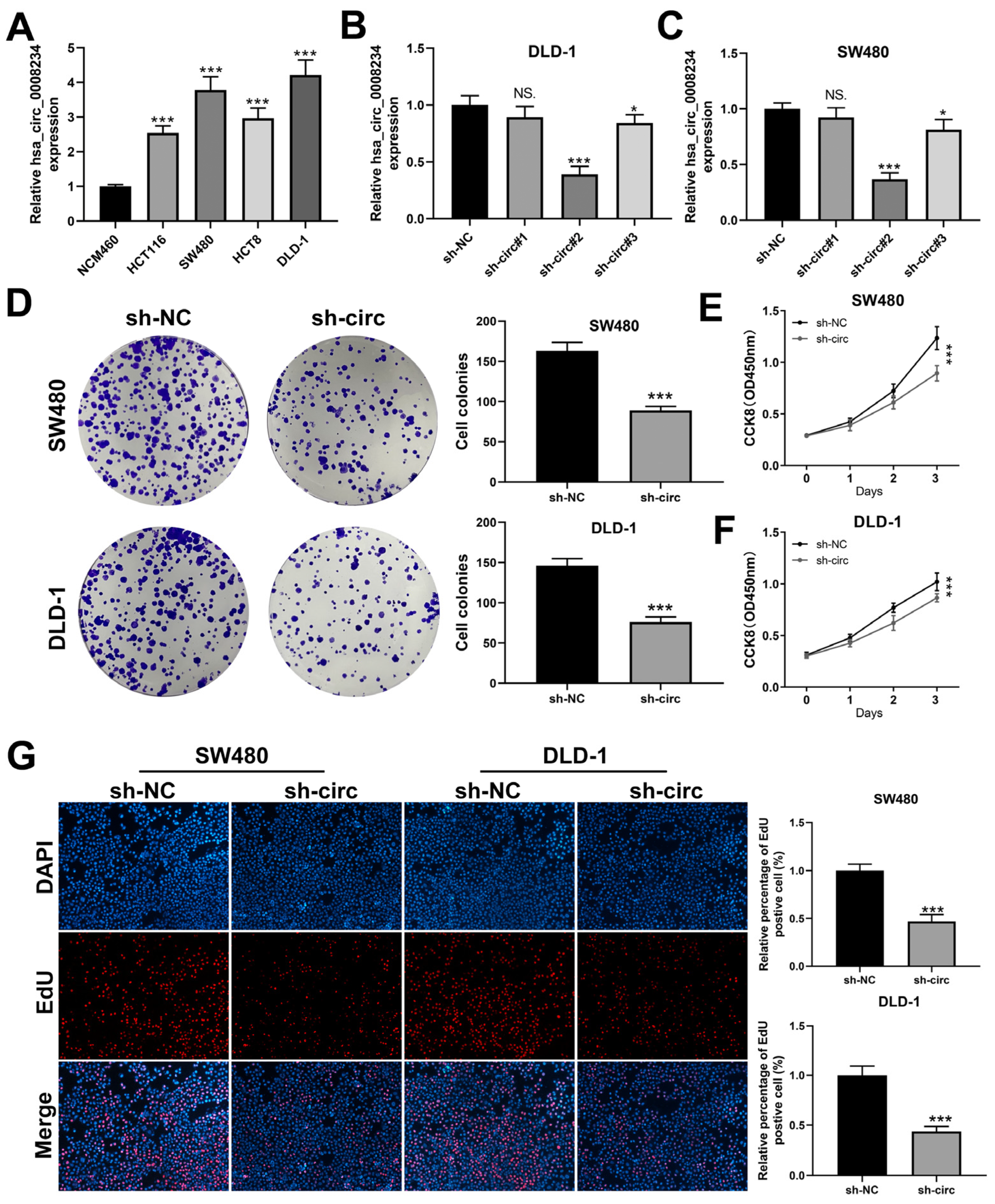
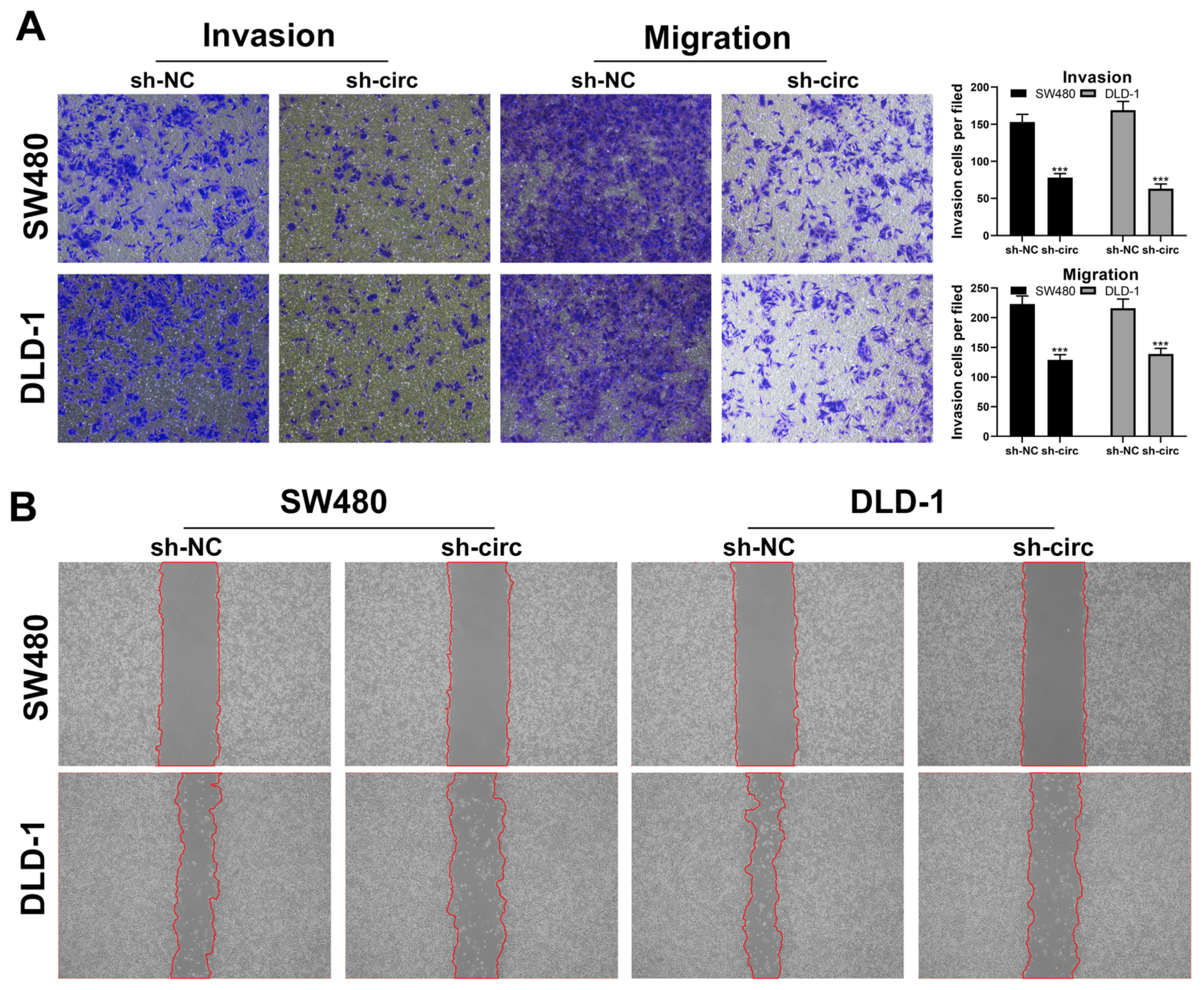
3.2. CircRNA hsa_circ_0008234 Promote the Proliferation, Invasion, and Migration of Colon Cancer Cells
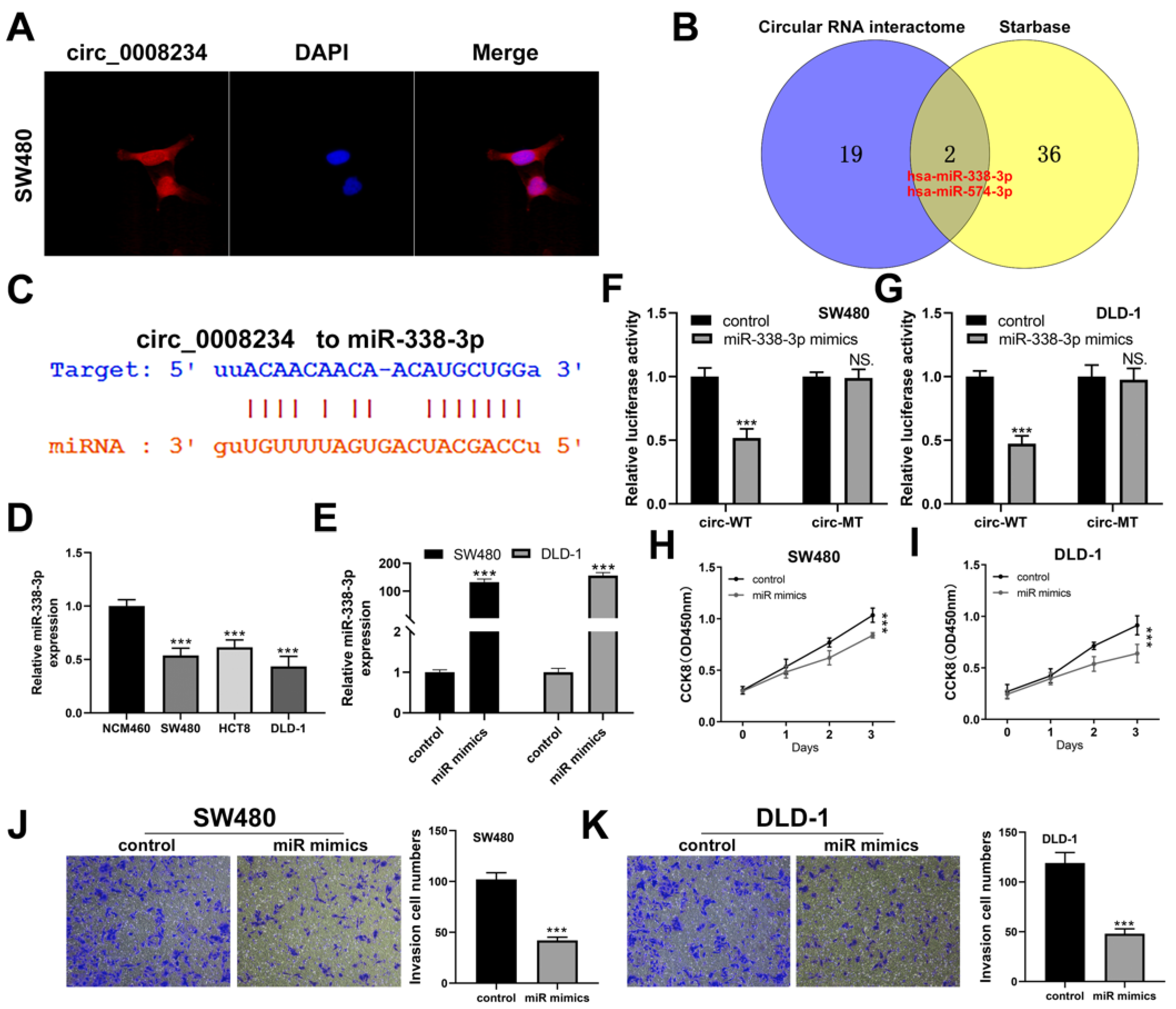
3.3. CircRNA hsa_circ_0008234 Functions as a miR-338-3p Sponge in Colon Cancer Cells
3.4. ETS1 Is the Targeted Gene of miR-338-3p
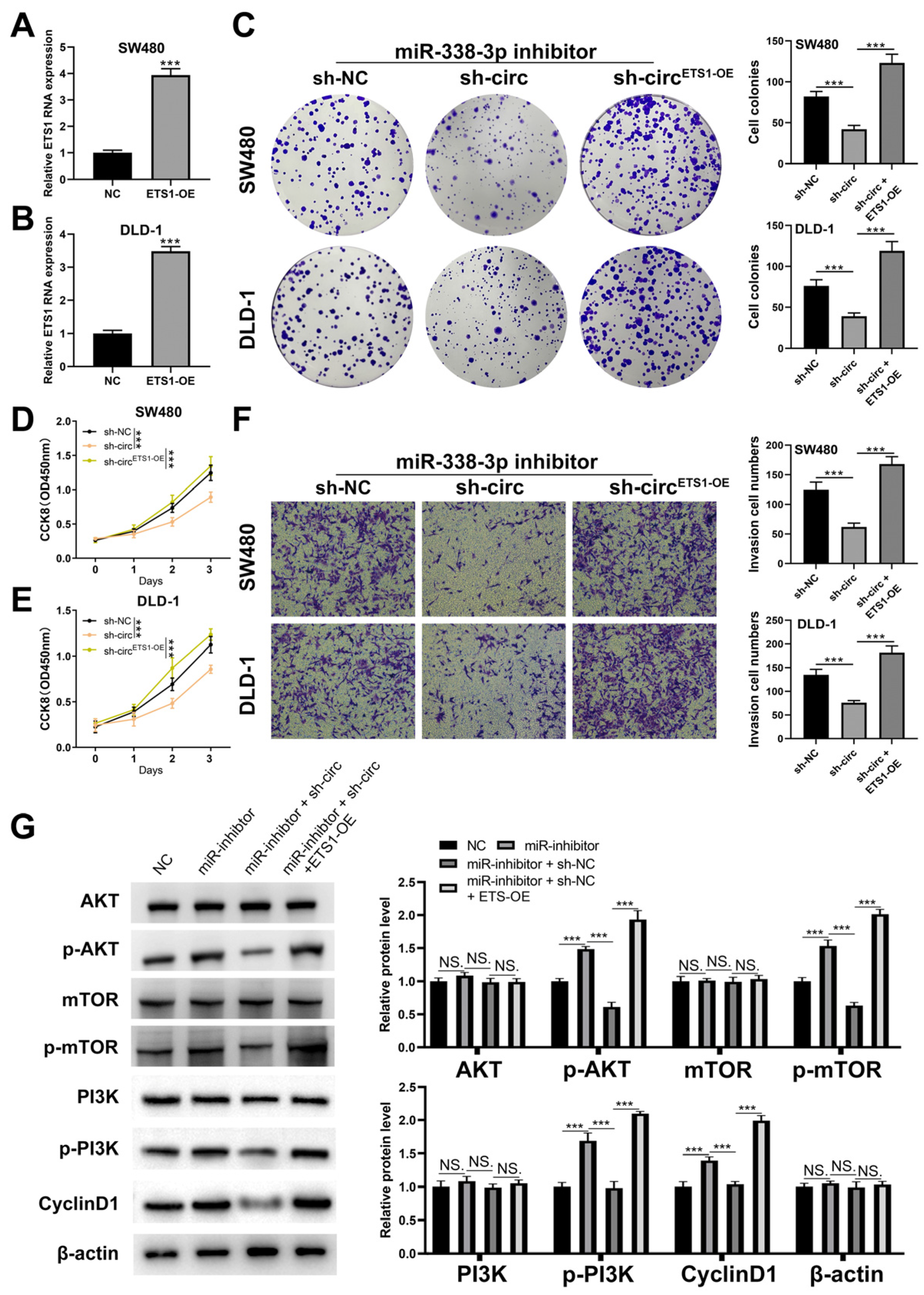
3.5. CircRNA hsa_circ_0008234 Regulates the PI3K/AKT/mTOR Signaling Pathway via the miR-338-3p/ETS1 Axis to Promote the Proliferation, Invasion, and Migration of Colon Cancer
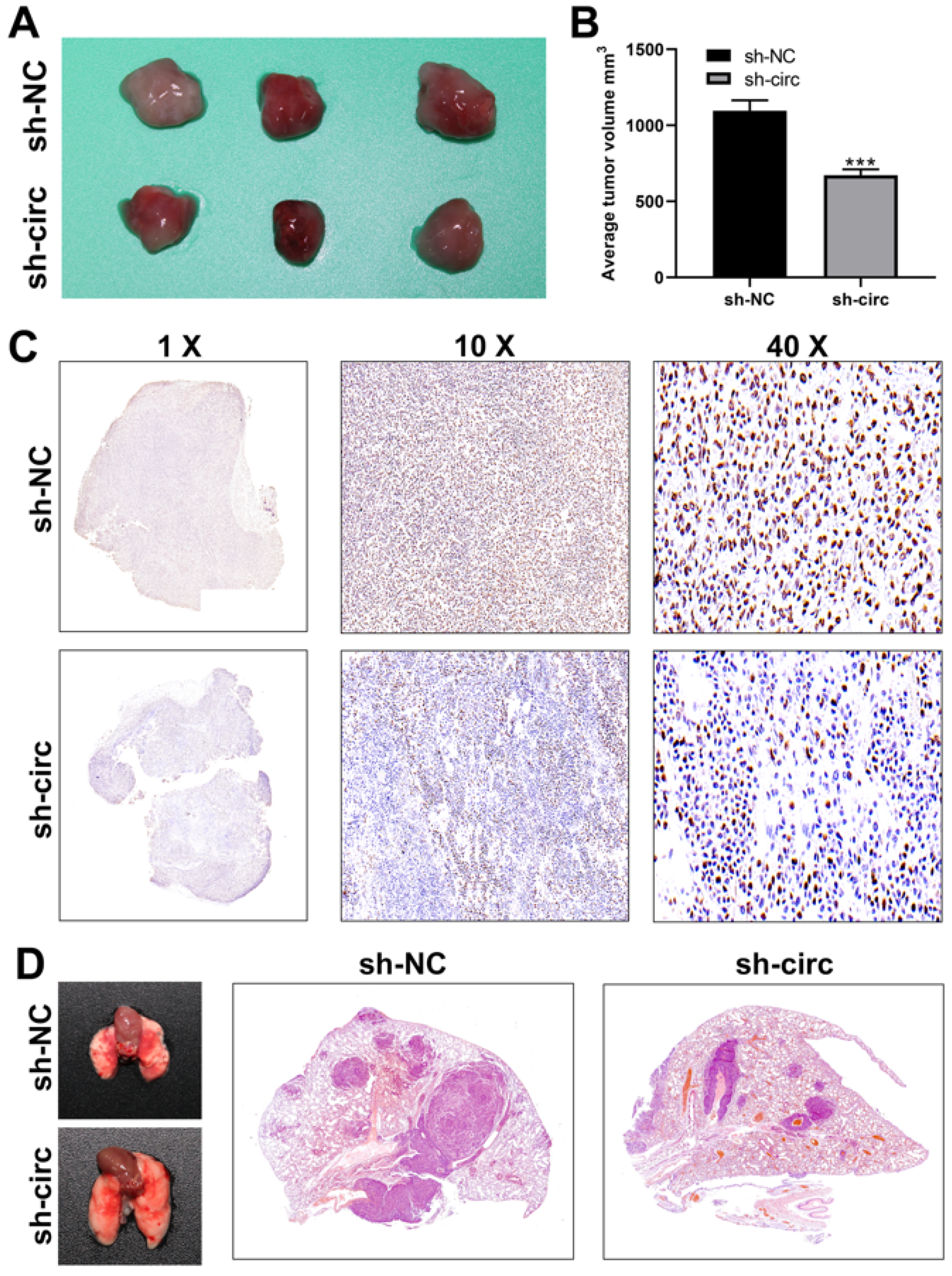
3.6. CircRNA hsa_circ_0008234 Promotes Colon Cancer Proliferation and Metastasis in Vivo
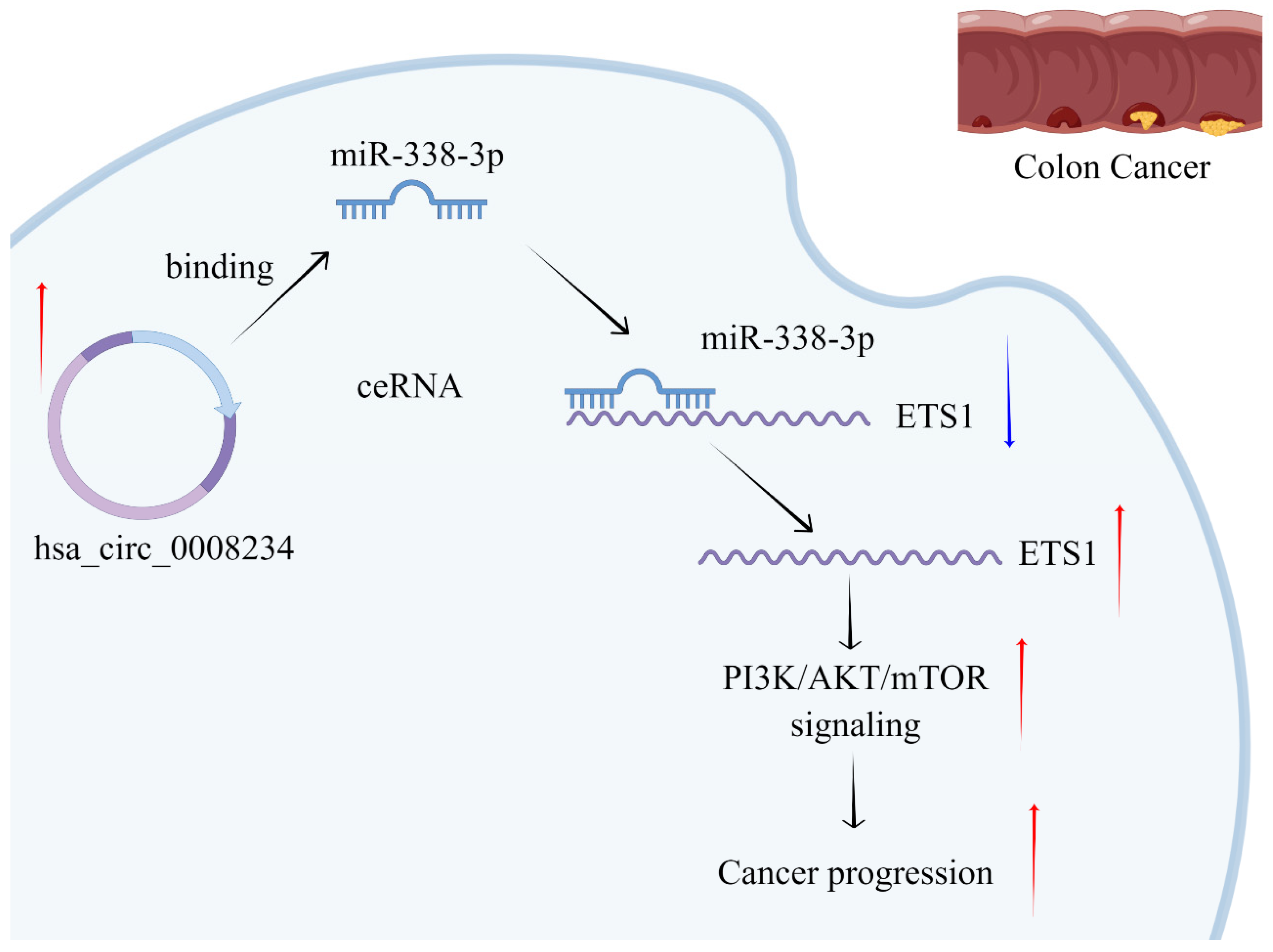
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| circRNAs | Circular RNAs |
| qRT-PCR | Quantitative real-time PCR |
| shRNAs | Short hairpin RNAs |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| FISH | Fluorescence in situ hybridization |
| RIP | RNA immunoprecipitation |
| IHC | Immunohistochemistry |
| GEO | Gene Expression Omnibus |
References
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G.; et al. Colon Cancer. Crit. Rev. Oncol./Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Modifiable risk factors for colon cancer. Gastroenterol. Clin. N. Am. 2002, 31, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Dickman, P.W.; De Wilt, J.H.; Verhoeven, R. Conditional Survival and Cure of Patients with Colon or Rectal Cancer: A Population-Based Study. J. Natl. Compr. Cancer Netw. 2020, 18, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.A.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular Rna: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Vicens, Q.; Westhof, E. Biogenesis of Circular RNAs. Cell 2014, 159, 13–14. [Google Scholar] [CrossRef]
- Xiao, M.S.; Ai, Y.; Wilusz, J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef]
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef]
- Wu, P.; Mo, Y.; Peng, M.; Tang, T.; Zhong, Y.; Deng, X.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol. Cancer 2020, 19, 22. [Google Scholar] [CrossRef]
- Huang, G.; Liang, M.; Liu, H.; Huang, J.; Li, P.; Wang, C.; Zhang, Y.; Lin, Y.; Jiang, X. CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell Death Dis. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Yang, F.; Zang, Y.; Liu, J.; Wang, X.; Xu, X.; Li, W.; Jia, J.; Liu, Z. Circular Rna Hsa_Circ_0004872 Inhibits Gastric Cancer Progression Via the Mir-224/Smad4/Adar1 Successive Regulatory Circuit. Mol. Cancer 2020, 19, 157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Zhou, Y.; Wang, Q.; Zheng, Z.; Xu, B.; Wu, C.; Zhou, Q.; Hu, W.; Wu, C.; et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol. Cancer 2019, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, F.-Q.; Sun, C.-M.; Huang, J.-H.; Zhang, H.-M.; Li, X.; Wang, G.-C.; Zhang, N.; Che, J.-P.; Zhang, W.-T.; et al. circPRRC2A promotes angiogenesis and metastasis through epithelial-mesenchymal transition and upregulates TRPM3 in renal cell carcinoma. Theranostics 2020, 10, 4395–4409. [Google Scholar] [CrossRef] [PubMed]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cai, J.; Lin, J.; Zhou, H.; Peng, J.; Liang, J.; Xia, L.; Yin, Q.; Zou, B.; Zheng, J.; et al. A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating Snail in colon cancer. Mol. Cancer 2020, 19, 71. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wu, D.; Min, Z.; Tan, J.; Yu, B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J. Exp. Clin. Cancer Res. 2020, 39, 203. [Google Scholar] [CrossRef]
- Yang, Z.; Quan, Y.; Chen, Y.; Huang, Y.; Huang, R.; Yu, W.; Wu, D.; Ye, M.; Min, Z.; Yu, B. Knockdown of RNA N6-methyladenosine methyltransferase METTL3 represses Warburg effect in colorectal cancer via regulating HIF-1α. Signal Transduct. Target. Ther. 2021, 6, 89. [Google Scholar] [CrossRef]
- Zou, T.; Duan, J.; Liang, J.; Shi, H.; Zhen, T.; Li, H.; Zhang, F.; Dong, Y.; Han, A. miR-338-3p suppresses colorectal cancer proliferation and progression by inhibiting MACC1. Int. J. Clin. Exp. Pathol. 2018, 11, 2256–2267. [Google Scholar]
- Meng, S.; Jian, Z.; Yan, X.; Li, J.; Zhang, R. LncRNA SNHG6 inhibits cell proliferation and metastasis by targeting ETS1 via the PI3K/AKT/mTOR pathway in colorectal cancer. Mol. Med. Rep. 2019, 20, 2541–2548. [Google Scholar] [CrossRef]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Personalizing Colon Cancer Adjuvant Therapy: Selecting Optimal Treatments for Individual Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-D.; Jiang, L.-H.; Sun, D.-W.; Hou, J.-C.; Ji, Z.-L. CircRNA: A novel type of biomarker for cancer. Breast Cancer 2018, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, Y.; Wu, J.; Wu, G. Hsa_circ_0008234 facilitates proliferation of cutaneous squamous cell carcinoma through targeting miR-127-5p to regulate ADCY7. Arch. Dermatol. Res. 2022, 314, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Q.; Lv, B.; Shang, Y.; Li, J.; Yang, L.; Yu, Z.; Luo, K.; Deng, X.; Min, L.; et al. CircFOXP1: A novel serum diagnostic biomarker for non-small cell lung cancer. Int. J. Biol. Markers 2022, 37, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, D.-H.; Wu, N.; Xiao, J.-H.; Wang, X.; Ma, W. ceRNA in cancer: Possible functions and clinical implications. J. Med. Genet. 2015, 52, 710–718. [Google Scholar] [CrossRef]
- Luan, X.; Wang, Y. LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p. J. Gynecol. Oncol. 2018, 29, e95. [Google Scholar] [CrossRef]
- Dittmer, J. The role of the transcription factor Ets1 in carcinoma. Semin. Cancer Biol. 2015, 35, 20–38. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, C.; Chen, J.; Chen, D.; Yang, B.; He, B.; Hu, W.; Zhang, Y.; Liu, H.; Dai, L.; et al. Wtap Facilitates Progression of Hepatocellular Carcinoma Via M6a-Hur-Dependent Epigenetic Silencing of Ets1. Mol. Cancer 2019, 18, 127. [Google Scholar] [CrossRef]
- Rodgers, J.J.; McClure, R.; Epis, M.R.; Cohen, R.J.; Leedman, P.J.; Harvey, J.M.; Thomas, M.A.; Bentel, J.M.; BioResource, A.P.C. ETS1 induces transforming growth factor β signaling and promotes epithelial-to-mesenchymal transition in prostate cancer cells. J. Cell. Biochem. 2019, 120, 848–860. [Google Scholar] [CrossRef]
- Gu, C.; Cai, J.; Xu, Z.; Zhou, S.; Ye, L.; Yan, Q.; Zhang, Y.; Fang, Y.; Liu, Y.; Tu, C.; et al. Mir-532-3p Suppresses Colorectal Cancer Progression by Disrupting the Ets1/Tgm2 Axis-Mediated Wnt/Β-Catenin Signaling. Cell Death Dis. 2019, 10, 739. [Google Scholar] [CrossRef]
- Xu, S.; Ge, J.; Zhang, Z.; Zhou, W. MiR-129 inhibits cell proliferation and metastasis by targeting ETS1 via PI3K/AKT/mTOR pathway in prostate cancer. Biomed. Pharmacother. 2017, 96, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Han, W.-Z.; Wang, J.-L.; Sun, N.-N.; Zhuang, M. MicroRNA-365 inhibits the progression of lung adenocarcinoma through targeting ETS1 and inactivating AKT/mTOR pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4836–4845. [Google Scholar] [PubMed]
- Wang, J.; Jiang, C.; Li, N.; Wang, F.; Xu, Y.; Shen, Z.; Yang, L.; Li, Z.; He, C. The Circepsti1/Mir-942-5p/Ltbp2 Axis Regulates the Progression of Oscc in the Background of Osf Via Emt and the Pi3k/Akt/Mtor Pathway. Cell Death Dis. 2020, 11, 682. [Google Scholar] [CrossRef]
- Ling, Z.; Fang, Z.; Wu, J.; Liu, J. The depletion of Circ-PRKDC enhances autophagy and apoptosis in T-cell acute lymphoblastic leukemia via microRNA-653-5p/Reelin mediation of the PI3K/AKT/mTOR signaling pathway. Kaohsiung J. Med. Sci. 2021, 37, 392–401. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H. Circrna Plod2 Promotes Tumorigenesis and Warburg Effect in Colon Cancer by the Mir-513a-5p/Six1/Ldha Axis. Cell Cycle. 2022, 21, 2484–2498. [Google Scholar] [CrossRef]
- Zhang, F.; Su, T.; Xiao, M. Runx3-Regulated Circrna Mettl3 Inhibits Colorectal Cancer Proliferation and Metastasis Via Mir-107/Per3 Axis. Cell Death Dis. 2022, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, T.W.; Lee, W.J.; Jeong, S.D.; Cho, Y.B.; Kim, H.H. Two Circppfia1s Negatively Regulate Liver Metastasis of Colon Cancer Via Mir-155-5p/Cdx1 and Hur/Rab36. Mol. Cancer 2022, 21, 197. [Google Scholar] [CrossRef]
- Min, L.; Wang, H.; Zeng, Y. Circrna_104916 Regulates Migration, Apoptosis and Epithelial-Mesenchymal Transition in Colon Cancer Cells. Front. Biosci. (Landmark Ed.) 2019, 24, 819–832. [Google Scholar]
- Zhao, G.; Dai, G.J. Hsa_Circrna_000166 Promotes Cell Proliferation, Migration and Invasion by Regulating Mir-330-5p/Elk1 in Colon Cancer. Onco. Targets Ther. 2020, 13, 5529–5539. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Huang, H.L.; Huang, R.Q.; Tian, C.; Zhu, H.B.; Dai, Y.H.; Li, Z.Y. Circrna_100859 Functions as an Oncogene in Colon Cancer by Sponging the Mir-217-Hif-1α Pathway. Aging 2020, 12, 13338–13353. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, Q. Circ_0085315 Promotes Cell Proliferation, Invasion, and Migration in Colon Cancer through Mir-1200/Map3k1 Signaling Pathway. Cell Cycle. 2022, 21, 1194–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Chen, B.; Yu, Z.; Zhang, J.; Zhang, Z.; Hu, C.; Bai, Y.; Ruan, X.; Wang, S.; et al. Circular Rna Circcspp1 Promotes the Occurrence and Development of Colon Cancer by Sponging Mir-431 and Regulating Rock1 and Zeb1. J. Transl. Med. 2022, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, Z.; Shan, J.; Gao, Y.; Liu, X.; Ma, D.; Li, Z. Hsa_Circ_0020095 Promotes Oncogenesis and Cisplatin Resistance in Colon Cancer by Sponging Mir-487a-3p and Modulating Sox9. Front. Cell Dev. Biol. 2020, 8, 604869. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xu, Z.; Zhang, J.; Wei, Y.; Cheng, L.; Wang, J. Circ_0038718 Promotes Colon Cancer Cell Malignant Progression Via the Mir-195-5p/Axin2 Signaling Axis and Also Effect Wnt/Β-Catenin Signal Pathway. BMC Genom. 2021, 22, 768. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Li, Y.; Xu, A.; Tang, W.; Yu, B. CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling. Cancers 2023, 15, 2068. https://doi.org/10.3390/cancers15072068
Wu D, Li Y, Xu A, Tang W, Yu B. CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling. Cancers. 2023; 15(7):2068. https://doi.org/10.3390/cancers15072068
Chicago/Turabian StyleWu, Dejun, Yuqin Li, Anjun Xu, Wenqing Tang, and Bo Yu. 2023. "CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling" Cancers 15, no. 7: 2068. https://doi.org/10.3390/cancers15072068
APA StyleWu, D., Li, Y., Xu, A., Tang, W., & Yu, B. (2023). CircRNA RNA hsa_circ_0008234 Promotes Colon Cancer Progression by Regulating the miR-338-3p/ETS1 Axis and PI3K/AKT/mTOR Signaling. Cancers, 15(7), 2068. https://doi.org/10.3390/cancers15072068




