The Novel IGF-1R Inhibitor PB-020 Acts Synergistically with Anti-PD-1 and Mebendazole against Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Cell Viability Assay and Combined Drug Effects
2.3. Western Blotting (WB)
2.4. Mouse Xenograft Tumor Models
2.4.1. HT-29 Xenograft Model
2.4.2. MC38 Xenograft Model
2.5. RNA Sequencing
2.6. Statistical Analyses
3. Results
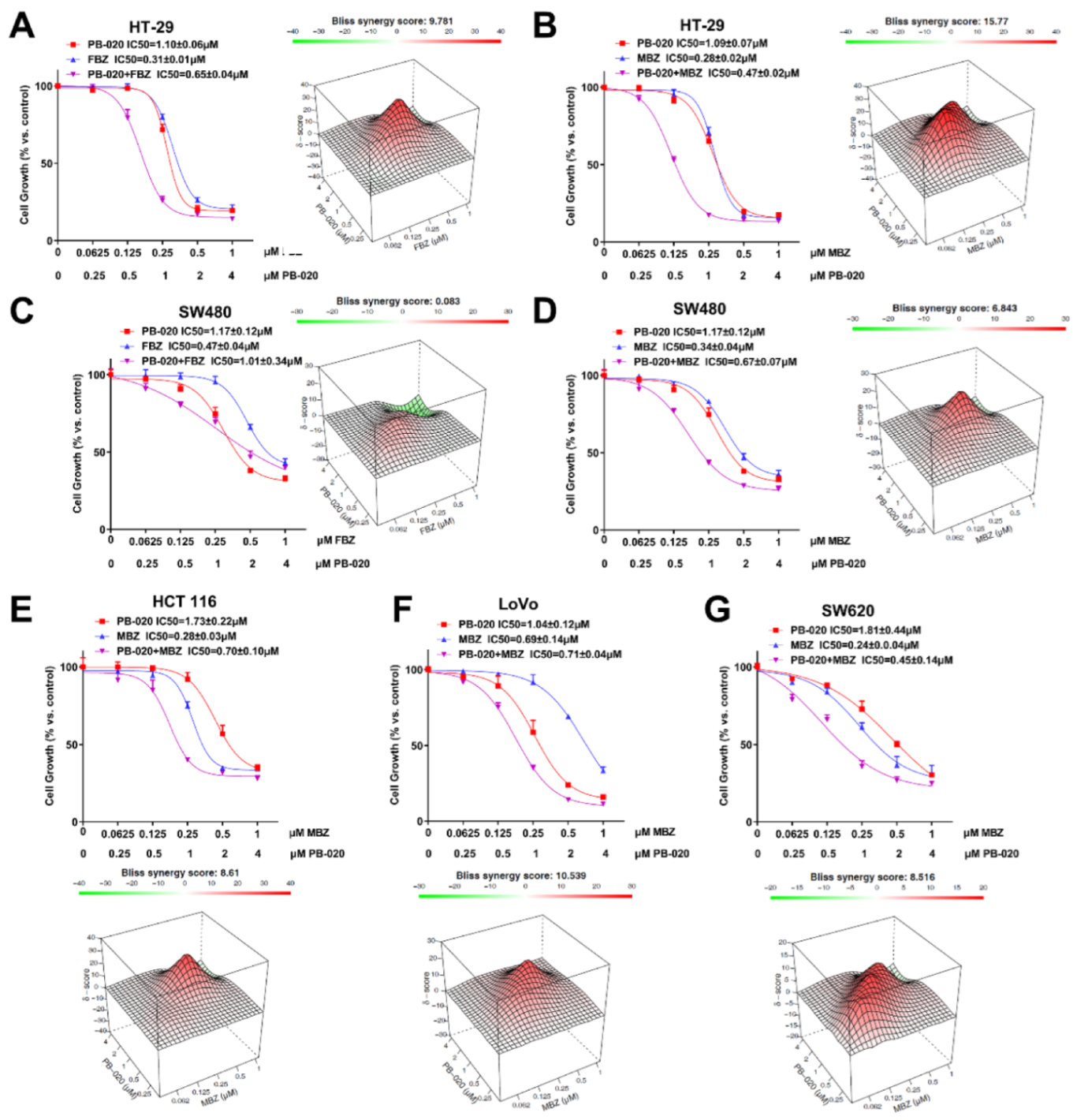
3.1. PB-020 in Combination with Multiple Anticancer Drugs Potently Reduced the Viability of CRC Cells
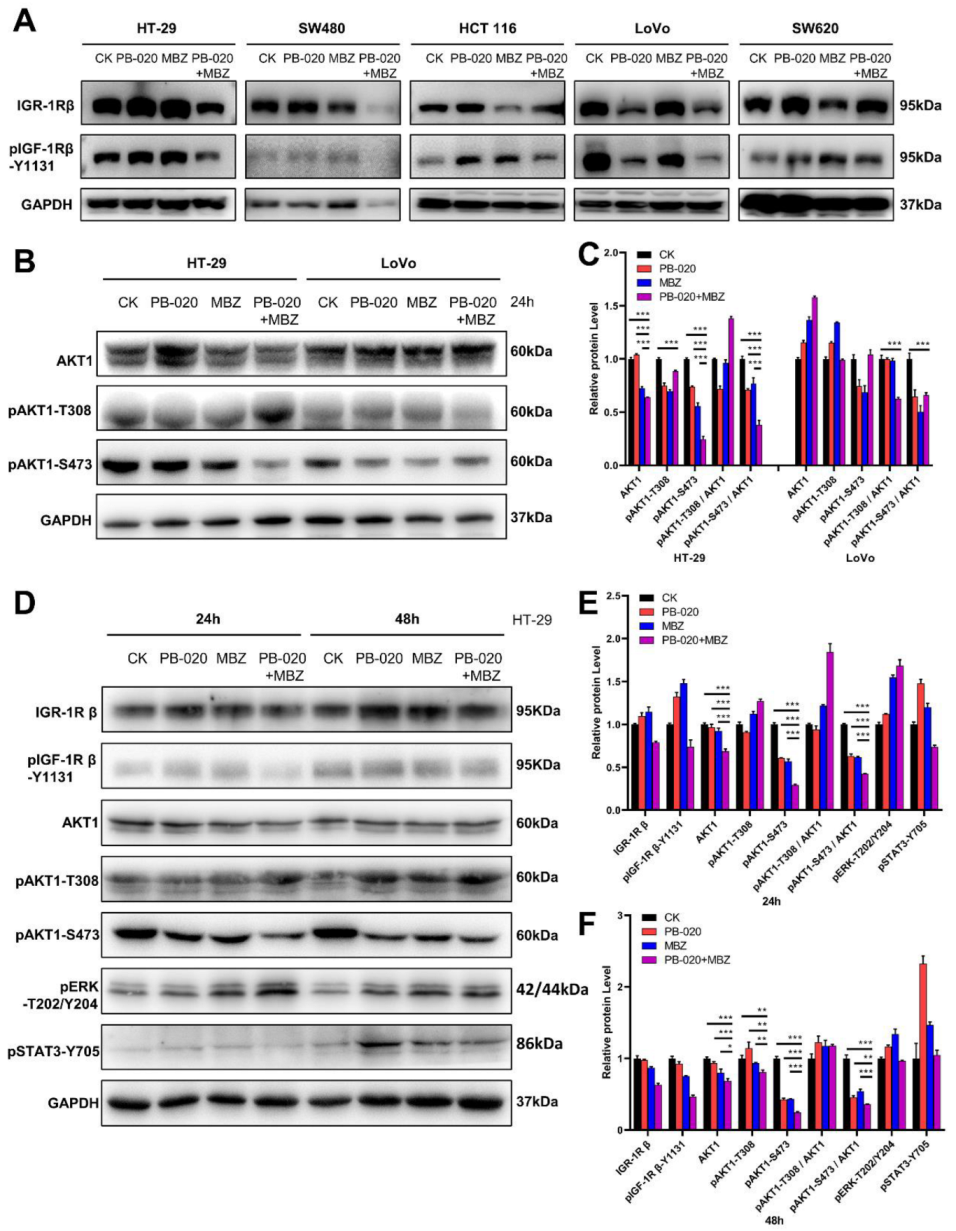
3.2. PB-020/MBZ Combination Potently Decreased the Level of pAKT1-S473
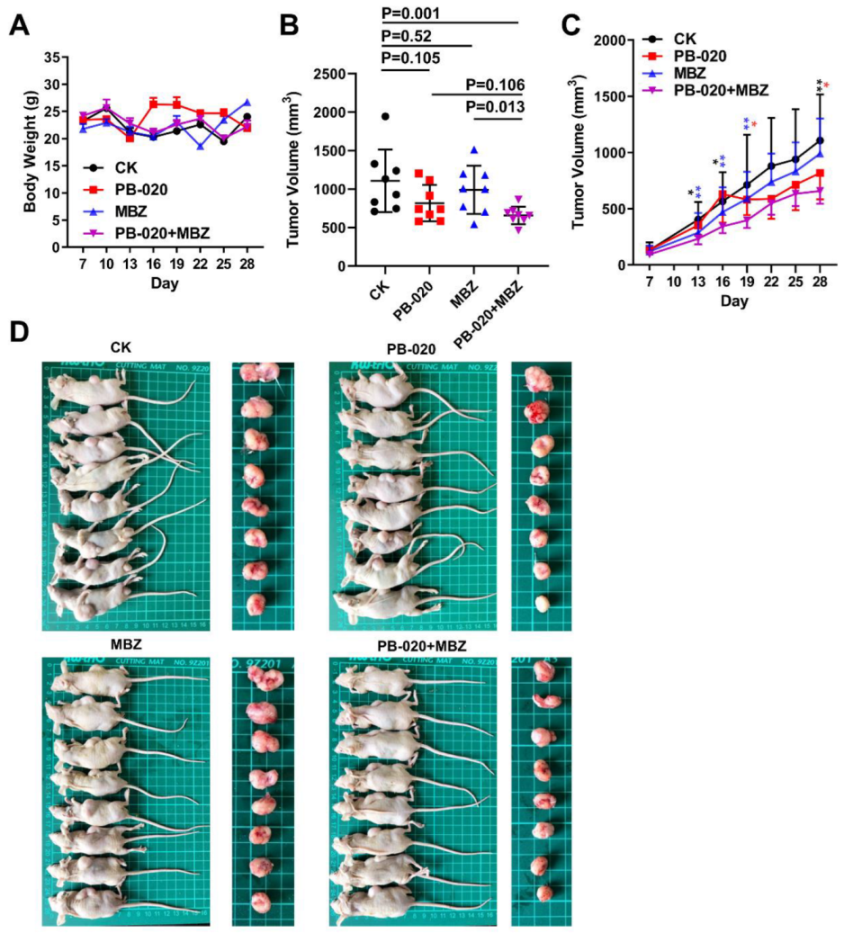
3.3. Orally Administered PB-020 in Combination with MBZ Inhibited Propagation of HT-29 Cell Xenografts In Vivo
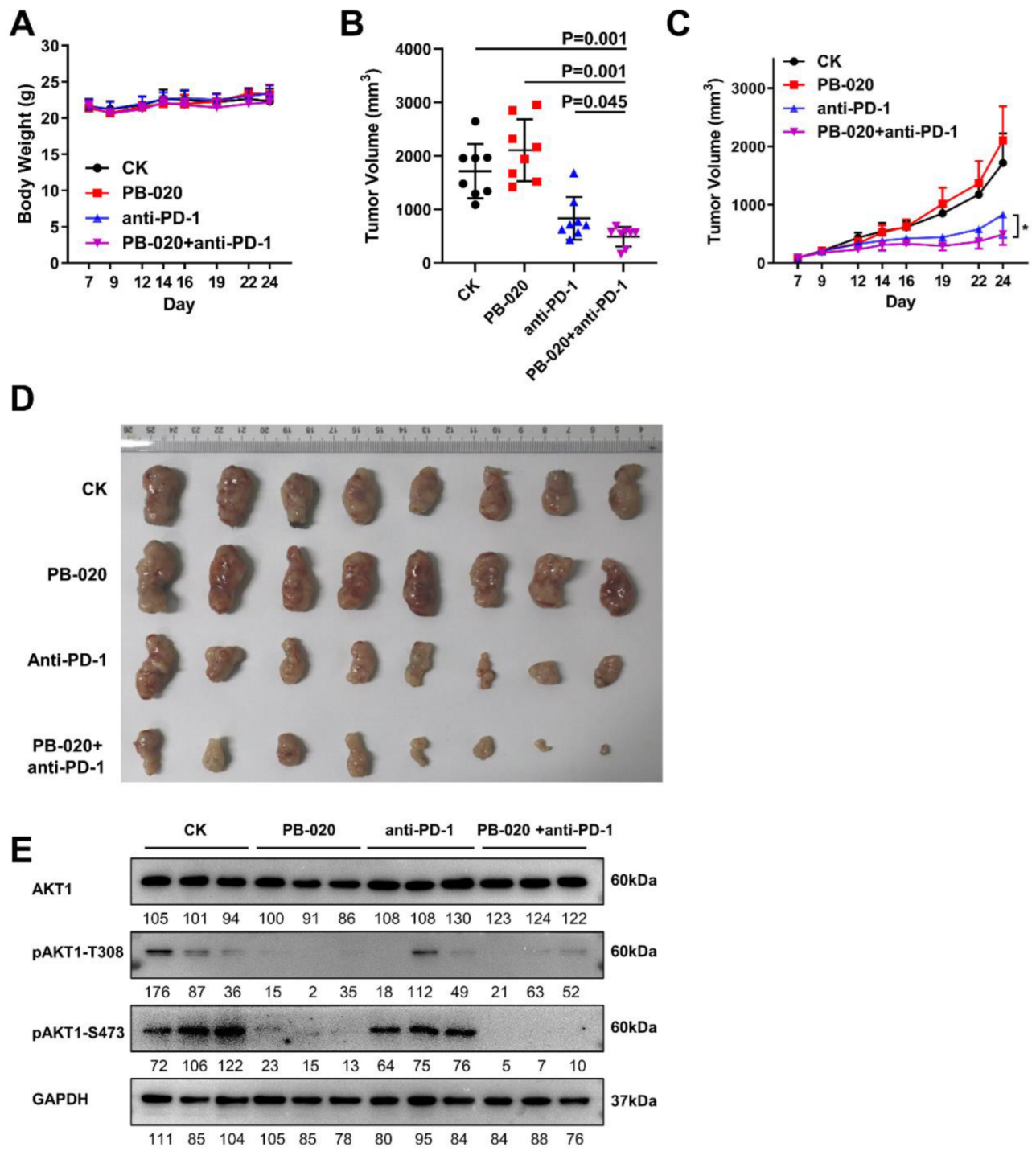
3.4. PB-020 in Combination with PD-1 Blockade Inhibited Propagation of MC38 Colorectal Cancer In Vivo
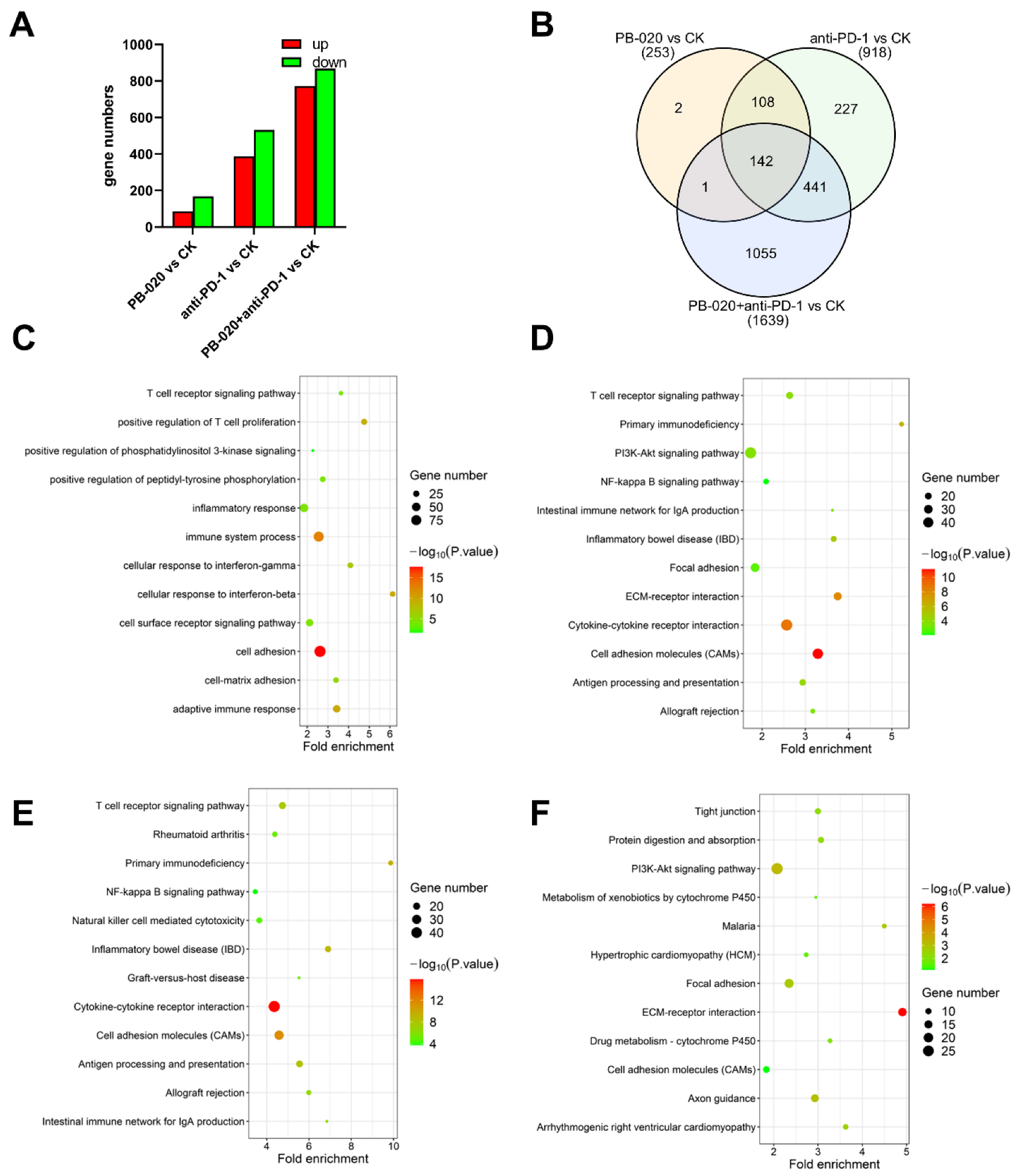
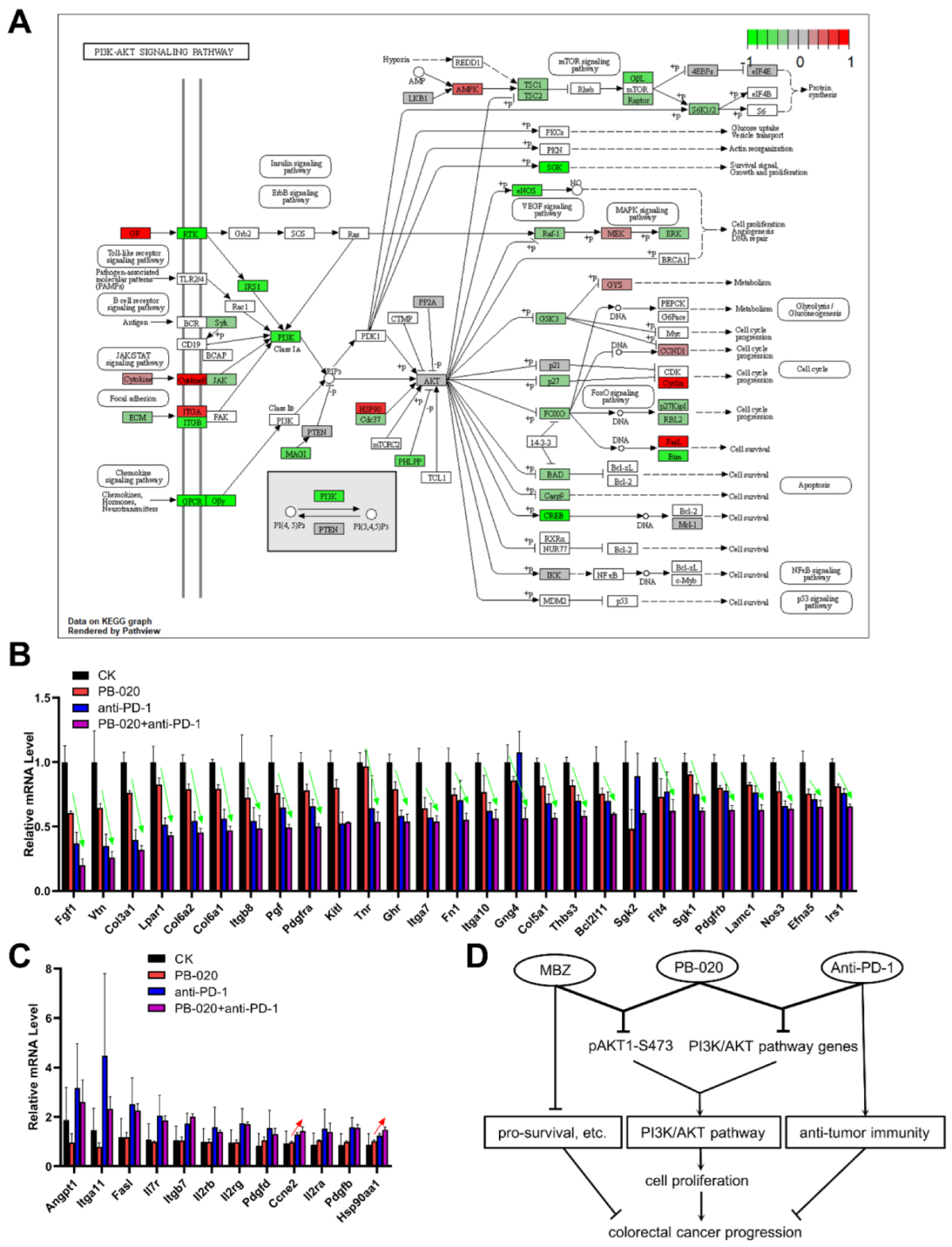
3.5. PB-020 in Combination with PD-1 Blockade Suppressed Transcription of PI3K/AKT Pathway Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Dual targeting of HER3 and EGFR in colorectal tumors might overcome anti-EGFR resistance. Crit. Rev. Oncol. Hematol. 2016, 101, 151–157. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Shen, B. Molecular Imaging of IGF-1R in Cancer. Mol. Imaging 2017, 16, 1536012117736648. [Google Scholar] [CrossRef]
- Shali, H.; Ahmadi, M.; Kafil, H.S.; Dorosti, A.; Yousefi, M. IGF1R and c-met as therapeutic targets for colorectal cancer. Biomed. Pharmacother. 2016, 82, 528–536. [Google Scholar] [CrossRef]
- Feng, X.; Aleem, E.; Lin, Y.; Axelson, M.; Larsson, O.; Stromberg, T. Multiple antitumor effects of picropodophyllin in colon carcinoma cell lines: Clinical implications. Int. J. Oncol. 2012, 40, 1251–1258. [Google Scholar] [CrossRef]
- Tian, A.; Kang, B.; Li, B.; Qiu, B.; Jiang, W.; Shao, F.; Gao, Q.; Liu, R.; Cai, C.; Jing, R.; et al. Oncogenic State and Cell Identity Combinatorially Dictate the Susceptibility of Cells within Glioma Development Hierarchy to IGF1R Targeting. Adv. Sci. 2020, 7, 2001724. [Google Scholar] [CrossRef]
- Florio, R.; Carradori, S.; Veschi, S.; Brocco, D.; Di Genni, T.; Cirilli, R.; Casulli, A.; Cama, A.; De Lellis, L. Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy. Pharmaceuticals 2021, 14, 372. [Google Scholar] [CrossRef]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef]
- Hanušová, V.; Skálová, L.; Králová, V.; Matoušková, P. The Effect of Flubendazole on Adhesion and Migration in SW480 and SW620 Colon Cancer Cells. Anticancer Agents Med. Chem. 2018, 18, 837–846. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, X.H. Integrative medicine in the era of cancer immunotherapy: Challenges and opportunities. J. Integr. Med. 2021, 19, 291–294. [Google Scholar] [CrossRef]
- Kong, M.Y.; Li, L.Y.; Lou, Y.M.; Chi, H.Y.; Wu, J.J. Chinese herbal medicines for prevention and treatment of colorectal cancer: From molecular mechanisms to potential clinical applications. J. Integr. Med. 2020, 18, 369–384. [Google Scholar] [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 pathway. Sci. Adv. 2020, 6, 2001724. [Google Scholar] [CrossRef]
- Yaghoubi, N.; Soltani, A.; Ghazvini, K.; Hassanian, S.M.; Hashemy, S.I. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed. Pharmacother. 2019, 110, 312–318. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, Y.; Zhao, S.; Mou, J.; Zeng, L.; Jiang, X.; Yang, C.; Huang, C.; Li, Y.; Lu, Y.; et al. Combination Foretinib and Anti-PD-1 Antibody Immunotherapy for Colorectal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 689727. [Google Scholar] [CrossRef]
- Lederer, S.; Dijkstra, T.M.H.; Heskes, T. Additive Dose Response Models: Defining Synergy. Front. Pharmacol. 2019, 10, 1384. [Google Scholar] [CrossRef]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- So, L.; Fruman, D.A. PI3K signalling in B- and T-lymphocytes: New developments and therapeutic advances. Biochem. J. 2012, 442, 465–481. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Tian, C.; He, Y.; Tian, Z.; Wan, Y.; Liu, T. Dual-target Inhibitors Based on BRD4: Novel Therapeutic Approaches for Cancer. Curr. Med. Chem. 2021, 28, 1775–1795. [Google Scholar] [CrossRef]
- Dallas, N.A.; Xia, L.; Fan, F.; Gray, M.J.; Gaur, P.; van Buren, G., 2nd; Samuel, S.; Kim, M.P.; Lim, S.J.; Ellis, L.M. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009, 69, 1951–1957. [Google Scholar] [CrossRef]
- Sekharam, M.; Zhao, H.; Sun, M.; Fang, Q.; Zhang, Q.; Yuan, Z.; Dan, H.C.; Boulware, D.; Cheng, J.Q.; Coppola, D. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/Bcl-x(L) pathway. Cancer Res. 2003, 63, 7708–7716. [Google Scholar]
- Zhang, Q.Y.; Wang, L.; Song, Z.Y.; Qu, X.J. Knockdown of type I insulin-like growth factor receptor inhibits human colorectal cancer cell growth and downstream PI3K/Akt, WNT/beta-catenin signal pathways. Biomed. Pharmacother. 2015, 73, 12–18. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zhu, J.; Zheng, H.; Chen, S.; Chen, L.; Yang, H.S. IGF-1R inhibition induces MEK phosphorylation to promote survival in colon carcinomas. Signal. Transduct. Target. Ther. 2020, 5, 153. [Google Scholar] [CrossRef]
- Mohmad-Saberi, S.E.; Hashim, Y.Z.; Mel, M.; Amid, A.; Ahmad-Raus, R.; Packeer-Mohamed, V. Metabolomics profiling of extracellular metabolites in CHO-K1 cells cultured in different types of growth media. Cytotechnology 2013, 65, 577–586. [Google Scholar] [CrossRef]
- Knuppel, A.; Fensom, G.K.; Watts, E.L.; Gunter, M.J.; Murphy, N.; Papier, K.; Perez-Cornago, A.; Schmidt, J.A.; Smith Byrne, K.; Travis, R.C.; et al. Circulating Insulin-like Growth Factor-I Concentrations and Risk of 30 Cancers: Prospective Analyses in UK Biobank. Cancer Res. 2020, 80, 4014–4021. [Google Scholar] [CrossRef]
- De Witt, M.; Gamble, A.; Hanson, D.; Markowitz, D.; Powell, C.; Al Dimassi, S.; Atlas, M.; Boockvar, J.; Ruggieri, R.; Symons, M. Repurposing Mebendazole as a Replacement for Vincristine for the Treatment of Brain Tumors. Mol. Med. 2017, 23, 50–56. [Google Scholar] [CrossRef]
- Blom, K.; Rubin, J.; Berglund, M.; Jarvius, M.; Lenhammar, L.; Parrow, V.; Andersson, C.; Loskog, A.; Fryknäs, M.; Nygren, P.; et al. Mebendazole-induced M1 polarisation of THP-1 macrophages may involve DYRK1B inhibition. BMC Res. Notes 2019, 12, 234. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 2009, 27, 83–117. [Google Scholar] [CrossRef]
- Marginean, E.C.; Melosky, B. Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part I-Colorectal Cancer: Microsatellite Instability, Testing, and Clinical Implications. Arch. Pathol. Lab. Med. 2018, 142, 17–25. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Hochster, H.S.; Bendell, J.C.; Cleary, J.M.; Foster, P.; Eckhardt, S.G. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)-high metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017, 35, 673. [Google Scholar] [CrossRef]
- Kim, Y.D.; Park, S.M.; Ha, H.C.; Lee, A.R.; Won, H.; Cha, H.; Cho, S.; Cho, J.M. HDAC Inhibitor, CG-745, Enhances the Anti-Cancer Effect of Anti-PD-1 Immune Checkpoint Inhibitor by Modulation of the Immune Microenvironment. J. Cancer 2020, 11, 4059–4072. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Esposito, D.L.; Aru, F.; Lattanzio, R.; Morgano, A.; Abbondanza, M.; Malekzadeh, R.; Bishehsari, F.; Valanzano, R.; Russo, A.; Piantelli, M.; et al. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS ONE 2012, 7, e36190. [Google Scholar] [CrossRef]
- Manzat Saplacan, R.M.; Balacescu, L.; Gherman, C.; Chira, R.I.; Craiu, A.; Mircea, P.A.; Lisencu, C.; Balacescu, O. The Role of PDGFs and PDGFRs in Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 4708076. [Google Scholar] [CrossRef]
- Fujino, S.; Miyoshi, N.; Ohue, M.; Takahashi, Y.; Yasui, M.; Hata, T.; Matsuda, C.; Mizushima, T.; Doki, Y.; Mori, M. Plateletderived growth factor receptorbeta gene expression relates to recurrence in colorectal cancer. Oncol. Rep. 2018, 39, 2178–2184. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Tang, H.; Jiang, Z.; You, L.; Liao, Y. Crosstalk between IGF-1R and other tumor promoting pathways. Curr. Pharm. Des. 2014, 20, 2912–2921. [Google Scholar] [CrossRef]
- Heske, C.M.; Yeung, C.; Mendoza, A.; Baumgart, J.T.; Edessa, L.D.; Wan, X.; Helman, L.J. The Role of PDGFR-beta Activation in Acquired Resistance to IGF-1R Blockade in Preclinical Models of Rhabdomyosarcoma. Transl. Oncol. 2016, 9, 540–547. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Zhang, X.; Wang, W.; She, S.; Chen, W.; Chen, C.; Wang, Y.; Pan, X.; Xu, O.; Wang, Y. The Novel IGF-1R Inhibitor PB-020 Acts Synergistically with Anti-PD-1 and Mebendazole against Colorectal Cancer. Cancers 2022, 14, 5747. https://doi.org/10.3390/cancers14235747
Kang B, Zhang X, Wang W, She S, Chen W, Chen C, Wang Y, Pan X, Xu O, Wang Y. The Novel IGF-1R Inhibitor PB-020 Acts Synergistically with Anti-PD-1 and Mebendazole against Colorectal Cancer. Cancers. 2022; 14(23):5747. https://doi.org/10.3390/cancers14235747
Chicago/Turabian StyleKang, Bo, Xiaobing Zhang, Weibing Wang, Shiqi She, Wenjie Chen, Cheng Chen, Yisha Wang, Xiaoyun Pan, Ouyuan Xu, and Yingjie Wang. 2022. "The Novel IGF-1R Inhibitor PB-020 Acts Synergistically with Anti-PD-1 and Mebendazole against Colorectal Cancer" Cancers 14, no. 23: 5747. https://doi.org/10.3390/cancers14235747
APA StyleKang, B., Zhang, X., Wang, W., She, S., Chen, W., Chen, C., Wang, Y., Pan, X., Xu, O., & Wang, Y. (2022). The Novel IGF-1R Inhibitor PB-020 Acts Synergistically with Anti-PD-1 and Mebendazole against Colorectal Cancer. Cancers, 14(23), 5747. https://doi.org/10.3390/cancers14235747





