Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunostaining of Periostin and Smad2/3
2.3. Immunohistochemical Determination
2.4. Statistical Analysis
3. Results
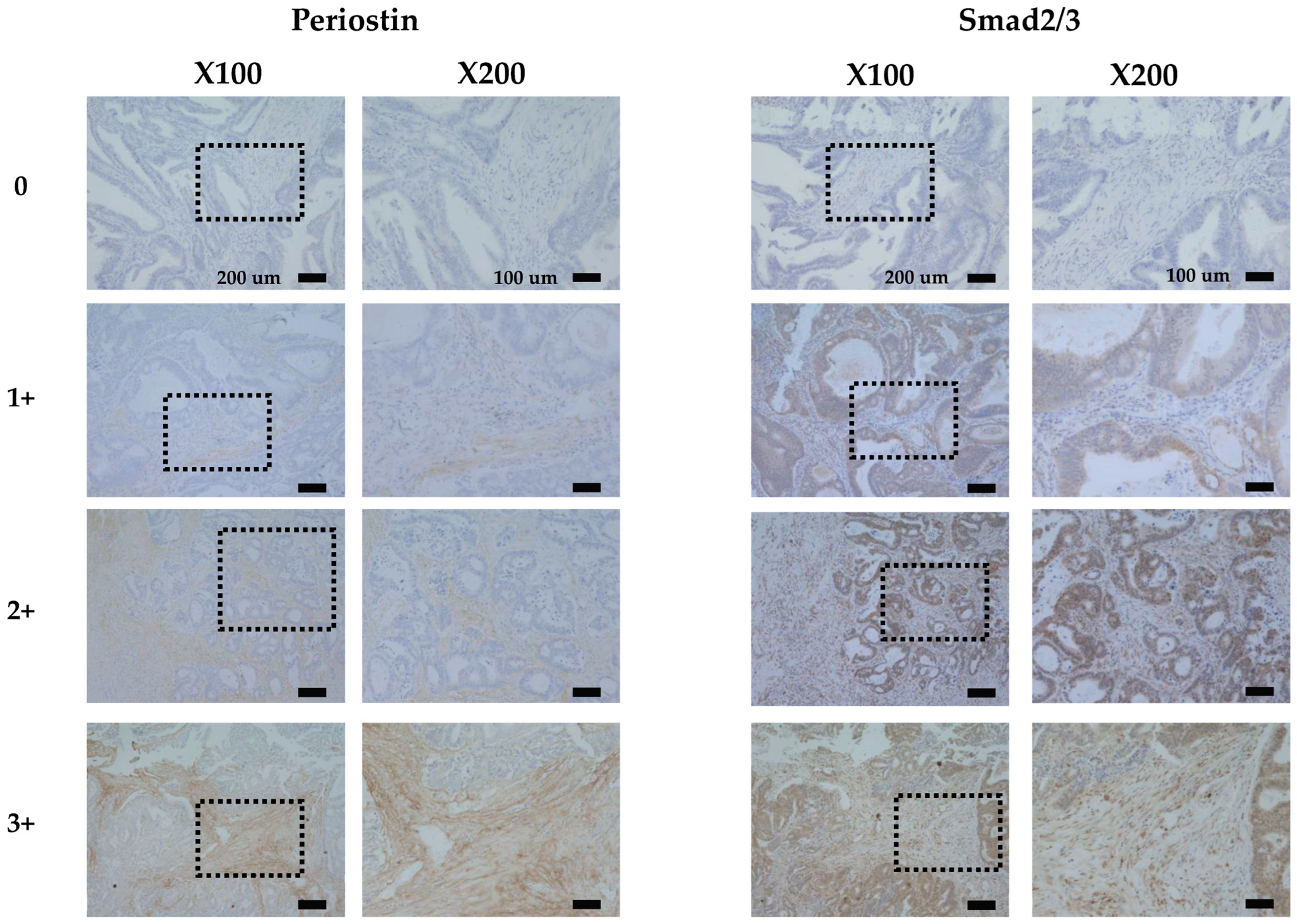
3.1. Immunostaining of Periostin and Smad2/3
3.2. The Relationship Between Clinicopathological Features and Periostin and Smad2/3 Expression
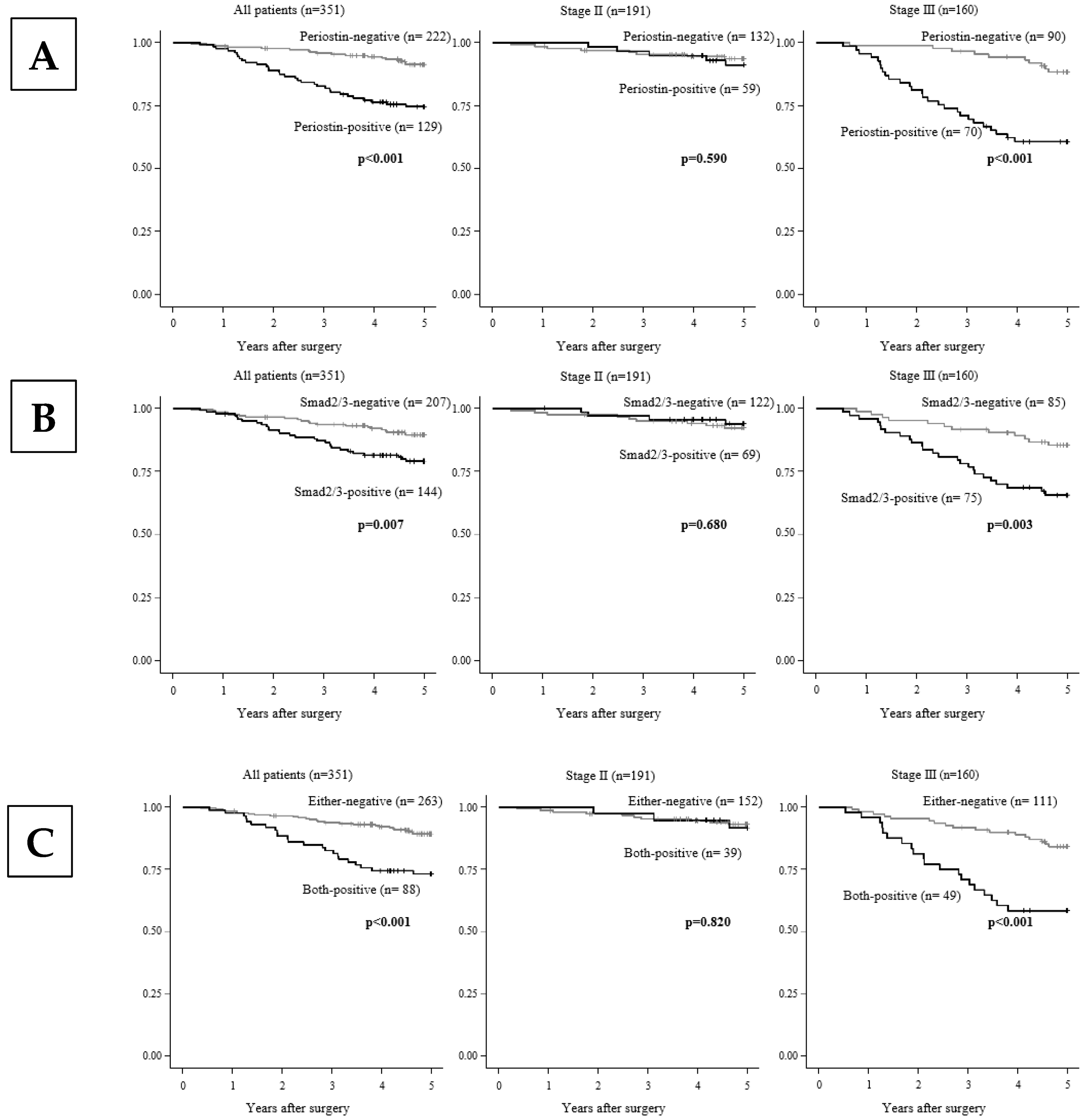
3.3. The Correlation Between the Patients’ Survival and the Periostin and/or Smad2/3 Expression
3.4. Univariate and Multivariate Analyses of Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 330145. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Kikuno, R.; Tezuka, K.-i.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tilman, G.; Mattiussi, M.; Brasseur, F.; van Baren, N.; Decottignies, A. Human periostin gene expression in normal tissues, tumors and melanoma: Evidences for periostin production by both stromal and melanoma cells. Mol. Cancer 2007, 6, 1–13. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Tischler, V.; Fritzsche, F.R.; Wild, P.J.; Stephan, C.; Seifert, H.-H.; Riener, M.-O.; Hermanns, T.; Mortezavi, A.; Gerhardt, J.; Schraml, P.; et al. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer 2010, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Ben, Q.W.; Jin, X.L.; Liu, J.; Cai, X.; Yuan, F.; Yuan, Y.Z. Periostin, a matrix specific protein, is associated with proliferation and invasion of pancreatic cancer. Oncol. Rep. 2011, 25, 709–716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, C.H.; Wang, W.; Lin, Y.; Qian, L.H.; Zhang, X.W.; Wang, Q.B.; Yu, L.K. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 2017, 8, 18746–18753. [Google Scholar] [CrossRef] [PubMed]
- Komura, M.; Wang, C.; Ito, S.; Kato, S.; Ueki, A.; Ebi, M.; Ogasawara, N.; Tsuzuki, T.; Kasai, K.; Kasugai, K. Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients. Int. J. Mol. Sci. 2024, 25, 2537. [Google Scholar] [CrossRef]
- Liu, B.; Wu, T.; Lin, B.; Liu, X.; Liu, Y.; Song, G.; Fan, C.; Ouyang, G. Periostin colon TGF-β loop contributes to tumour-stroma crosstalk in liver metastatic outgrowth of colorectal cancer. Br. J. Cancer 2024, 130, 358–368. [Google Scholar] [CrossRef]
- Ueki, A.; Komura, M.; Koshino, A.; Wang, C.; Nagao, K.; Homochi, M.; Tsukada, Y.; Ebi, M.; Ogasawara, N.; Tsuzuki, T. Stromal POSTN Enhances Motility of Both Cancer and Stromal Cells and Predicts Poor Survival in Colorectal Cancer. Cancers 2023, 15, 606. [Google Scholar] [CrossRef]
- Michaylira, C.Z.; Wong, G.S.; Miller, C.G.; Gutierrez, C.M.; Nakagawa, H.; Hammond, R.; Klein-Szanto, A.J.; Lee, J.-S.; Kim, S.B.; Herlyn, M. Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res. 2010, 70, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Mochizuki, S.; Horiuchi, K.; Tsujimoto, H.; Kouzu, K.; Kishi, Y.; Okada, Y.; Ueno, H. Periostin derived from cancer-associated fibroblasts promotes esophageal squamous cell carcinoma progression via ADAM17 activation. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166669. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Nakamura, I.; Dhanasekaran, R.; Iguchi, E.; Tolosa, E.J.; Romecin, P.A.; Vera, R.E.; Almada, L.L.; Miamen, A.G.; Chaiteerakij, R. Transcriptional Induction of Periostin by a Sulfatase 2–TGFβ1–SMAD Signaling Axis Mediates Tumor Angiogenesis in Hepatocellular Carcinoma. Cancer Res. 2017, 77, 632–645. [Google Scholar] [CrossRef]
- Dzieran, J.; Fabian, J.; Feng, T.; Coulouarn, C.; Ilkavets, I.; Kyselova, A.; Breuhahn, K.; Dooley, S.; Meindl-Beinker, N.M. Comparative analysis of TGF-β/Smad signaling dependent cytostasis in human hepatocellular carcinoma cell lines. PLoS ONE 2013, 8, e72252. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-B.; Fu, X.; Liu, Y.; Wang, Z.-C.; Liu, S.-Y.; Li, Y.-C.; Sun, H.-J. Disrupted cardiac fibroblast BCAA catabolism contributes to diabetic cardiomyopathy via a periostin/NAP1L2/SIRT3 axis. Cell. Mol. Biol. Lett. 2023, 28, 93. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Yang, Y.; Yang, S.; Dong, Z.; Du, L.; Wang, L.; Wang, C. Periostin Expression and Its Prognostic Value for Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 12108–12118. [Google Scholar] [CrossRef]
- Oh, H.J.; Bae, J.M.; Wen, X.-Y.; Cho, N.-Y.; Kim, J.H.; Kang, G.H. Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer. JPTM 2017, 51, 306–313. [Google Scholar] [CrossRef]
- Sueyama, T.; Kajiwara, Y.; Mochizuki, S.; Shimazaki, H.; Shinto, E.; Hase, K.; Ueno, H. Periostin as a key molecule defining desmoplastic environment in colorectal cancer. Virchows Arch. 2021, 478, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Singsuksawat, E.; Sumransub, N.; Pongpaibul, A.; Trakarnsanga, A.; Thuwajit, P.; Thuwajit, C. Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT-dependent pathway in colorectal cancer cell migration. J. Cell. Mol. Med. 2020, 24, 12421–12432. [Google Scholar] [CrossRef]
- Moniuszko, T.; Wincewicz, A.; Koda, M.; Domysławska, I.; Sulkowski, S. Role of periostin in esophageal, gastric and colon cancer. Oncol. Lett. 2016, 12, 783–787. [Google Scholar] [CrossRef]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and Characterization of a Novel Protein, Periostin, with Restricted Expression to Periosteum and Periodontal Ligament and Increased Expression by Transforming Growth Factor β. J. Bone Miner. Res. 2009, 14, 1239–1249. [Google Scholar] [CrossRef]
- Brown, K.A.; Pietenpol, J.A.; Moses, H.L. A tale of two proteins: Differential roles and regulation of Smad2 and Smad3 in TGF-β signaling. J. Cell. Biochem. 2007, 101, 9–33. [Google Scholar] [CrossRef]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β signaling in cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Huang, Z.; Byrd, O.; Tan, S.; Hu, K.; Knight, B.; Lo, G.; Taylor, L.; Wu, Y.; Berchuck, A.; Murphy, S.K. Periostin facilitates ovarian cancer recurrence by enhancing cancer stemness. Sci. Rep. 2023, 13, 21382. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.-F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Waisberg, J.; Viana, L.D.S.; Junior, R.J.A.; Silva, S.R.M.; Denadai, M.V.A.; Margeotto, F.B.; De Souza, C.S.; Matos, D. Overexpression of the ITGAV gene is associated with progression and spread of colorectal cancer. Anticancer Res. 2014, 34, 5599–5607. [Google Scholar]
- Denadai, M.V.; Viana, L.S.; Affonso Jr, R.J.; Silva, S.R.; Oliveira, I.D.; Toledo, S.R.; Matos, D. Expression of integrin genes and proteins in progression and dissemination of colorectal adenocarcinoma. BMC Clin. Pathol. 2013, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
| All Cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinicopathologic Features | Periostin | p-Value | Smad2/3 | p-Value | Co-Expression | p-Value | ||||
| Positive (n = 129) | Negative (n = 222) | Positive (n = 144) | Negative (n = 207) | Both-Positive (n = 88) | Either-Negative (n = 263) | |||||
| Age | <65 | 52 (38%) | 85 (62%) | 0.734 | 53 (39%) | 84 (61%) | 0.506 | 31 (23%) | 106 (77%) | 0.449 |
| ≥65 | 77 (36%) | 137 (64%) | 91 (43%) | 123 (57%) | 57 (27%) | 157 (73%) | ||||
| Gender | Female | 56 (33%) | 116 (67%) | 0.122 | 73 (42%) | 99 (58%) | 0.664 | 38 (22%) | 134 (78%) | 0.22 |
| Male | 73 (41%) | 106 (59%) | 71 (40%) | 108 (60%) | 50 (28%) | 129 (72%) | ||||
| Histological type | Well | 114 (35%) | 209 (65%) | 0.066 | 129 (40%) | 194 (60%) | 0.167 | 77 (24%) | 246 (76%) | 0.108 |
| Poor | 15 (54%) | 13 (46%) | 15 (54%) | 13 (46%) | 11 (39%) | 17 (61%) | ||||
| T stage | T1 | 72 (32%) | 155 (68%) | 0.011 | 94 (41%) | 133 (59%) | 0.91 | 52 (23%) | 175 (77%) | 0.246 |
| T2, T3, and T4 | 57 (46%) | 67 (54%) | 50 (40%) | 74 (60%) | 36 (29%) | 88 (71%) | ||||
| Lymph node metastasis | Negative | 59 (31%) | 133 (69%) | 0.011 | 70 (36%) | 122 (64%) | 0.064 | 39 (20%) | 153 (80%) | 0.026 |
| Positive | 70 (44%) | 89 (56%) | 74 (47%) | 85 (53%) | 49 (31%) | 110 (69%) | ||||
| Lymphatic invasion | Negative | 28 (29%) | 68 (71%) | 0.082 | 36 (38%) | 60 (62%) | 0.466 | 18 (19%) | 78 (81%) | 0.099 |
| Positive | 101 (40%) | 154 (60%) | 108 (42%) | 147 (58%) | 70 (27%) | 185 (73%) | ||||
| Venous invasion | Negative | 92 (33%) | 186 (67%) | 0.006 | 108 (39%) | 170 (61%) | 0.111 | 62 (22%) | 216 (78%) | 0.023 |
| Positive | 37 (51%) | 36 (49%) | 36 (49%) | 37 (51%) | 26 (36%) | 47 (64%) | ||||
| Location | Left | 81 (34%) | 156 (66%) | 0.158 | 99 (42%) | 138 (58%) | 0.729 | 56 (24%) | 181 (76%) | 0.43 |
| Right | 48 (42%) | 66 (58%) | 45 (39%) | 69 (61%) | 32 (28%) | 82 (72%) | ||||
| Relapse | Negative | 88 (33%) | 179 (67%) | 0.01 | 101 (38%) | 166 (62%) | 0.032 | 58 (22%) | 209 (78%) | 0.014 |
| Positive | 41 (49%) | 43 (51%) | 43 (51%) | 41 (49%) | 30 (36%) | 54 (64%) | ||||
| Stage | 2 | 59 (31%) | 132 (69%) | 0.015 | 69 (36%) | 122 (64%) | 0.049 | 39 (20%) | 152 (80%) | 0.035 |
| 3 | 70 (44%) | 90 (56%) | 75 (47%) | 85 (53%) | 49 (31%) | 111 (69%) | ||||
| Periostin | Negative | 56 (25%) | 166 (75%) | <0.001 | ||||||
| Positive | 88 (68%) | 41 (32%) | ||||||||
| Stage 2 | ||||||||||
| Clinicopathologic Features | Periostin | p-value | Smad2/3 | p-value | Co-Expression | p-value | ||||
| Positive (n = 59) | Negative (n = 132) | Positive (n = 69) | Negative (n = 122) | Both-Positive (n = 39) | Either-Negative (n = 152) | |||||
| Age | <65 | 23 (32%) | 50 (68%) | 1 | 24 (33%) | 49 (67%) | 0.536 | 12 (16%) | 61 (84%) | 0.356 |
| ≥65 | 36 (31%) | 82 (69%) | 45 (38%) | 73 (62%) | 27 (23%) | 91 (77%) | ||||
| Gender | Female | 23 (26%) | 65 (74%) | 0.211 | 35 (40%) | 53 (60%) | 0.366 | 15 (17%) | 73 (83%) | 0.368 |
| Male | 36 (35%) | 67 (65%) | 34 (33%) | 69 (67%) | 24 (23%) | 79 (77%) | ||||
| Histological type | Well | 56 (30%) | 128 (70%) | 0.679 | 67 (36%) | 117 (64%) | 1 | 38 (21%) | 146 (79%) | 1 |
| Poor | 3 (43%) | 4 (57%) | 2 (29%) | 5 (71%) | 1 (14%) | 6 (86%) | ||||
| T stage | T1 | 37 (28%) | 94 (72%) | 0.244 | 50 (38%) | 81 (62%) | 0.421 | 26 (20%) | 105 (80%) | 0.847 |
| T2, T3, and T4 | 22 (37%) | 38 (63%) | 19 (32%) | 41 (68%) | 13 (22%) | 47 (78%) | ||||
| Lymph node metastasis | Negative | 59 (31%) | 131 (69%) | 1 | 69 (36%) | 121 (64%) | 1 | 39 (21%) | 151 (79%) | 1 |
| Positive | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | ||||
| Lymphatic invasion | Negative | 22 (28%) | 56 (72%) | 0.528 | 27 (35%) | 51 (65%) | 0.761 | 13 (17%) | 65 (83%) | 0.362 |
| Positive | 37 (33%) | 76 (67%) | 42 (37%) | 71 (63%) | 26 (23%) | 87 (77%) | ||||
| Venous invasion | Negative | 48 (30%) | 113 (70%) | 0.52 | 59 (37%) | 102 (63%) | 0.837 | 33 (20%) | 128 (80%) | 1 |
| Positive | 11 (37%) | 19 (63%) | 10 (33%) | 20 (67%) | 6 (20%) | 24 (80%) | ||||
| Location | Left | 33 (27%) | 91 (73%) | 0.101 | 46 (37%) | 78 (63%) | 0.754 | 24 (19%) | 100 (81%) | 0.707 |
| Right | 26 (39%) | 41 (61%) | 23 (34%) | 44 (66%) | 15 (22%) | 52 (78%) | ||||
| Relapse | Negative | 52 (31%) | 115 (69%) | 1 | 60 (36%) | 107 (64%) | 1 | 33 (20%) | 134 (80%) | 0.589 |
| Positive | 7 (29%) | 17 (71%) | 9 (38%) | 15 (62%) | 6 (25%) | 18 (75%) | ||||
| Periostin | Negative | 30 (23%) | 102 (77%) | <0.001 | ||||||
| Stage 3 | ||||||||||
| Clinicopathologic Features | Periostin | p-value | Smad2/3 | p-value | Co-Expression | p-value | ||||
| Positive (n = 70) | Negative (n = 90) | Positive (n = 75) | Negative (n = 85) | Both-Positive (n = 49) | Either-Negative (n = 111) | |||||
| Age | <65 | 29 (45%) | 35 (55%) | 0.748 | 29 (45%) | 35 (55%) | 0.872 | 19 (30%) | 45 (70%) | 0.863 |
| ≥65 | 41 (43%) | 55 (57%) | 46 (48%) | 50 (52%) | 30 (31%) | 66 (69%) | ||||
| Gender | Female | 33 (39%) | 51 (61%) | 0.265 | 38 (45%) | 46 (55%) | 0.751 | 23 (27%) | 61 (73%) | 0.393 |
| Male | 37 (49%) | 39 (51%) | 37 (49%) | 39 (51%) | 26 (34%) | 50 (66%) | ||||
| Histological type | Well | 58 (42%) | 81 (58%) | 0.239 | 62 (45%) | 77 (55%) | 0.163 | 39 (28%) | 100 (72%) | 0.08 |
| Poor | 12 (57%) | 9 (43%) | 13 (62%) | 8 (38%) | 10 (48%) | 11 (52%) | ||||
| T stage | T1 | 35 (36%) | 61 (64%) | 0.034 | 44 (46%) | 52 (54%) | 0.75 | 26 (27%) | 70 (73%) | 0.294 |
| T2, T3, and T4 | 35 (55%) | 29 (45%) | 31 (48%) | 33 (52%) | 23 (36%) | 41 (64%) | ||||
| Lymph node metastasis | Negative | 0 (0%) | 2 (100%) | 0.505 | 1 (50%) | 1 (50%) | 1 | 0 (0%) | 2 (100%) | 1 |
| Positive | 70 (44%) | 88 (56%) | 74 (47%) | 84 (53%) | 49 (31%) | 109 (69%) | ||||
| Lymphatic invasion | Negative | 6 (33%) | 12 (67%) | 0.452 | 9 (50%) | 9 (50%) | 0.807 | 5 (28%) | 13 (72%) | 1 |
| Positive | 64 (45%) | 78 (55%) | 66 (46%) | 76 (54%) | 44 (31%) | 98 (69%) | ||||
| Venous invasion | Negative | 44 (38%) | 73 (62%) | 0.012 | 49 (42%) | 68 (58%) | 0.049 | 29 (25%) | 88 (75%) | 0.012 |
| Positive | 26 (60%) | 17 (40%) | 26 (60%) | 17 (40%) | 20 (47%) | 23 (53%) | ||||
| Location | Left | 48 (42%) | 65 (58%) | 0.727 | 53 (47%) | 60 (53%) | 1 | 32 (28%) | 81 (72%) | 0.35 |
| Right | 22 (47%) | 25 (53%) | 22 (47%) | 25 (53%) | 17 (36%) | 30 (64%) | ||||
| Relapse | Negative | 36 (36%) | 64 (64%) | 0.014 | 41 (41%) | 59 (59%) | 0.072 | 25 (25%) | 75 (75%) | 0.053 |
| Positive | 34 (57%) | 26 (43%) | 34 (57%) | 26 (43%) | 24 (40%) | 36 (60%) | ||||
| Periostin | Negative | 26 (29%) | 64 (71%) | <0.001 | ||||||
| Positive | 49 (70%) | 21 (30%) | ||||||||
| All Cases | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Parameter | Hazard Ratio | 95%CI | p-Value | Hazard Ratio | 95%CI | p-Value |
| Periostin | 2.949 | 1.851–4.697 | <0.001 | 2.518 | 1.475–4.299 | <0.001 |
| Smad2/3 | 2.214 | 1.389–3.53 | <0.001 | 1.291 | 0.757–2.201 | 0.348 |
| Age | 1.805 | 1.091–2.987 | 0.021 | 2.045 | 1.222–3.42 | 0.006 |
| Gender | 1.031 | 0.653–1.63 | 0.895 | |||
| Histological type | 2.91 | 1.597–5.302 | <0.001 | 2.76 | 1.484–5.133 | 0.001 |
| T stage | 1.233 | 0.773–1.968 | 0.38 | |||
| Lymph node metastasis | 2.422 | 1.507–3.893 | <0.001 | 1.832 | 1.104–3.042 | 0.019 |
| Lymphatic invasion | 2.317 | 1.22–4.398 | 0.01 | 1.639 | 0.834–3.219 | 0.152 |
| Venous invasion | 1.91 | 1.167–3.127 | 0.01 | 1.215 | 0.721–2.049 | 0.465 |
| Location | 1.196 | 0.738–1.938 | 0.467 | |||
| Stage 2 | ||||||
| Parameter | Hazard Ratio | 95%CI | p-Value | |||
| Periostin | 2.336 | 1.057–5.163 | 0.036 | |||
| Smad2/3 | 1.507 | 0.661–3.438 | 0.33 | |||
| Age | 1.371 | 0.595–3.161 | 0.459 | |||
| Gender | 0.625 | 0.282–1.385 | 0.247 | |||
| Histological type | 1.928 | 0.45–8.262 | 0.377 | |||
| T stage | 0.862 | 0.359–2.073 | 0.74 | |||
| Lymph node metastasis | 0 | 0-Inf | 0.997 | |||
| Lymphatic invasion | 1.161 | 0.51–2.64 | 0.722 | |||
| Venous invasion | 2.029 | 0.807–5.097 | 0.132 | |||
| Location | 1.005 | 0.432–2.338 | 0.99 | |||
| Stage 3 | ||||||
| Parameter | Hazard Ratio | 95%CI | p-Value | Hazard Ratio | 95%CI | p-Value |
| Periostin | 2.964 | 1.654–5.313 | <0.001 | 2.681 | 1.381–5.207 | 0.004 |
| Smad2/3 | 2.382 | 1.33–4.265 | 0.003 | 1.454 | 0.749–2.823 | 0.268 |
| Age | 2.259 | 1.197–4.264 | 0.012 | 2.55 | 1.341–4.852 | 0.004 |
| Gender | 1.517 | 0.864–2.665 | 0.147 | |||
| Histological type | 2.456 | 1.254–4.811 | 0.009 | 2.868 | 1.449–5.676 | 0.002 |
| T stage | 1.307 | 0.744–2.297 | 0.351 | |||
| Lymph node metastasis | 0.329 | 0.08–1.364 | 0.126 | |||
| Lymphatic invasion | 3.538 | 0.859–14.569 | 0.08 | |||
| Venous invasion | 1.524 | 0.845–2.747 | 0.161 | |||
| Location | 1.503 | 0.834–2.71 | 0.175 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Wang, Q.; Kanei, S.; Kawabata, K.; Nishikubo, H.; Aoyama, R.; Zhu, Z.; Imanishi, D.; Sakuma, T.; Maruo, K.; et al. Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3. Cancers 2025, 17, 551. https://doi.org/10.3390/cancers17030551
Fan C, Wang Q, Kanei S, Kawabata K, Nishikubo H, Aoyama R, Zhu Z, Imanishi D, Sakuma T, Maruo K, et al. Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3. Cancers. 2025; 17(3):551. https://doi.org/10.3390/cancers17030551
Chicago/Turabian StyleFan, Canfeng, Qiang Wang, Saki Kanei, Kyoka Kawabata, Hinano Nishikubo, Rika Aoyama, Zhonglin Zhu, Daiki Imanishi, Takashi Sakuma, Koji Maruo, and et al. 2025. "Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3" Cancers 17, no. 3: 551. https://doi.org/10.3390/cancers17030551
APA StyleFan, C., Wang, Q., Kanei, S., Kawabata, K., Nishikubo, H., Aoyama, R., Zhu, Z., Imanishi, D., Sakuma, T., Maruo, K., Tsujio, G., Yamamoto, Y., Fukuoka, T., & Yashiro, M. (2025). Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3. Cancers, 17(3), 551. https://doi.org/10.3390/cancers17030551






