Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Irradiation Procedure
2.3. Blood Sampling
2.4. Histological Processing
2.5. Statistical Analysis
3. Results
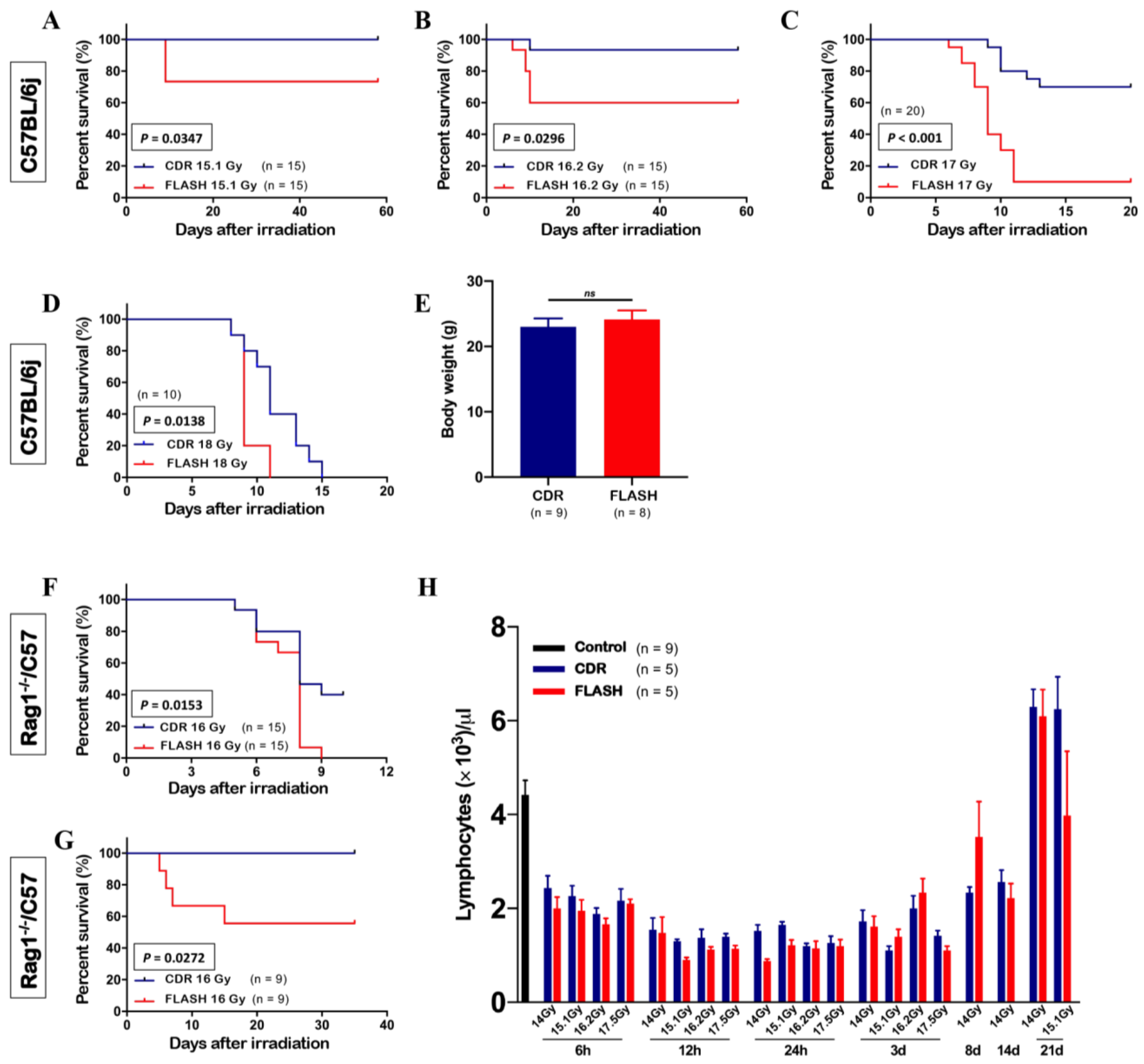
3.1. Abdominal FLASH Irradiation Decreased Survival
3.2. FLASH Irradiation Did Not Preserve Circulating Lymphocytes
3.3. Abdominal FLASH Irradiation Did Not Spare the Number of Proliferating Crypt Cells or the Muscularis Externa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra293. [Google Scholar] [CrossRef] [PubMed]
- Labarbe, R.; Hotoiu, L.; Barbier, J.; Favaudon, V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother. Oncol. 2020, 153, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Radiotherapy Using High-Intensity Pulsed Radiation Beams (FLASH): A Radiation-Chemical Perspective. Radiat. Res. 2020, 194, 607–617. [Google Scholar] [CrossRef]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH effect depends on oxygen concentration. Br. J. Radiol. 2020, 93, 20190702. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Labarbe, R.; Limoli, C.L. Model studies of the role of oxygen in the FLASH effect. Med. Phys. 2022, 49, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Spring Kong, F.M. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother. Oncol. 2020, 149, 55–62. [Google Scholar] [CrossRef]
- Dewey, D.L.; Boag, J.W. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature 1959, 183, 1450–1451. [Google Scholar] [CrossRef]
- Epp, E.R.; Weiss, H.; Santomasso, A. The oxygen effect in bacterial cells irradiated with high-intensity pulsed electrons. Radiat. Res. 1968, 34, 320–325. [Google Scholar] [CrossRef]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of mammalian cells exposed to x rays at ultra-high dose-rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef]
- Town, C.D. Effect of High Dose Rates on Survival of Mammalian Cells. Nature 1967, 215, 847–848. [Google Scholar] [CrossRef]
- Hornsey, S.; Bewley, D.K. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971, 19, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Venkatesulu, B.P.; Sharma, A.; Pollard-Larkin, J.M.; Sadagopan, R.; Symons, J.; Neri, S.; Singh, P.K.; Tailor, R.; Lin, S.H.; Krishnan, S. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci. Rep. 2019, 9, 17180. [Google Scholar] [CrossRef] [PubMed]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Levy, K.; Natarajan, S.; Wang, J.; Chow, S.; Eggold, J.T.; Loo, P.E.; Manjappa, R.; Melemenidis, S.; Lartey, F.M.; Schüler, E.; et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci. Rep. 2020, 10, 21600. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.A.; Casey, K.M.; Wang, J.; Blaney, A.; Manjappa, R.; Breitkreutz, D.; Skinner, L.; Dutt, S.; Ko, R.B.; Bush, K.; et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat. Res. 2020, 194, 618–624. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gaide, O.; Herrera, F.; Jeanneret Sozzi, W.; Gonçalves Jorge, P.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.F.; Gondré, M.; et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91. [Google Scholar] [CrossRef]
- Patriarca, A.; Fouillade, C.; Auger, M.; Martin, F.; Pouzoulet, F.; Nauraye, C.; Heinrich, S.; Favaudon, V.; Meyroneinc, S.; Dendale, R.; et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 619–626. [Google Scholar] [CrossRef]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cascio, E.; Li, C.; Yang, Q.; Gerweck, L.E.; Huang, P.; Gottschalk, B.; Flanz, J.; Schuemann, J. FLASH Investigations Using Protons: Design of Delivery System, Preclinical Setup and Confirmation of FLASH Effect with Protons in Animal Systems. Radiat. Res. 2020, 194, 656–664. [Google Scholar] [CrossRef]
- Bialkowska, A.B.; Ghaleb, A.M.; Nandan, M.O.; Yang, V.W. Improved Swiss-rolling Technique for Intestinal Tissue Preparation for Immunohistochemical and Immunofluorescent Analyses. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Adusumilli, P.; Konatam, M.L.; Gundeti, S.; Bala, S.; Maddali, L.S. Treatment Challenges and Survival Analysis of Human Epidermal Growth Factor Receptor 2-positive Breast Cancer in Real World. Indian. J. Med. Paediatr. Oncol. 2017, 38, 22–27. [Google Scholar] [CrossRef]
- Durante, M.; Bräuer-Krisch, E.; Hill, M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br. J. Radiol. 2018, 91, 20170628. [Google Scholar] [CrossRef]
- Westermann, J.; Pabst, R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin. Investig. 1992, 70, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Trepel, F. Number and distribution of lymphocytes in man. A critical analysis. Klin. Wochenschr. 1974, 52, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Lee, C.; Wouters, S.; Tullis, I.D.C.; Verslegers, M.; Mysara, M.; Then, C.K.; Smart, S.C.; Hill, M.A.; Muschel, R.J.; et al. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1250–1261. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Zhang, W.; Wang, J.; Xiao, D.; Ren, H.; Wang, T.; Gao, F.; Liu, Z.; Zhou, K.; et al. FLASH X-ray spares intestinal crypts from pyroptosis initiated by cGAS-STING activation upon radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2208506119. [Google Scholar] [CrossRef]
- Evans, T.; Cooley, J.; Wagner, M.; Yu, T.; Zwart, T. Demonstration of the FLASH Effect Within the Spread-out Bragg Peak After Abdominal Irradiation of Mice. Int. J. Part. Ther. 2022, 8, 68–75. [Google Scholar] [CrossRef]
- Zhang, Q.; Gerweck, L.E.; Cascio, E.W.; Yang, Q.; Huang, P.; Niemierko, A.; Bertolet, A.; Nesteruk, K.P.; McNamara, A.L.; Schuemann, J. Proton FLASH effects on mouse skin at different oxygen tensions. Phys. Med. Biol. 2023, 68, 055010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Gerweck, L.E.; Cascio, E.; Gu, L.; Yang, Q.; Dong, X.; Huang, P.; Bertolet, A.; Nesteruk, K.P.; Sung, W.; et al. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers 2023, 15, 2269. https://doi.org/10.3390/cancers15082269
Zhang Q, Gerweck LE, Cascio E, Gu L, Yang Q, Dong X, Huang P, Bertolet A, Nesteruk KP, Sung W, et al. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers. 2023; 15(8):2269. https://doi.org/10.3390/cancers15082269
Chicago/Turabian StyleZhang, Qixian, Leo E. Gerweck, Ethan Cascio, Liqun Gu, Qingyuan Yang, Xinyue Dong, Peigen Huang, Alejandro Bertolet, Konrad Pawel Nesteruk, Wonmo Sung, and et al. 2023. "Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine" Cancers 15, no. 8: 2269. https://doi.org/10.3390/cancers15082269
APA StyleZhang, Q., Gerweck, L. E., Cascio, E., Gu, L., Yang, Q., Dong, X., Huang, P., Bertolet, A., Nesteruk, K. P., Sung, W., McNamara, A. L., & Schuemann, J. (2023). Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers, 15(8), 2269. https://doi.org/10.3390/cancers15082269







