Mannose Inhibits the Pentose Phosphate Pathway in Colorectal Cancer and Enhances Sensitivity to 5-Fluorouracil Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analysis of PMI mRNA Expression and Protein Levels in CRC
2.2. Cell Lines and Cell Culture Conditions
2.3. Drugs and Compounds
2.4. cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Thiazolyl Blue Tetrazolium Bromide Assay
2.6. Trypan Blue Cell Viability Assay
2.7. Sulforhodamine B Assay
2.8. Total Dehydrogenase Activity
2.9. Nitroblue Tetrazolium Reduction Assay
2.10. Cell Cycle Analysis
2.11. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
2.12. Western Blotting
2.13. Mouse Xenograft Studies
2.14. Statistical Analysis
3. Results
3.1. PMI Levels Are Low in Human Colorectal Cancer Tissues
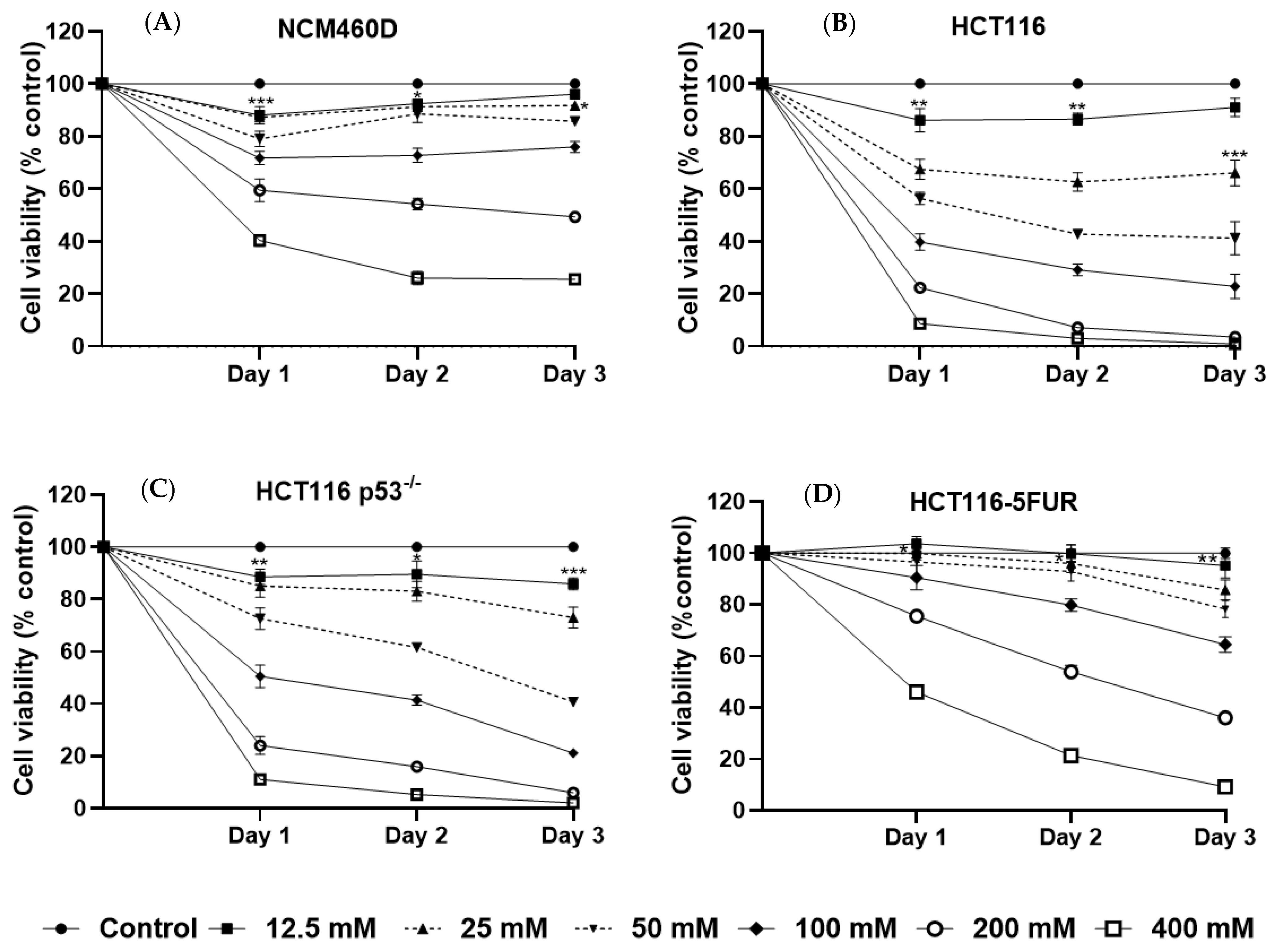
3.2. Mannose Inhibits the Growth of Human CRC Cells with Different p53 and 5-FU Resistance Statuses
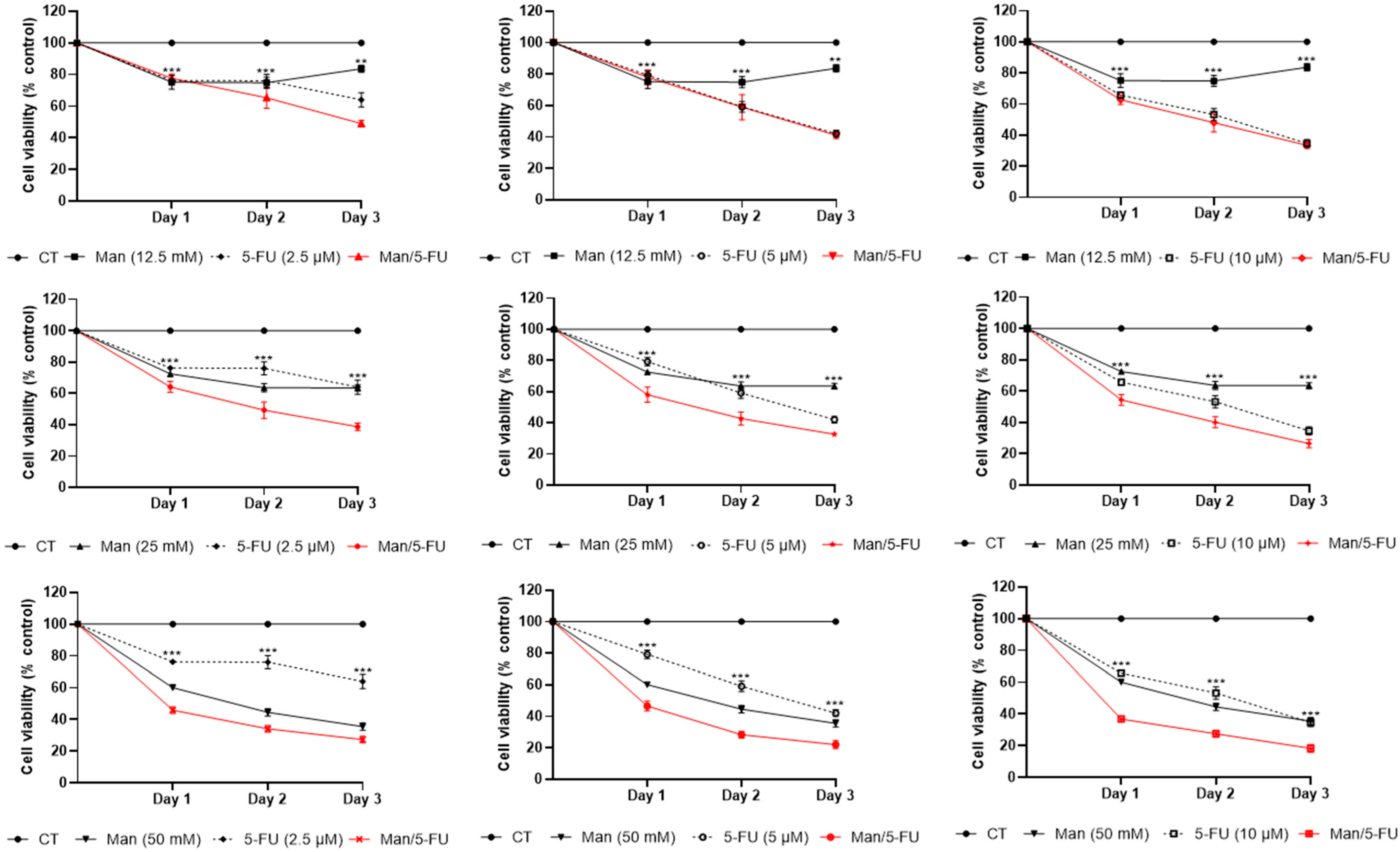
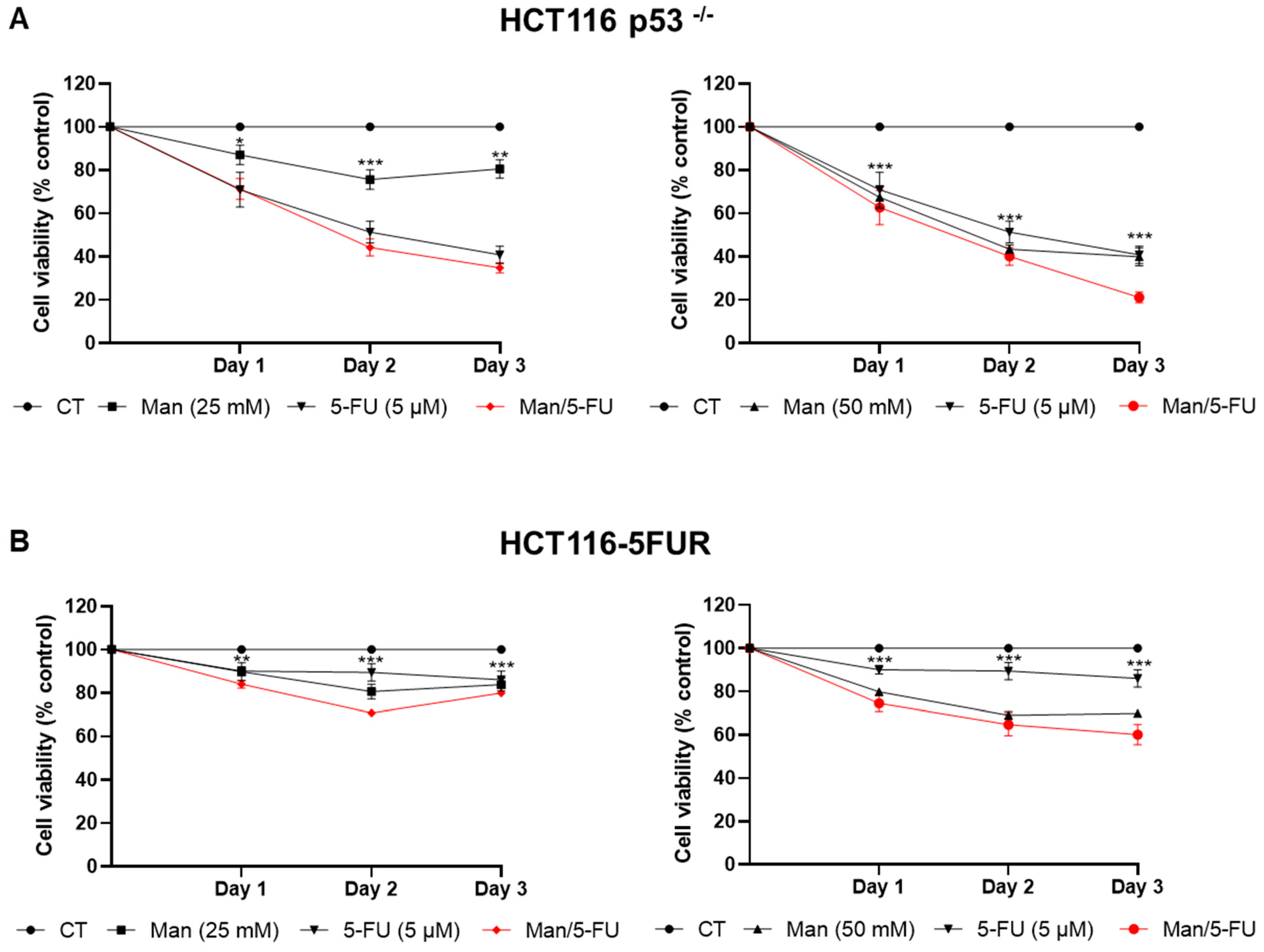
3.3. Mannose Synergizes with 5-FU in Reducing Colorectal Cancer Cell Viability
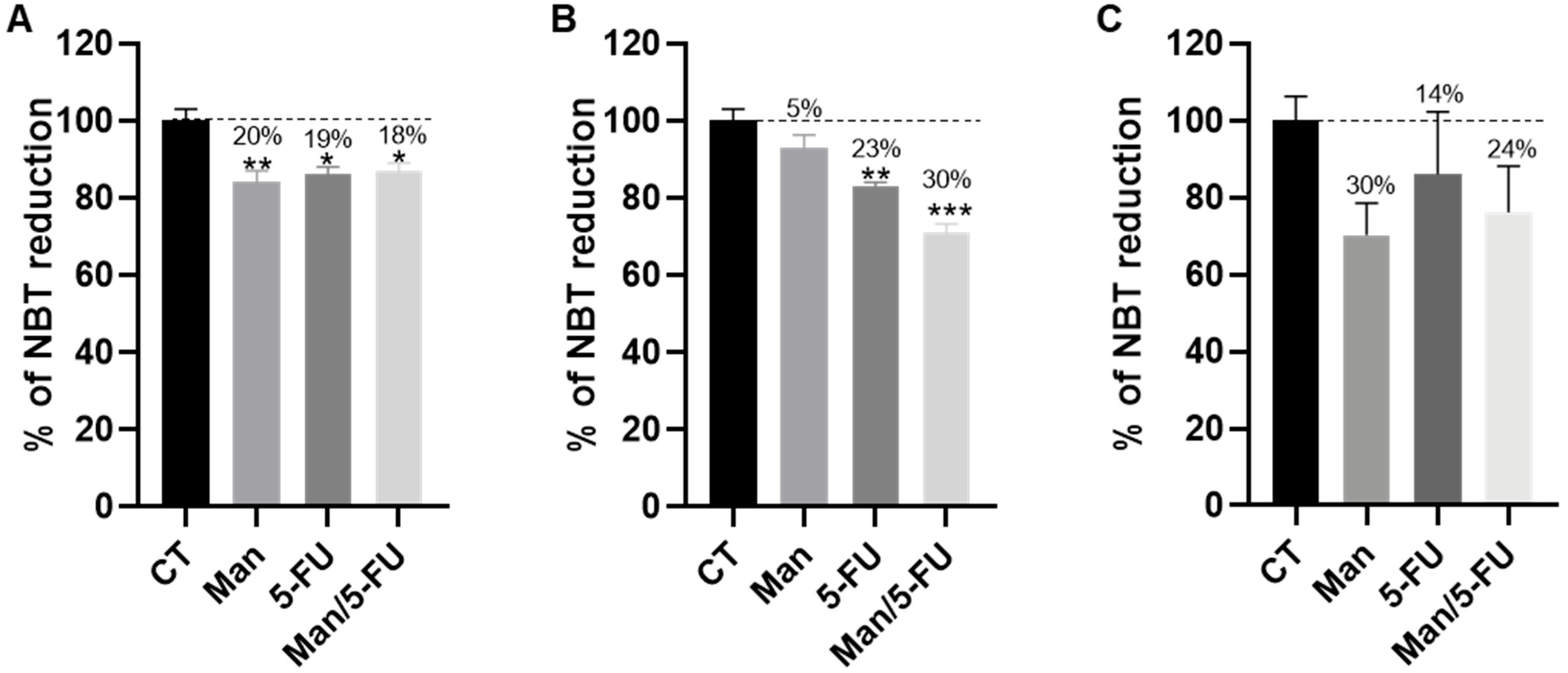
3.4. Mannose Alone or in Combination with 5-FU Reduces the Total Dehydrogenase Activity of Key PPP Enzymes in CRC Cells
3.5. Mannose Alone or in Combination with 5-FU Induces ROS Production in Colorectal Cancer Cells
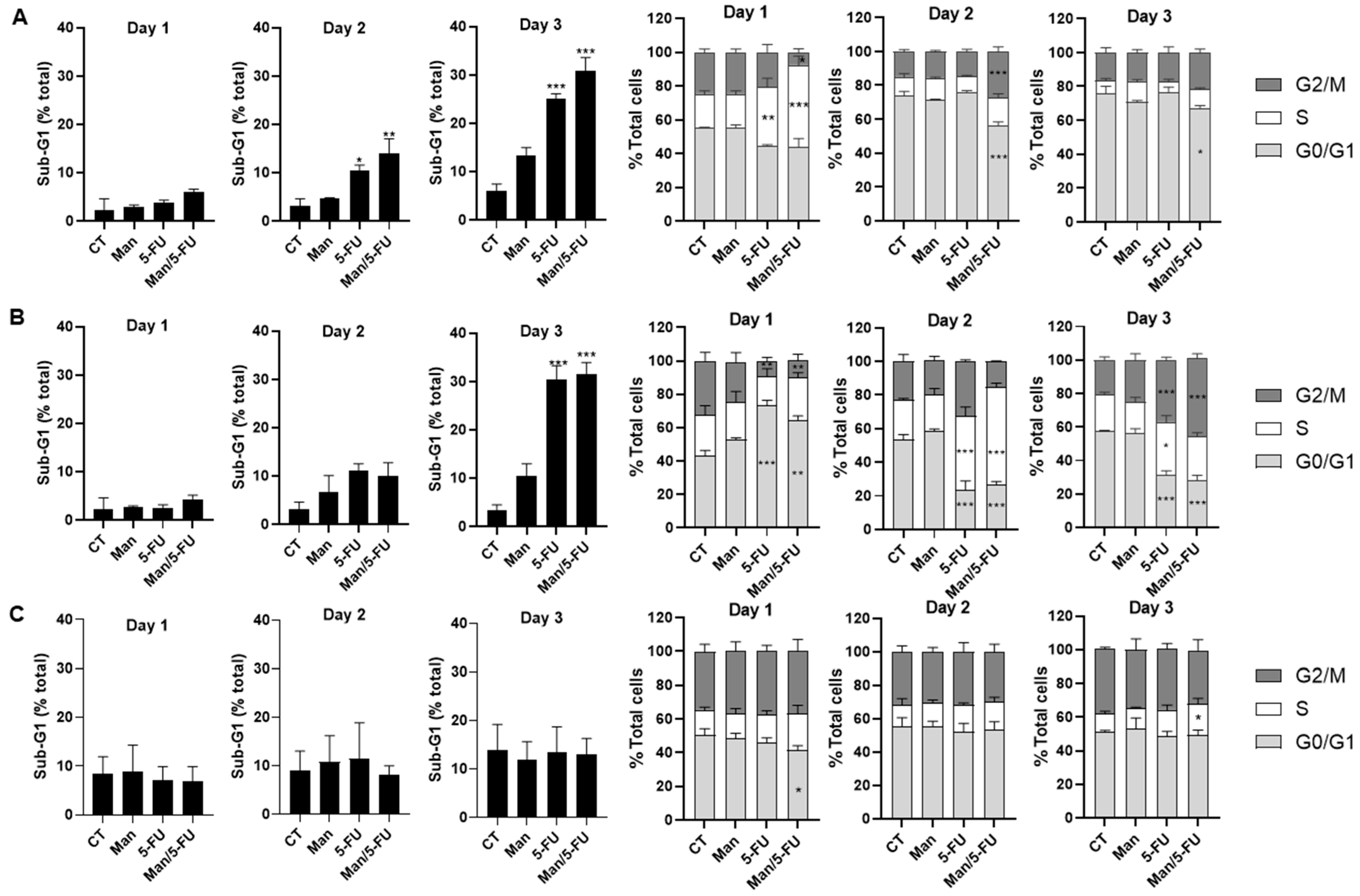
3.6. Mannose in Combination with 5-FU Causes S-Phase Cell Cycle Arrest and Sub-G1 Accumulation in Colorectal Cancer Cells
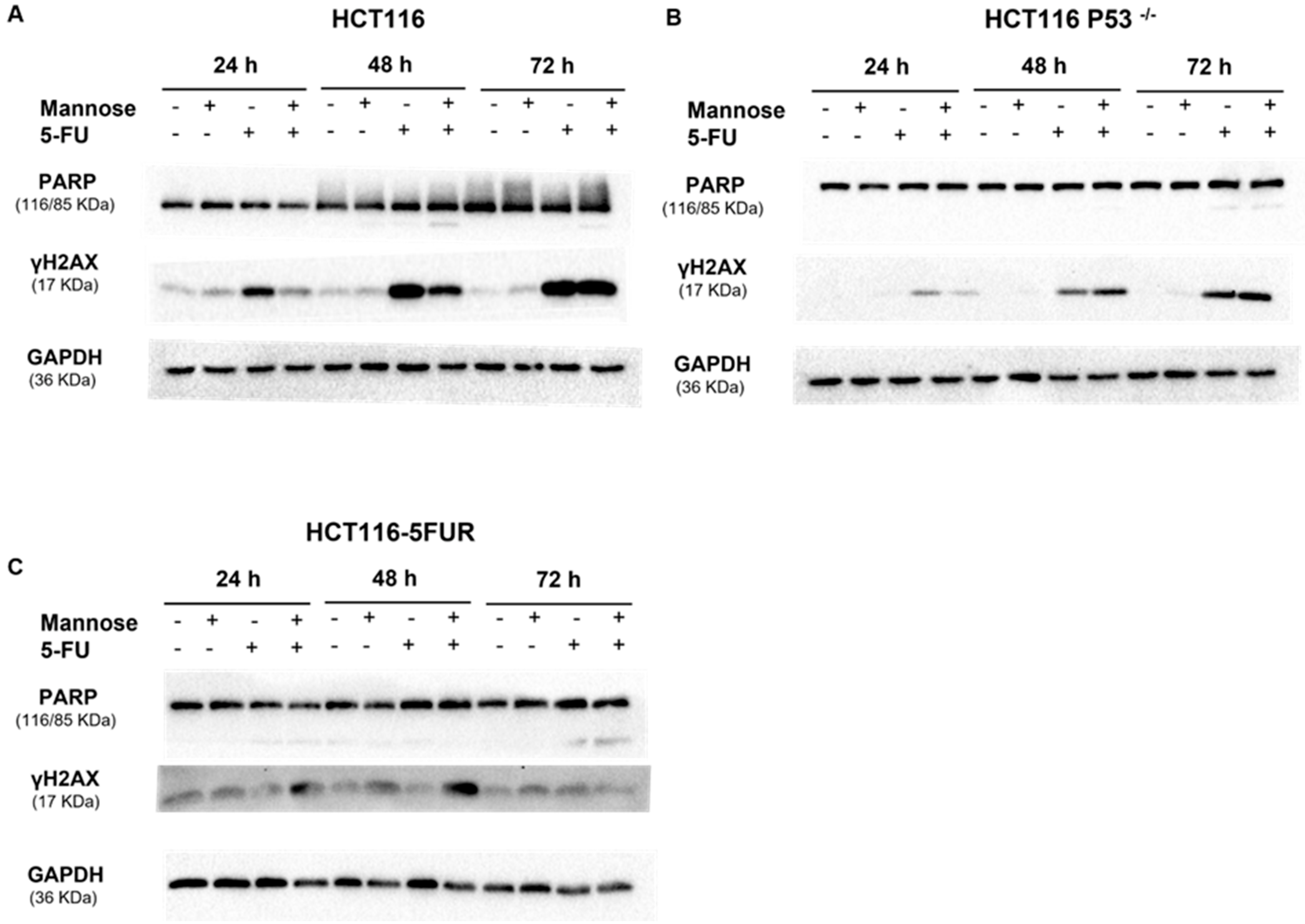
3.7. 5-FU Alone or in Combination with Mannose Induces DNA Damage in Colorectal Cancer Cells
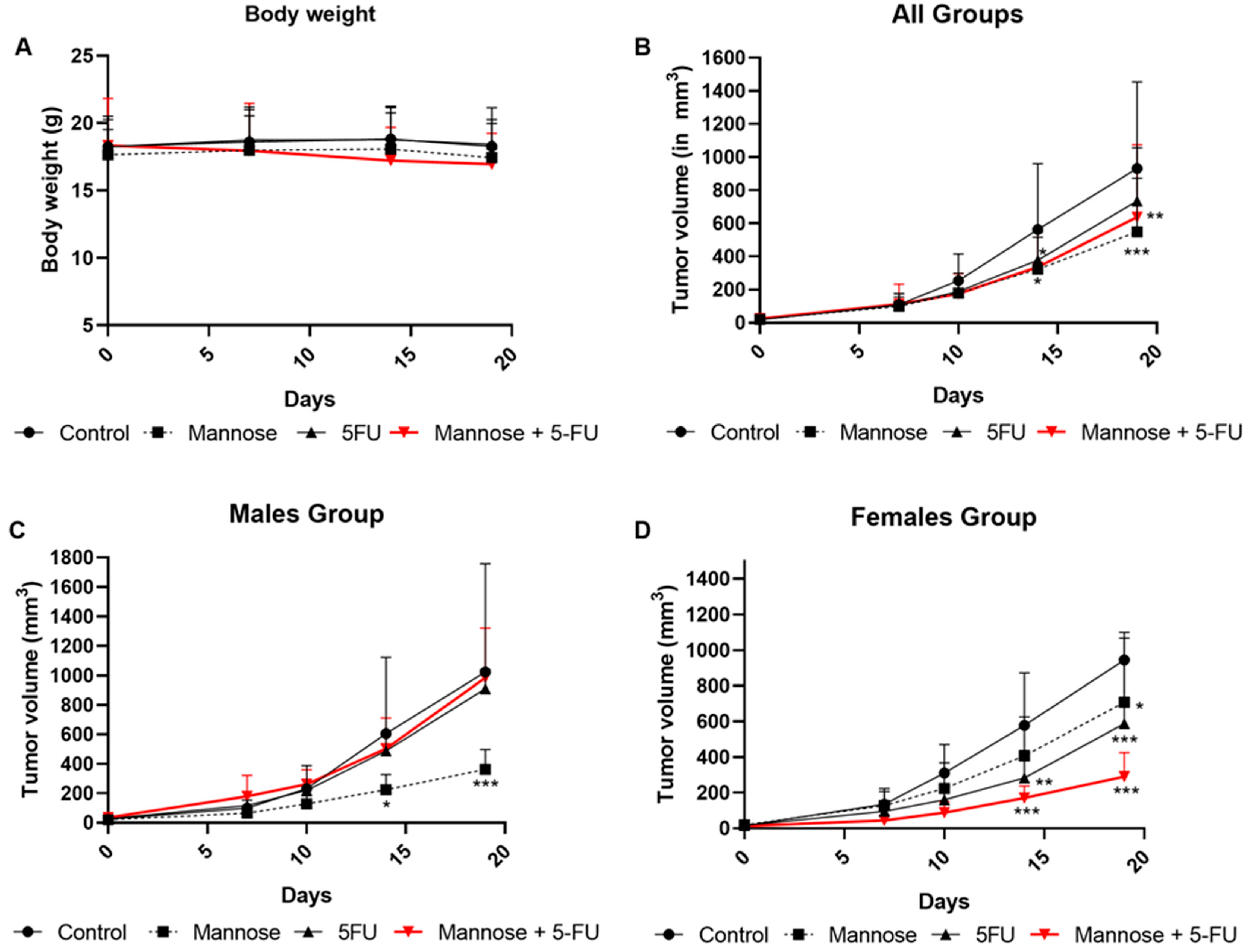
3.8. Mannose Alone or in Combination with 5-FU Reduces Tumor Growth in Xenografted Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Kurasaka, C.; Ogino, Y.; Sato, A. Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 2916. [Google Scholar] [CrossRef]
- Suetsugu, T.; Mori, R.; Futamura, M.; Fukada, M.; Tanaka, H.; Yasufuku, I.; Sato, Y.; Iwata, Y.; Imai, T.; Imai, H.; et al. Mechanism of acquired 5FU resistance and strategy for overcoming 5FU resistance focusing on 5FU metabolism in colon cancer cell lines. Oncol. Rep. 2021, 45, 27. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhong, X.; He, X.; Wang, Y.; Hu, Z.; Huang, H.; Zhao, S.; Wei, P.; Li, D. Warburg effect in colorectal cancer: The emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 2022, 15, 160. [Google Scholar] [CrossRef]
- Brown, R.E.; Short, S.P.; Williams, C.S. Colorectal Cancer and Metabolism. Curr. Colorectal Cancer Rep. 2018, 14, 226–241. [Google Scholar] [CrossRef]
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kinoshita, M.; Yamada, A.; Kawano, S.; Liu, H.S.; Kamimura, S.; Nakagawa, M.; Nagasawa, S.; Taguchi, T.; Yamada, S.; et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 2021, 112, 4944–4956. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Zhang, H.; Li, H. Mannose Attenuates Colitis-Associated Colorectal Tumorigenesis by Targeting Tumor-Associated Macrophages. J. Cancer Prev. 2022, 27, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ichikawa, M.; Freeze, H.H. Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 2014, 453, 220–228. [Google Scholar] [CrossRef]
- Nan, F.; Sun, Y.; Liang, H.; Zhou, J.; Ma, X.; Zhang, D. Mannose: A Sweet Option in the Treatment of Cancer and Inflammation. Front. Pharmacol. 2022, 13, 1825. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.; Sakamaki, J.I.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef]
- Abdel-Samad, R.; Aouad, P.; Gali-Muhtasib, H.; Sweidan, Z.; Hmadi, R.; Kadara, H.; D’Andrea, E.L.; Fucci, A.; Pisano, C.; Darwiche, N. Mechanism of action of the atypical retinoid ST1926 in colorectal cancer: DNA damage and DNA polymerase α. Am. J. Cancer Res. 2018, 8, 39–55. [Google Scholar]
- Hong, Y.; Downey, T.; Eu, K.W.; Koh, P.K.; Cheah, P.Y. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis 2010, 27, 83–90. [Google Scholar]
- Skrzypczak, M.; Goryca, K.; Rubel, T.; Paziewska, A.; Mikula, M.; Jarosz, D.; Pachlewski, J.; Oledzki, J.; Ostrowsk, J. Modeling Oncogenic Signaling in Colon Tumors by Multidirectional Analyses of Microarray Data Directed for Maximization of Analytical Reliability. PLoS ONE 2010, 5, e13091. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Z.; Huang, Z.; Wang, R.; Yang, A.; Liao, L.; Du, J. Antitumor and immunomodulatory effects of low-dose 5-FU on hepatoma 22 tumor-bearing mice. Oncol. Lett. 2014, 7, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Inoue Kichi Tanaka, G.; Akimoto, K.; Kubota, K. Augmented Pentose Phosphate Pathway Plays Critical Roles in Colorectal Carcinomas. Oncology 2015, 88, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Cao, D.; Cui, R.; Xia, L.; Hua, X.; Lu, Y.; Han, S. Mannose impairs lung adenocarcinoma growth and enhances the sensitivity of A549 cells to carboplatin. Cancer Manag. Res. 2020, 12, 11077–11083. [Google Scholar] [CrossRef]
- Fei, Y.Q.; Shi, R.T.; Zhou, Y.F.; Wu, J.Z.; Song, Z. Mannose inhibits proliferation and promotes apoptosis to enhance sensitivity of glioma cells to temozolomide through Wnt/β-catenin signaling pathway. Neurochem. Int. 2022, 157, 105348. [Google Scholar] [CrossRef]
- Liu, F.; Xu, X.; Li, C.; Li, C.; Li, Y.; Yin, S.; Yu, S.; Chen, X.Q. Mannose synergizes with chemoradiotherapy to cure cancer via metabolically targeting HIF-1 in a novel triple-negative glioblastoma mouse model. Clin. Transl. Med. 2020, 10, e226. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; He, B. Mannose shows antitumour properties against lung cancer via inhibiting proliferation, promoting cisplatin mediated apoptosis and reducing metastasis. Mol. Med. Rep. 2020, 22, 2957–2965. [Google Scholar] [CrossRef]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- De Moura Sperotto, N.D.; Silva, R.B.M.; Perelló, M.A.; Borsoi, A.F.; da Silva Dadda, A.; Roth, C.D.; Freitas, R.D.S.; de Souza, A.P.D.; de Freitas, D.D.N.; Picada, J.N.; et al. Targeting thymidine phosphorylase inhibition in human colorectal cancer xenografts. Biomed. Pharmacother. 2021, 139, 111672. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Control | Mannose | 5-FU | Mannose/5-FU |
|---|---|---|---|---|
| Number of mice | 12 (6 M, 6 F) | 13 (6 M, 7 F) | 13 (6 M, 7 F) | 12 (6 M, 6 F) |
| 24 Hours | 48 Hours | 72 Hours | ||||||
|---|---|---|---|---|---|---|---|---|
| Mannose (mM) | 5-FU (µM) | CI | Mannose (mM) | 5-FU (µM) | CI | Mannose (mM) | 5-FU (µM) | CI |
| 12.5 | 2.5 | 2.11715 | 12.5 | 2.5 | 1.12495 | 12.5 | 2.5 | 0.89611 |
| 12.5 | 5 | 3.04617 | 12.5 | 5 | 1.22017 | 12.5 | 5 | 1.03834 |
| 12.5 | 10 | 0.73937 | 12.5 | 10 | 1.13527 | 12.5 | 10 | 1.24165 |
| 25 | 2.5 | 0.72892 | 25 | 2.5 | 0.79519 | 25 | 2.5 | 0.88039 |
| 25 | 5 | 0.48905 | 25 | 5 | 0.76761 | 25 | 5 | 0.96374 |
| 25 | 10 | 0.42279 | 25 | 10 | 0.94199 | 25 | 10 | 1.10926 |
| 50 | 2.5 | 0.29071 | 50 | 2.5 | 0.68224 | 50 | 2.5 | 0.94712 |
| 50 | 5 | 0.33398 | 50 | 5 | 0.56759 | 50 | 5 | 0.91218 |
| 50 | 10 | 0.15323 | 50 | 10 | 0.70285 | 50 | 10 | 0.95026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hadeethi, S.; El-Baba, C.; Araji, K.; Hayar, B.; Cheikh, I.A.; El-Khoury, R.; Usta, J.; Darwiche, N. Mannose Inhibits the Pentose Phosphate Pathway in Colorectal Cancer and Enhances Sensitivity to 5-Fluorouracil Therapy. Cancers 2023, 15, 2268. https://doi.org/10.3390/cancers15082268
Al Hadeethi S, El-Baba C, Araji K, Hayar B, Cheikh IA, El-Khoury R, Usta J, Darwiche N. Mannose Inhibits the Pentose Phosphate Pathway in Colorectal Cancer and Enhances Sensitivity to 5-Fluorouracil Therapy. Cancers. 2023; 15(8):2268. https://doi.org/10.3390/cancers15082268
Chicago/Turabian StyleAl Hadeethi, Sadaf, Chirine El-Baba, Khaled Araji, Berthe Hayar, Israa Ahmad Cheikh, Riyad El-Khoury, Julnar Usta, and Nadine Darwiche. 2023. "Mannose Inhibits the Pentose Phosphate Pathway in Colorectal Cancer and Enhances Sensitivity to 5-Fluorouracil Therapy" Cancers 15, no. 8: 2268. https://doi.org/10.3390/cancers15082268
APA StyleAl Hadeethi, S., El-Baba, C., Araji, K., Hayar, B., Cheikh, I. A., El-Khoury, R., Usta, J., & Darwiche, N. (2023). Mannose Inhibits the Pentose Phosphate Pathway in Colorectal Cancer and Enhances Sensitivity to 5-Fluorouracil Therapy. Cancers, 15(8), 2268. https://doi.org/10.3390/cancers15082268









