Simple Summary
Despite advances in treatment, the treatment outcome of pancreatic cancer still remains poor. Local progression can be a significant cause of several morbidities in pancreatic cancer, and dose escalation is needed. Stereotactic body proton beam therapy (SBPT) can give higher dose while minimizing dose at organ at risk with Bragg peak. The purpose of the present study was to investigate the feasibility of SBPT in pancreatic cancer. SBPT, administered in five fractions of a total 50–60 GyRBE, was performed mostly after induction chemotherapy. Grade 3 or higher gastroduodenal toxicities occurred in 6.1% of cases. The 2-year overall survival and local control rates were 67.6% and 73.0%. SBPT showed favourable treatment outcomes and treatment-related toxicities. It could be a promising alternative to radical surgery.
Abstract
Background/Purpose: This study aimed to evaluate the clinical outcomes of stereotactic body proton beam therapy (SBPT) for pancreatic cancer. Methods: This retrospective study included 49 patients who underwent SBPT for pancreatic cancer between 2017 and 2020. Survival outcomes, bowel-related toxicities, and failure patterns were analysed. SBPT was performed after induction chemotherapy in 44 (89.8%) patients. The dose-fractionation scheme included 60 gray (Gy) relative biological effectiveness (RBE) in five fractions (n = 42, 85.7%) and 50 GyRBE in five fractions (n = 7, 14.3%). The median follow-up was 16.3 months (range, 1.8–45.0 months). Results: During follow-up, the best responses were complete response, partial response, and stable disease in four (8.2%), 13 (26.5%), and 31 (63.3%) patients, respectively. The 2-year overall survival, progression-free survival, and local control (LC) rates were 67.6%, 38.0%, and 73.0%, respectively. Grade ≥ 3 gastroduodenal (GD) toxicity occurred in three (6.1%) patients. Among them, one patient underwent endoscopic haemostasis. The other two patients received surgical management. They were followed up without disease progression for >30 months after SBPT. Overall, there was no significant dosimetric difference between the grade ≥ 2 and lower toxicity groups. Conclusions: SBPT provides relatively high LC rates with acceptable toxicities in pancreatic cancer.
1. Introduction
Pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide and is predicted to become the third leading cause by 2025 [1]. Its prognosis remains poor, with a 5-year survival rate of approximately 10%, despite advances in pancreatic cancer treatment [2,3].
Currently, several modalities are used to treat pancreatic cancer. Although surgical resection is the backbone of management as the only curative treatment, treatment failure is common, even in early disease. Therefore, neoadjuvant and/or adjuvant therapies are recommended. Furthermore, in patients with unresectable disease, systemic chemotherapy with or without radiotherapy (RT), is generally adopted [4].
However, the systemic recurrence rate is approximately 50%, and the 5-year overall survival (OS) rate is only 25%, even after surgical resection with (neo) adjuvant therapy [5]. In locally advanced pancreatic cancer (LAPC), the distant metastasis rate within 3 months of diagnosis, even during initial treatment, is 30–50%. These facts emphasise the need for intensive systemic therapy. However, local progression (LP) is an important issue that cannot be overlooked. The local recurrence rate after curative surgical resection with (neo)adjuvant therapy has been reported to be 20–60% [6]. Additionally, systemic chemotherapy alone has been reported to have a higher LP rate, and 30–40% patients with pancreatic cancer die from locally progressive disease (PD) without distant metastases. Furthermore, LP can be a significant cause of several morbidities including biliary and/or gastroduodenal (GD) obstruction, bleeding, and perforation [7]. These findings also highlight the importance of combining RT with systemic therapy, thereby maximising local control (LC) in the treatment of pancreatic cancer, especially in unresectable disease [8].
The pancreas is surrounded by radiosensitive organs such as the duodenum, stomach, and small bowel. This makes it difficult to deliver sufficient radiation doses to pancreatic tumours. With advances in RT techniques, it has become possible to lower the dose to the surrounding organs and administer a higher dose to the pancreas. However, a high degree of caution is required for high dose administration to the pancreas, while simultaneously ensuring lower doses to other regions, and the treatment results need to be improved [9,10,11].
Stereotactic body RT (SBRT), which delivers a high dose in a few fractions, can shorten the treatment course, increase quality of life, minimize the interruption of intensive systemic therapy, and deliver a highly conformal ablative dose to the target with a sharp dose fall-off. This results in minimal exposure of at-risk organs. Proton beam therapy (PBT) offers a better dosimetric advantage. Protons travel a finite distance into the tissue determined by their energy and release most of that energy at a well-defined depth, forming a Bragg peak. Beyond the Bragg peak, there is little or no additional deposited dose. Consequently, radiation exposure to surrounding normal organs can be minimised [12]. Several studies have demonstrated that the radiation exposure of normal organs including liver, duodenum, and small bowel can be reduced using proton beam than photon beam in the treatment planning [12,13,14,15,16,17,18]. Based on the advantages of PBT, the application of stereotactic body PBT (SBPT) with chemotherapy is expected to improve LC in the management of pancreatic cancer. However, to date, limited studied have evaluated the treatment outcomes of SBPT in pancreatic cancer.
Therefore, we aimed to analyse the efficacy and safety of SBPT in pancreatic cancer. The relationship between GD toxicities and dose—volume histograms (DVHs) was analysed.
2. Materials and Methods
2.1. Patients
We retrospectively reviewed the medical records of patients with histologically confirmed pancreatic adenocarcinoma who underwent SBPT at our institution between January 2017 and December 2020. The institutional review board of Samsung Medical Center approved this retrospective study (no. 2021-07-038-001). We defined SBPT as the equivalent total dose delivered in 2 gray (Gy) fractions (EQD2) (alpha/beta ratio = 10 Gy) of ≥80 Gy relative biological effectiveness (RBE) in five fractions. Patients with a history of previous abdominal RT and those who received an incomplete planned SBPT course were excluded. Among the 65 patients with pancreatic cancer who underwent PBT during the study period, 49 met the inclusion criteria (Figure S1). The survival curves calculated from the start date of PBT to the last follow-up or event for all 65 patients are shown in Figure S2.
Each patient underwent an initial workup including physical examination, complete blood count analysis, standard blood chemistry evaluation, carbohydrate antigen (CA) 19-9 measurement, pancreatic protocol computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT. Patients were staged according to the eighth edition of the American Joint Committee on Cancer staging system.
2.2. Treatment
During the study period, we treated patients according to our institution’s treatment protocol for pancreatic cancer, based on the National Comprehensive Cancer Network (NCCN) guidelines [4]. Patients with resectable disease in terms of the disease extent and medical aspects underwent surgical resection. Neoadjuvant chemotherapy with or without chemoradiotherapy (CRT) followed by surgery was considered for borderline resectable tumours. In cases of locally advanced unresectable pancreatic cancer or controlled minimally metastatic disease, chemotherapy was administered for 4–6 months. X-ray therapy or PBT was also administered if there was no disease progression or only LP. Additionally, X-ray therapy or PBT was considered when surgery was unsuitable because of the patient’s medical condition, refusal to undergo surgery, or local recurrence after surgery. SBPT was preferentially considered in cases without bowel invasion at the time of diagnosis.
Before CT scan, patients underwent at least once respiratory training to breath as regularly and as shallowly as possible. The planning CT was taken on a Discovery CT590 (General Electric Healthcare, Chicago, Illinois, USA). All patients underwent four-dimensional CT (4D-CT) simulation in the supine position, with a slice thickness of 2.5 mm. After obtaining non-enhanced 4D-CT images for SBPT planning, an intravenous contrast agent was injected to improve the accuracy of delineation of the target and normal organs For planning, maximum intensity projection images were generated from 4D-CT scans and used for target delineation.
The gross tumour volume (GTV) included all identified gross lesions and metastatic lymph nodes based on diagnostic CT, MRI, PET-CT, and simulation CT findings. The clinical target volume (CTV) was defined as the GTV [19]. The internal target volume (ITV) was delineated based on all respiratory phases using 4D-CT in all patients, except two patients. These two patients were treated with a gating plan, and the ITV was delineated based on 30–70% respiratory phases. The simultaneous integrated boost (SIB) technique was used in two-thirds of the PBT plans. The low-risk planning target volume (PTV) was delineated by adding a 5-mm margin to the ITV to account for setup uncertainties. High-risk PTV was delineated by removing the planning organ-at-risk volume from the ITV by adding a 3-mm margin. In cases treated with the SIB technique, the prescribed dose to the low-risk PTV was 50–65% of the high-risk PTV according to the surrounding normal organ.
RayStation (RaySearch Laboratories, Stockholm, Sweden) was used for treatment planning. The proton plans for all patients treated with the free-breathing technique were calculated on PTVs defined from MIP images. The dose distribution and dose-volume histograms (DVHs) for calculated plan on the MIP were verified with the max- and min-inhalation phases of the 4DCT datasets. Additionally, all plans were robustly optimized to PTV using 5 mm setup uncertainty and 3.5% range uncertainty. PBT was delivered using a proton therapy system (Sumitomo, Tokyo, Japan) at Samsung Medical Center. In all cases, proton therapy was delivered using the pencil beam line-scanning method, a passive scanning technique. Line scanning proceeded in the lateral direction, and after the line reached the lateral edge, the next line was scanned in the opposite direction. Three proton beams were irradiated in the posterior–anterior, right posterior oblique, and left posterior oblique directions to reduce the effects of the range and setup uncertainty. The dose distribution in the target was optimised by adjusting line and layer spacings (Figure 1). A spot size of 70% was used for all patients because of the high-quality plan [20]. Daily cone-beam CT and/or orthogonal kilovoltage X-ray images provided by VeriSuite (MedCom, Darmstadt, Germany) were obtained and matched with simulation images mainly based on the vertebral bodies before every treatment session.
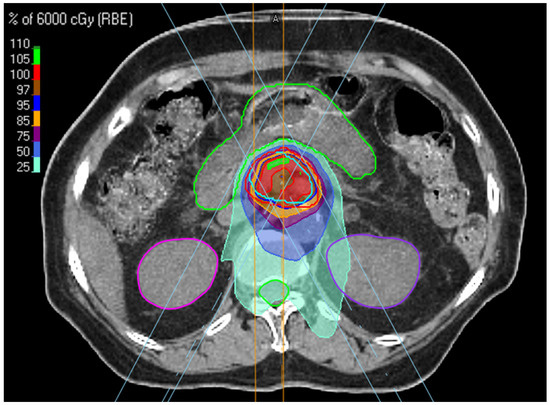
Figure 1.
Beam arrangement and dose distribution in SBPT planning.
2.3. Endpoints and Statistical Analysis
Patients underwent regular follow-up examinations including physical examination, haematologic studies, and CT. The endpoints included OS, progression-free survival (PFS), and LC. All endpoints were calculated from the start date of SBPT to the date of the last follow-up or event. Additionally, OS and PFS were calculated from the start date of the treatment course to the last follow-up or event, respectively. Tumour response to RT was evaluated using the Revised Response Evaluation Criteria in Solid Tumours guidelines (version 1.1). The best response was recorded from treatment initiation to disease progression or recurrence. Failure patterns after SBPT were classified as locoregional, peritoneal seeding, or distant haematogenous metastases. Treatment-related toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Survival outcomes were analysed using the Kaplan–Meier method and compared using the log-rank test for univariate analysis. The Cox proportional hazard regression model was used for univariate and multivariate analyses. Statistical significance was set at p < 0.05. Multivariable analysis was performed on variables with a probability value of <0.2 or on those that were considered relevant. Statistical analyses were performed using SPSS Statistics version 27.0 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Patient Characteristics
The baseline characteristics of all patients are summarised in Table 1. The median patient age was 61 years (range, 49–90 years). In terms of resectability, 81.5% cases were unresectable, 10.3% were borderline resectable, and 8.2% were resectable. The head, body, and tail of the pancreas were the primary tumour locations in 23 (46.9%), 22 (44.9%), and four (8.2%) patients, respectively.

Table 1.
Characteristics of patients (n = 49).
Eight (16.3%) patients underwent surgery before or after SBPT; two patients underwent surgery as they responded to induction chemotherapy and SBPT. The other patients underwent surgery during the previous treatment course.
Approximately 90% patients received induction chemotherapy prior to SBPT. Most patients (n = 31, 70.5%) received either FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) or modified FOLFIRINOX therapy. The median chemotherapy to SBPT interval for these patients was 6.7 months (range, 2.1–23.4 months). Partial response (PR), stable disease (SD), and PD were observed in 32.7%, 51.0%, and 6.1% patients, respectively.
The dose fractionation scheme used was 60 GyRBE in five fractions (n = 42, 85.7%) or 50 GyRBE in five fractions (n = 7, 14.3%). The median PTV was 79.0 cm3 (range, 20.5–291.8 cm3).
3.2. Survival Outcomes and Prognostic Factors
The median follow-up after SBPT was 16.3 months (range, 1.8–45.0 months). The 1-year OS, PFS, and LC rates were 90.7%, 54.3% and 88.6%, respectively. The 2-year OS, PFS, and LC rates were 67.6%, 38.0% and 73.0%, respectively. Additionally, we calculated OS and PFS from the start of chemotherapy or the start of SBPT if chemotherapy was not administered. Using this calculation, the 1-year OS and PFS rates were 95.6% and 76.5%, respectively. The 2-year OS and PFS rates were 78.9% and 42.3%, respectively (Figure 2).
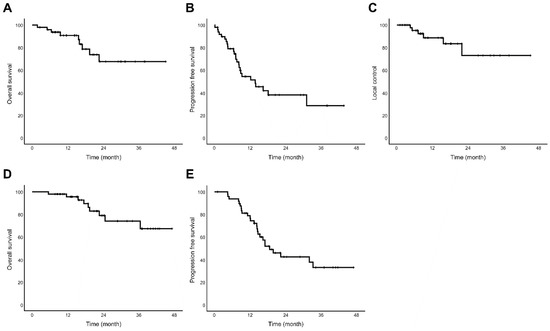
Figure 2.
Kaplan–Meier survival curves of all enrolled patients treated with stereotactic body proton beam therapy (n = 49) (A) Overall survival (B) Progression-free survival (C) Local control rate (D) Overall survival from start date of treatment course (E) Progression-free survival from start date of treatment course.
Age (hazard ratio, 1.10; 95% confidence interval, 1.01–1.20; p = 0.024) was an independent prognostic factor for PFS (Table 2). The univariate and multivariate Cox proportional hazard models for OS and LC are presented in Table 3 and Table 4, respectively.

Table 2.
Univariable and multivariable Cox proportional hazard model for progression-free survival (n = 49).

Table 3.
Univariable and multivariable Cox proportional hazard model for overall survival (n = 49).

Table 4.
Univariate and multivariate Cox proportional hazard models for local control (n = 49).
3.3. Treatment Response and Patterns of Failure
Regarding anti-tumour activity, the best response after SBPT was complete response (CR) in four (8.2%) patients, PR in 13 (26.5%) patients, SD in 31 (63.3%) patients, and PD in one (2.0%) patient. During follow-up, 26 (53.1%) patients developed PD. Figure 3 shows the failure patterns after SBPT. Locoregional failure was observed in 12 (24.5%) patients. Peritoneal seeding and distant haematogenous metastases occurred in 13 (26.5%) and 14 (28.6%) patients, respectively.
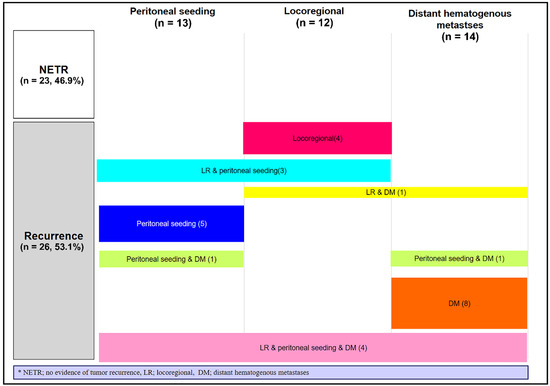
Figure 3.
Failure patterns after stereotactic body proton beam therapy (n = 49).
3.4. Treatment-Related Gastroduodenal Toxicity
All patients tolerated SBPT well, but 16 experienced treatment-related GD toxicities during follow-up. Grade ≥ 3 GD toxicities occurred in three (6.1%) patients. One patient had GD perforation due to a severe GD ulcer (Table S1). Among the 13 patients who experienced grade 1 or 2 GD toxicities, 10 improved spontaneously or with medication and three patients showed a similar degree of symptoms during follow-up. One patient with grade 3 toxicity underwent endoscopic haemostasis. Two patients with grade 4 toxicity underwent emergency surgery. One patient underwent primary repair of a gastric perforation and another underwent total gastrectomy. Postoperatively, no significant SBPT-related complications occurred during follow-up (Figure S3).
The relationship between the GD toxicities and DVH values is presented in Table 5. The dosimetric values were converted into EQD2 values. Overall, the dosimetric values (Dmax, D5cc, and D10cc in EQD2) were not significantly different between the grade ≥ 2 and lower toxicity groups.

Table 5.
Relationship between dosimetric parameters and Grade 2 or higher gastroduodenal toxicities in stereotactic body proton beam therapy (n = 49).
4. Discussion
The role of RT in pancreatic cancer is controversial because of conflicting results, particularly in LAPC [6,21,22]. Therefore, to clarify the role of CRT in LAPC, the phase III randomised LAP07 trial compared CRT after induction chemotherapy with chemotherapy alone. CRT did not lead to increased OS, but it significantly prolonged the treatment-free period and time to LP [9,22]. Consequently, RT has been considered an acceptable treatment option for LAPC, especially in patients with controlled disease after systemic chemotherapy and/or who cannot tolerate further systemic chemotherapy according to the current American Society of Clinical Oncology (ASCO) and NCCN guideline [4,23].
However, the LC rate with conventional fractionated CRT is low (50–60%). This shifts the question from whether RT is beneficial to how to make RT more effective in pancreatic cancer. Therefore, advanced techniques, such as intensity-modulated RT or SBRT, that escalate radiation doses to primary tumours have been attracting attention [21,22].
SBRT delivers a more ablative radiation dose to the tumour volume while reducing the dose to the surrounding normal at-risk organs. Furthermore, a short treatment duration can improve quality of life and make it easier to combine with other modalities [6]. Early SBRT studies used single-fraction SBRT, reporting high 1-year LC and OS rates of 84–100% and 21–50%, respectively. However, the incidence of grade ≥ 2 late GD toxicities was also high (20–44%) [6,24,25,26,27,28]. Subsequently, multi-fractionated SBRT was used to reduce toxicity. In previous studies, it reduced late GD toxicities with comparable LC rates compared with single-fraction SBRT [6,24,29]. Mahadevan et al. analysed the treatment outcomes of SBRT with chemotherapy in 39 patients with LAPC who received SBRT of 24–30 Gy in three fractions. The median OS and PFS were 20 and 15 months, respectively. The 1-year freedom from LP (FFLP) rate was 85.0%. Grade ≥ 3 late GD toxicity occurred in only 9% patients [30]. In another recent study, Jung et al. analysed the treatment outcomes of SBRT with chemotherapy in 95 patients with LAPC who received SBRT of 24–36 Gy in four fractions. The median OS and PFS were 16.7 and 10.2 months, respectively. The 1-year OS and PFS rates were 67.4% and 42.9%, respectively. The 1-year FFLP rate was 80.1%. Grade ≥ 3 late GD toxicity occurred in only 3% cases [24]. Moreover, several other studies on fractionated SBRT have reported favourable 1-year and 2-year OS rates of 35–85% and 0–50% and a 1-year FFLP rate of 40–100%, with a minimal toxicity rate of 0–23% [29]. Based on these advantages, SBRT can be considered an alternative to conventional fractionated CRT in experienced centres.
PBT offers further clinical advantages over SBRT and conventional RT with X-ray. It has a unique depth-dose distribution with a sharp dose peak (Bragg peak) at a specific depth of tissue, which enables substantial reductions in doses delivered to the normal tissues proximal and distal to the target volume. Due to this characteristic dosimetric profile, it is possible to deliver escalated dose to the tumor and minimize low to medium dose exposed volume of normal tissues [10,29,31]. In several studies comparing proton and photon plan in pancreatic cancer, the PBT plans showed significant lower doses to normal organs like liver, duodenum, small bowel. Dose reduction to radiosensitive normal organs can translate to a potential decline in both acute and long-term RT related toxicities and consequently fewer interruptions to intensive and aggressive systemic therapy [12,13,14,15,16,17,18]. In addition, PBT can reduce radiation induced lymphopenia, known as an unfavourable prognostic factor, by decreasing low dose irradiated volume of normal organs like spine and vessels [32,33].
Terashima et al. analysed 50 patients with LAPC treated with PBT (50–70.2 GyRBE in 25–26 fractions) with concurrent gemcitabine and showed favourable outcomes in terms of the 1-year OS, recurrence-free survival (RFS), and LP-free survival rates (76.8%, 64.3% and 81.7%, respectively) and grade ≥ 3 late toxicity rate (10%) [34]. Kim et al. analysed 40 patients with localised pancreatic cancer treated with SBPT (30–45 GyRBE in 10 fractions) with or without induction chemotherapy and showed favourable outcomes in terms of 1-year OS, RFS, and FFLP rates (75.7%, 33.2% and 64.8%, respectively) and grade ≥ 3 late toxicity rate (0%) [10].
In our study, we aimed to report the survival outcomes and GD toxicities associated with SBPT in pancreatic cancer. We analysed 49 patients with pancreatic cancer treated with SBPT and prescribed 50–60 GyRBE in five fractions according to the proximity of the normal organs. The 1-year OS, PFS, and LC rates were 90.7%, 54.3% and 88.6%, respectively, while the 2-year OS, PFS, and LC rates were 67.6%, 38.0% and 73.0%, respectively. The 1- and 2-year OS and PFS rates, which were calculated from the date of treatment initiation, were 95.6% and 78.9% and 76.5% and 42.3%, respectively. These results were favourable compared to those of the LAP07 trial and those of other SBRT or proton studies [9,29]. In our study, a longer chemotherapy to RT interval (≥4 months vs. <4 months) was an independent favourable prognostic factor for OS. This result is consistent with that of a previous study that emphasises the importance of induction chemotherapy [35]. This may be because longer induction chemotherapy regimens provide sufficient time for sterilisation of micrometastases or better selection of an effective patient population for RT for local disease.
The incidence of grade ≥ 3 GD toxicities was 6.1% (n = 3), which is similar to that reported in previous SBRT studies (0–15.6%). Considering that the prescribed doses to the target volume were higher than those in most previous SBRT studies that administered 20–50 Gy in three to seven fractions, SBPT had significant dosimetric advantages, with superior survival outcomes [29,30,36,37,38,39,40]. Moreover, some patients experienced severe GD toxicities, but they recovered with proper management without lasting complications.
This study has some limitations. First, this was a retrospective study with a small number of patients and a short follow-up period. Additional prospective studies with larger sample sizes and longer follow-up periods are required. Second, oesophagogastroduodenoscopy was only performed when the patient was symptomatic. Therefore, it is likely that the toxicity-reducing effect of SBPT, along with its favourable treatment outcomes, has been overestimated. Third, significant heterogeneity was observed in the patient population in terms of disease status, combined treatment regimens, RT timing, and other factors. Finally, we only included patients treated with SBPT; hence, we cannot directly compare the outcomes with those of patients who underwent treatment using other modalities such as conventional CRT or SBRT. Therefore, we cannot conclude that SBPT is superior to other RT techniques such as conventional CRT in terms of its toxicity profile. In this regard, another study comparing proton- and photon-based therapies may be helpful.
5. Conclusions
The present data show that SBPT can be a very effective and safe treatment option for LC in a well-selected patient population. Future studies should focus on developing an optimal scheme for chemotherapy and SBPT in terms of timing, regimen, dose, field, and other factors. Careful patient selection considering the risks and benefits should continue when administering SBPT in patients with pancreatic cancer, especially those with tumours with GD invasion.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/cancers14194556/s1, Figure S1: Patient inclusion and exclusion criteria. Figure S2: Kaplan–Meier survival curves of all patients treated with proton beam therapy (n = 65) (A) Overall survival (B) Progression-free survival (C) Local control rate. Figure S3: (A) Abdominal computed tomography (CT) of a patient who underwent primary repair of gastric perforation (B) Abdominal CT and endoscopic findings in a patient who underwent total gastrectomy. Table S1: Treatment-related gastroduodenal toxicities after stereotactic body proton beam therapy (n = 49).
Author Contributions
Conceptualization, J.I.Y. and H.C.P.; methodology, H.S., J.I.Y. and H.C.P.; formal analysis, H.S., J.I.Y. and H.C.P.; investigation, all authors; resources, J.I.Y., H.C.P., G.S.Y., S.C., J.O.P., K.T.L., K.H.L., J.K.L., J.K.P., J.S.H., I.W.H. and S.H.S.; data curation, H.S.; writing—original draft preparation, H.S. and J.I.Y.; writing—review and editing, J.I.Y., G.S.Y. and H.C.P.; supervision, J.I.Y. and H.C.P.; project administration, J.I.Y. and H.C.P.; funding acquisition, J.I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (NRF-2020R1F1A1073205).
Institutional Review Board Statement
This study was approved by the Health Institutional Review Boards of Samsung Medical Center (SMC no. 2021-07-038-001).
Informed Consent Statement
The requirement for informed consent was waived because of the retrospective nature of this study.
Data Availability Statement
Data availability is limited due to institutional data protection law and confidentiality of patient data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Won, Y.-J.; Lee, J.J.; Jung, K.-W.; Kim, H.-J.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res. Treat 2022, 54, 330–344. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.-E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Blakaj, A.; Stein, S.M.; Khan, S.A.; Johung, K.L. Review and current state of radiation therapy for locally advanced pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2018, 9, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Iacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 Gene Status of the Primary Carcinoma Correlates with Patterns of Failure in Patients with Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.; Chang, B.W. New possibilities and potential benefits for local control in locally recurrent pancreatic cancer. J. Gastrointest. Oncol. 2013, 4, 340–342. [Google Scholar]
- Hammel, P.; Huguet, F.; van Laethem, J.-L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, W.J.; Woo, S.M.; Kim, H.; Oh, E.S.; Lee, J.H.; Han, S.-S.; Park, S.-J.; Suh, Y.-G.; Moon, S.H.; et al. Effectiveness and Safety of Simultaneous Integrated Boost-Proton Beam Therapy for Localized Pancreatic Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818783879. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Rutenberg, M.S.; Nichols, R.C. Proton beam radiotherapy for pancreas cancer. J. Gastrointest. Oncol. 2019, 11, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.; Badiyan, S.N.; Yam, M.; Li, Z.; Langen, K.; Regine, W.; Morris, C.; Snider Iii, J.; Mehta, M.; Huh, S.; et al. Pencil beam scanning versus passively scattered proton therapy for unresectable pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 687–693. [Google Scholar] [CrossRef]
- Kobeissi, J.M.; Simone, C.B.; Lin, H.; Hilal, L.; Hajj, C. Proton Therapy in the Management of Pancreatic Cancer. Cancers 2022, 14, 2789. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.C.; Huh, S.N.; Prado, K.L.; Yi, B.Y.; Sharma, N.K.; Ho, M.W.; Hoppe, B.S.; Mendenhall, N.P.; Li, Z.; Regine, W.F. Protons Offer Reduced Normal-Tissue Exposure for Patients Receiving Postoperative Radiotherapy for Resected Pancreatic Head Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Mayekar, S.U.; Zhai, H.; Both, S.; Apisarnthanarax, S.; Metz, J.M.; Plastaras, J.P.; Ben-Josef, E. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Med. Phys. 2014, 41, 081711. [Google Scholar] [CrossRef]
- Verma, V.; Lin, S.H.; Simone Ii, C.B.; Mehta, M.P. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: A systematic review. J. Gastrointest. Oncol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Zurlo, A.; Lomax, A.; Hoess, A.; Bortfeld, T.; Russo, M.; Goitein, G.; Valentini, V.; Marucci, L.; Capparella, R.; Loasses, A. The role of proton therapy in the treatment of large irradiation volumes: A comparative planning study of pancreatic and biliary tumors. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 277–288. [Google Scholar] [CrossRef]
- Brunner, T.B.; Haustermans, K.; Huguet, F.; Morganti, A.G.; Mukherjee, S.; Belka, C.; Krempien, R.; Hawkins, M.A.; Valentini, V.; Roeder, F. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother. Oncol. 2021, 154, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.S.; Yu, J.I.; Cho, S.; Jung, S.H.; Han, Y.; Park, S.; Oh, Y.; Lee, B.; Park, H.C.; Lim, D.H.; et al. Comparison of clinical outcomes between passive scattering versus pencil-beam scanning proton beam therapy for hepatocellular carcinoma. Radiother. Oncol. 2020, 146, 187–193. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Lawrence, T.S. Radiotherapy: The importance of local control in pancreatic cancer. Nat. Rev. Clin. Oncol. 2011, 9, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yu, W.; Rosati, L.M.; Herman, J.M. Advances of stereotactic body radiotherapy in pancreatic cancer. Chin. J. Cancer Res. 2015, 27, 349–357. [Google Scholar] [PubMed]
- Balaban, E.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Mukherjee, S.; Crane, C.H.; Javle, M.M.; Eads, J.R.; Allen, P.; Ko, A.H.; et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2654–2668. [Google Scholar] [CrossRef]
- Jung, J.; Yoon, S.M.; Park, J.H.; Seo, D.W.; Lee, S.S.; Kim, M.H.; Lee, S.K.; Park, D.H.; Song, T.J.; Ryoo, B.Y.; et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS ONE 2019, 14, e0214970. [Google Scholar] [CrossRef]
- Koong, A.C.; Christofferson, E.; Le, Q.T.; Goodman, K.A.; Ho, A.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Greco, R.; Norton, J.; et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Koong, A.C.; Le, Q.T.; Ho, A.; Fong, B.; Fisher, G.; Cho, C.; Ford, J.; Poen, J.; Gibbs, I.C.; Mehta, V.K.; et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1017–1021. [Google Scholar] [CrossRef]
- Schellenberg, D.; Goodman, K.A.; Lee, F.; Chang, S.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Quon, A.; Desser, T.S.; Norton, J.; et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A.; et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 181–188. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Short, M.; Wilson, P.; Bezak, E. Clinical Limitations of Photon, Proton and Carbon Ion Therapy for Pancreatic Cancer. Cancers 2020, 12, 163. [Google Scholar] [CrossRef]
- Mahadevan, A.; Miksad, R.; Goldstein, M.; Sullivan, R.; Bullock, A.; Buchbinder, E.; Pleskow, D.; Sawhney, M.; Kent, T.; Vollmer, C.; et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e615–e622. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, W.J.; Woo, S.M.; Oh, E.S.; Youn, S.H.; Jang, H.Y.; Han, S.S.; Park, S.J.; Suh, Y.G.; Moon, S.H.; et al. Efficacy and feasibility of proton beam radiotherapy using the simultaneous integrated boost technique for locally advanced pancreatic cancer. Sci. Rep. 2020, 10, 21712. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Myoung Noh, J.; Lee, W.; Park, B.; Park, H.; Young Park, J.; Pyo, H. Proton beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: A comparative analysis of proton versus photon therapy. Radiother. Oncol. 2021, 156, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.T.; Herman, J.M.; Dholakia, A.S.; Moningi, S.; Lu, Y.; Rosati, L.M.; Hacker-Prietz, A.; Assadi, R.K.; Saeed, A.M.; Pawlik, T.M.; et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients with Unresectable Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Terashima, K.; Demizu, Y.; Hashimoto, N.; Jin, D.; Mima, M.; Fujii, O.; Niwa, Y.; Takatori, K.; Kitajima, N.; Sirakawa, S.; et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. 2012, 103, 25–31. [Google Scholar] [CrossRef]
- Faisal, F.; Tsai, H.-L.; Blackford, A.; Olino, K.; Xia, C.; De Jesus-Acosta, A.; Le, D.T.; Cosgrove, D.; Azad, N.; Rasheed, Z.; et al. Longer Course of Induction Chemotherapy Followed by Chemoradiation Favors Better Survival Outcomes for Patients with Locally Advanced Pancreatic Cancer. Am. J. Clin. Oncol. 2016, 39, 18. [Google Scholar] [CrossRef]
- Chuong, M.D.; Springett, G.M.; Freilich, J.M.; Park, C.K.; Weber, J.M.; Mellon, E.A.; Hodul, P.J.; Malafa, M.P.; Meredith, K.L.; Hoffe, S.E.; et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 516–522. [Google Scholar] [CrossRef]
- Gurka, M.K.; Kim, C.; He, A.R.; Charabaty, A.; Haddad, N.; Turocy, J.; Johnson, L.; Jackson, P.; Weiner, L.M.; Marshall, J.L.; et al. Stereotactic Body Radiation Therapy (SBRT) Combined with Chemotherapy for Unresected Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2017, 40, 152. [Google Scholar] [CrossRef]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S.; et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef]
- Hoyer, M.; Roed, H.; Sengelov, L.; Traberg, A.; Ohlhuis, L.; Pedersen, J.; Nellemann, H.; Kiil Berthelsen, A.; Eberholst, F.; Engelholm, S.A.; et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother. Oncol. 2005, 76, 48–53. [Google Scholar] [CrossRef]
- Macchia, G.; Morganti, A.G.; Cilla, S.; Ippolito, E.; Massaccesi, M.; Picardi, V.; Mattiucci, G.C.; Bonomo, P.; Tambaro, R.; Pacelli, F.; et al. Quality of life and toxicity of stereotactic radiotherapy in pancreatic tumors: A case series. Cancer Investig. 2012, 30, 149–155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).