Precision Surgery of Colorectal Liver Metastases in the Current Era: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
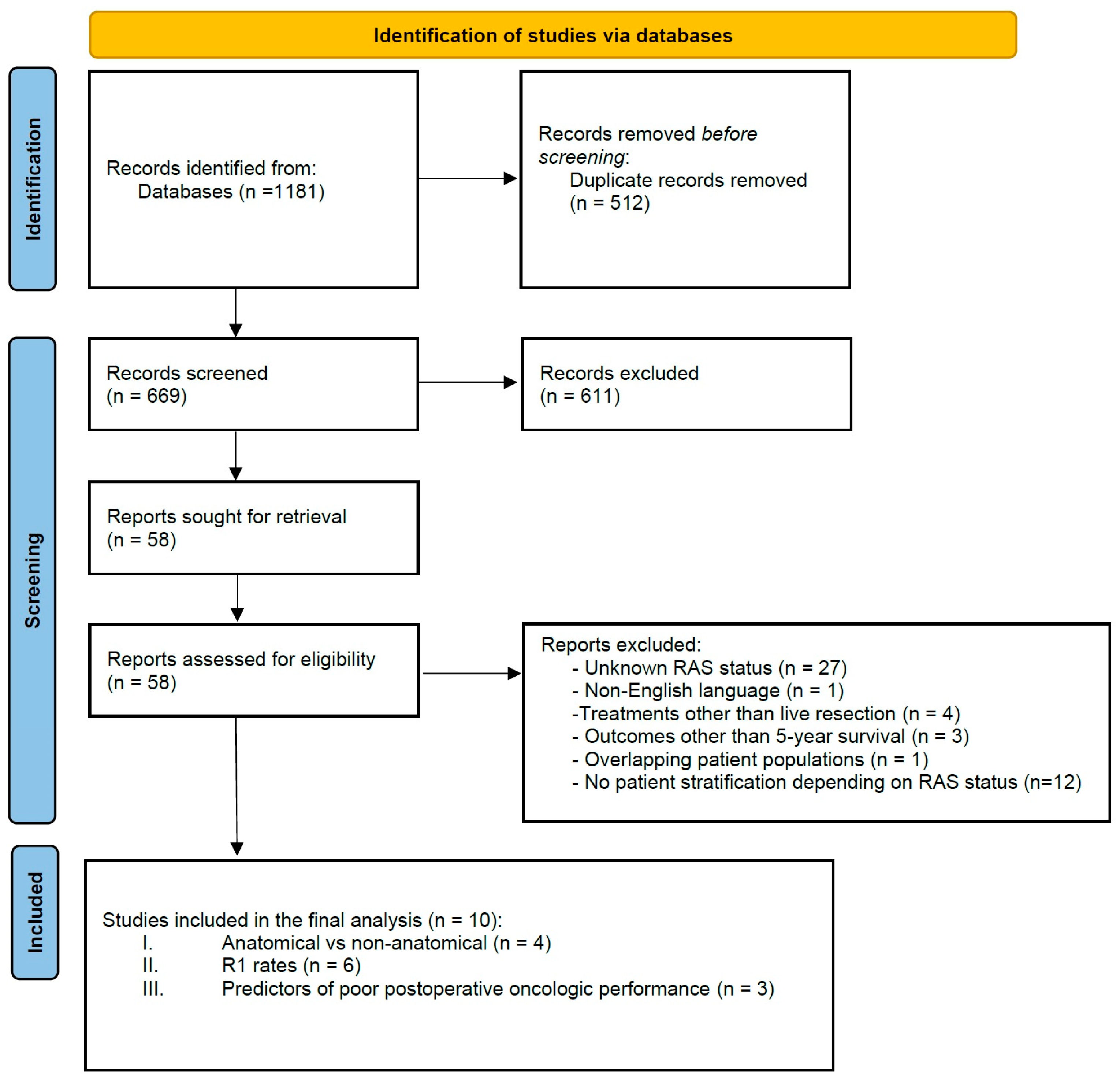
2. Materials and Methods
2.1. Literature Search and Strategy
2.2. Outcomes of Interest, Data Extraction and Synthesis
2.3. Inclusion and Exclusion Criteria
3. Results
3.1. Anatomical Versus Non-Anatomical Resections
3.2. R1 Resection Rates in Mutated and Wild-Type RAS Patients
3.3. Impact of RAS Status on Survival after Incomplete Resection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, R.P.; Kokudo, N.; Folprecht, G.; Mise, Y.; Unno, M.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J. Colorectal Liver Metastases: A Critical Review of State of the Art. Liver Cancer 2016, 6, 66–71. [Google Scholar] [CrossRef]
- Abbas, S.; Lam, V.; Hollands, M. Ten-year survival after liver resection for colorectal metastases: Systematic review and meta-analysis. ISRN Oncol. 2011, 2011, 763245. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Kim, Y.; Samaha, M.; Buettner, S.; Amini, N.; Antoniou, E.; Pawlik, T.M. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2016, 20, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Schulick, R.D.; Choti, M.A. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008, 13, 51–64. [Google Scholar] [CrossRef]
- Sakamoto, K.; Honda, G.; Beppu, T.; Kotake, K.; Yamamoto, M.; Takahashi, K.; Endo, I.; Hasegawa, K.; Itabashi, M.; Hashiguchi, Y.; et al. Comprehensive data of 3525 patients newly diagnosed with colorectal liver metastasis between 2013 and 2014: 2nd report of a nationwide survey in Japan. J. Hepato-Biliary Pancreat. Sci. 2020, 27, 555–562. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, J.A.M.; van der Stok, E.P.; Mekenkamp, L.J.; Wiering, B.; Koopman, M.; Punt, C.J.A.; Verhoef, C.; de Wilt, J.H. Management of liver metastases in colorectal cancer patients: A retrospective case-control study of systemic therapy versus liver resection. Eur. J. Cancer 2016, 59, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Russolillo, N.; Ferrero, A.; Langella, S.; Sperti, E.; Capussotti, L. Evolution of long-term outcome of liver resection for colorectal metastases: Analysis of actual 5-year survival rates over two decades. Ann. Surg. Oncol. 2012, 19, 2035–2044. [Google Scholar] [CrossRef]
- Hamady, Z.Z.R.; Lodge, J.P.A.; Welsh, F.K.; Toogood, G.J.; White, A.; John, T.; Rees, M. One-millimeter cancer-free margin is curative for colorectal liver metastases: A propensity score case-match approach. Ann. Surg. 2014, 259, 543–548. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Andreatos, N.; Tzanninis, I.G.; Sasaki, K.; Psaltopoulou, T.; Wang, J.; Buettner, S.; He, J.; et al. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann. Surg. 2018, 267, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Shirai, Y.; Ajioka, Y.; Nagakura, S.; Suda, T.; Hatakeyama, K. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer 2002, 94, 1642–1647. [Google Scholar] [CrossRef]
- Karagkounis, G.; Torbenson, M.S.; Daniel, H.D.; Azad, N.S.; Diaz, L.A.J.; Donehower, R.C.; Hirose, K.; Ahuja, N.; Pawlik, T.M.; Choti, M.A. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013, 119, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Harmoush, S.; Kopetz, S.E.; Ahrar, K.; Shin Chun, Y.; Conrad, C.; Aloia, T.A.; Gupta, S.; et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br. J. Surg. 2017, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Zimmitti, G.; Shindoh, J.; Mise, Y.; Kopetz, S.; Loyer, E.M.; Andreou, A.; Cooper, A.B.; Kaur, H.; Aloia, T.A.; Maru, D.M.; et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann. Surg. Oncol. 2015, 22, 834–842. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Mise, Y.; Chung, M.H.; Chun, Y.S.; Kopetz, S.E.; Passot, G.; Conrad, C.; Maru, D.M.; Aloia, T.A.; Vauthey, J.-N. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2016, 23, 2635–2643. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Ye, M.; Weng, W.; Tan, C.; Ni, S.; Huang, D.; Sheng, W.; Wang, L. KRAS Mutation Predicted More Mirometastases and Closer Resection Margins in Patients with Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2020, 27, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.; Cameron, J.L.; He, J.; et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Choi, M.; Han, D.H.; Choi, J.S.; Choi, G.H. Can the presence of KRAS mutations guide the type of liver resection during simultaneous resection of colorectal liver metastasis? Ann. Hepato-Biliary Pancreat. Surg. 2022, 26, 125–132. [Google Scholar] [CrossRef]
- Kawai, T.; Ishii, T.; Uchida, Y.; Sato, A.; Naito, S.; Kitaguchi, K.; Komatsubara, T.; Nakamura, I.; Ogiso, S.; Fukumitsu, K.; et al. Impact of anatomical liver resection on patient survival in KRAS-wild-type colorectal liver metastasis: A multicenter retrospective study. Surgery 2022, 172, 1133–1140. [Google Scholar] [CrossRef]
- Joechle, K.; Vreeland, T.J.; Vega, E.A.; Okuno, M.; Newhook, T.E.; Panettieri, E.; Chun, Y.S.; Tzeng, C.-W.D.; Aloia, T.A.; Lee, J.E.; et al. Anatomic Resection Is Not Required for Colorectal Liver Metastases with RAS Mutation. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 1033–1039. [Google Scholar] [CrossRef]
- Hatta, A.A.Z.; Pathanki, A.M.; Hodson, J.; Sutcliffe, R.P.; Marudanayagam, R.; Roberts, K.J.; Chatzizacharias, N.; Isaac, J.; Muiesan, P.; Taniere, P.; et al. The effects of resection margin and KRAS status on outcomes after resection of colorectal liver metastases. HPB 2021, 23, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.-W.; Yan, X.-L.; Li, J.; Wang, K.; Xing, B.-C. Sub-millimeter surgical margin is acceptable in patients with good tumor biology after liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Procopio, F.; Viganò, L.; Cimino, M.; Donadon, M.; Del Fabbro, D.; Torzilli, G. Does KRAS mutation status impact the risk of local recurrence after R1 vascular resection for colorectal liver metastasis? An observational cohort study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, H.; Tranberg, K.G.; Andersson, R.; Lundstedt, C.; Hägerstrand, I.; Ranstam, J.; Bengmark, S. Determinants of survival in liver resection for colorectal secondaries. Br. J. Surg. 1986, 73, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715–722, discussion 722–724. [Google Scholar] [CrossRef]
- Sadot, E.; Groot Koerkamp, B.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: Surgical technique or biologic surrogate? Ann. Surg. 2015, 262, 475–476. [Google Scholar] [CrossRef]
- Holdhoff, M.; Schmidt, K.; Diehl, F.; Aggrawal, N.; Angenendt, P.; Romans, K.; Edelstein, D.L.; Torbenson, M.; Kinzler, K.W.; Vogelstein, B.; et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin. cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 3551–3557. [Google Scholar] [CrossRef]
- Kokudo, N.; Miki, Y.; Sugai, S.; Yanagisawa, A.; Kato, Y.; Sakamoto, Y.; Yamamoto, J.; Yamaguchi, T.; Muto, T.; Makuuchi, M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: Minimum surgical margins for successful resection. Arch. Surg. 2002, 137, 833–840. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.-Y.; Yan, X.-L.; Li, J.; Wang, K.; Xing, B.-C. Development of a model to predict pathologic response to chemotherapy in patients with colorectal liver metastases. J. Gastrointest. Oncol. 2021, 12, 1498–1508. [Google Scholar] [CrossRef]
- Mentha, G.; Terraz, S.; Morel, P.; Andres, A.; Giostra, E.; Roth, A.; Rubbia-Brandt, L.; Majno, P. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br. J. Surg. 2009, 96, 95–103. [Google Scholar] [CrossRef]
- Truant, S.; Séquier, C.; Leteurtre, E.; Boleslawski, E.; Elamrani, M.; Huet, G.; Duhamel, A.; Hebbar, M.; Pruvot, F.-R. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2015, 17, 176–184. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, R.P.; Palese, C.; Jarnagin, W.R.; Sun, R.L.; Blumgart, L.H.; Fong, Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2000, 4, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; De Man, M.; Laurent, S.; Smeets, P.; Tomassini, F.; Ariotti, R.; Hoorens, A.; van Dorpe, J.; Varin, O.; Geboes, K.; et al. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.J.; Zheng, J.; Capanu, M.; Haviland, D.; Paty, P.; Dematteo, R.P.; D’Angelica, M.; Fong, Y.; Jarnagin, W.R.; Allen, P.J.; et al. Response to neoadjuvant chemotherapy does not predict overall survival for patients with synchronous colorectal hepatic metastases. Ann. Surg. Oncol. 2009, 16, 1844–1851. [Google Scholar] [CrossRef]
- Tang, H.; Li, B.; Zhang, H.; Dong, J.; Lu, W. Comparison of Anatomical and Nonanatomical Hepatectomy for Colorectal Liver Metastasis: A Meta-Analysis of 5207 Patients. Sci. Rep. 2016, 6, 32304. [Google Scholar] [CrossRef]
- Deng, G.; Li, H.; Jia, G.-Q.; Fang, D.; Tang, Y.-Y.; Xie, J.; Chen, K.-F.; Chen, Z.-Y. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: A systematic review and meta-analysis. Cancer Med. 2019, 8, 6165–6175. [Google Scholar] [CrossRef]
- Lee, K.S.; Suchett-Kaye, I.; Abbadi, R.; Finch-Jones, M.; Pope, I.; Strickland, A.; Rees, J. Microscopic resection margins adversely influence survival rates after surgery for colorectal liver metastases: An open ambidirectional Cohort Study. Int. J. Surg. 2020, 83, 8–14. [Google Scholar] [CrossRef]
- Nishioka, Y.; Paez-Arango, N.; Boettcher, F.O.; Kawaguchi, Y.; Newhook, T.E.; Chun, Y.S.; Tzeng, C.-W.D.; Tran Cao, H.S.; Lee, J.E.; Vreeland, T.J.; et al. Neither Surgical Margin Status nor Somatic Mutation Predicts Local Recurrence After R0-intent Resection for Colorectal Liver Metastases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2022, 26, 791–801. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Andreatos, N.; Kim, Y.; Merath, K.; Wagner, D.; Wilson, A.; Buettner, S.; Amini, N.; Antoniou, E.; et al. KRAS Mutation Status Dictates Optimal Surgical Margin Width in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2017, 24, 264–271. [Google Scholar] [CrossRef]
- Passot, G.; Denbo, J.W.; Yamashita, S.; Kopetz, S.E.; Chun, Y.S.; Maru, D.; Overman, M.J.; Brudvik, K.W.; Conrad, C.; Aloia, T.A.; et al. Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? An analysis of 524 patients undergoing curative liver resection. Surgery 2017, 161, 332–340. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef]
- Gagnière, J.; Dupré, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gönen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Zeindl-Eberhart, E.; Kirchner, T.; Jung, A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol. Res. Pract. 2009, 205, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Morikawa, T.; Liao, X.; Lochhead, P.; Kuchiba, A.; Yamauchi, M.; Qian, Z.R.; Nishihara, R.; Meyerhardt, J.A.; Haigis, K.M.; et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 4753–4763. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.; Norman, A.R.; Cunningham, D.; Oates, J.R.; Clarke, P.A. Kirsten ras mutations in patients with colorectal cancer: The multicenter “RASCAL” study. J. Natl. Cancer Inst. 1998, 90, 675–684. [Google Scholar] [CrossRef]
- Margonis, G.A.; Kim, Y.; Spolverato, G.; Ejaz, A.; Gupta, R.; Cosgrove, D.; Anders, R.; Karagkounis, G.; Choti, M.A.; Pawlik, T.M. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015, 150, 722–729. [Google Scholar] [CrossRef]
- Margonis, G.A.; Kim, Y.; Sasaki, K.; Samaha, M.; Amini, N.; Pawlik, T.M. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer 2016, 122, 2698–2707. [Google Scholar] [CrossRef]
| Study | Year of Publication | Country of Origin | Type of Study | Total Number of Patients | mutRAS, n (%) | Synchronous Presentation, n (%) | Number of Tumors (Median, Range) | Tumor Size (Median, Range) | Extrahepatic Disease, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Choi et al. [18] | 2022 | Korean | Retrospective | 250 | 94 (37.6) | 250 (100) | 2.85 ± 3.00 * | 2.37 ± 1.80 * | 0 |
| Kawai et al. [19] | 2022 | Japan | Retrospective | 290 | 104 (35.9) | 167 (57.6) | 1.6 ± 1.7 * | 2.5 ± 2.5 * | NR |
| Joechle et al. [20] | 2019 | USA | Retrospective, PSM | 360 | 150 (41.7) | 253 (70) | 1 (1–9) | 2 (0.1–9.5) | 55 (15) |
| Margonis et al. [16] | 2017 | USA | Retrospective | 389 | 140 (35.9) | 223 (57.3) | 2 (1–3) | 2.5 (1.6–4.0) | NR |
| Brudvik et al. [14] | 2016 | USA | Retrospective | 633 | 229 (36.2) | 446 (70.5) | NR | NR | NR |
| Zhang et al. [15] | 2020 | China | Retrospective | 251 | 130 (51.8) | 61 (24) | NR | NR | 28 (11.2) |
| Hatta et al. [21] | 2020 | UK | Retrospective | 500 | 152 (30.4) | 233 (51.7) | 2 (1–3) | 3 (2–5) | NR |
| Margonis et al. [3] | 2016 | USA | Retrospective | 485 | 178 (36.7) | 277 (57.1) | 2 (1–3) | 2.5 (1.5–4) | NR |
| Xu et al. [22] | 2019 | China | Retrospective | 214 | 100 (46.7) | NR | NR | NR | NR |
| Procopio et al. [23] | 2020 | Italy | Retrospective | 340 | 150 (44.1) | NR | NR | NR | NR |
| Anatomic vs. Non-Anatomic | ||||||
|---|---|---|---|---|---|---|
| mutRAS | wtRAS | |||||
| Study | LS-DFS Hazard Ratio | 5-Year LS-DFS (%) | p-Value | LS-DFS Hazard Ratio | 5-Year LS-DFS (%) | p-Value |
| Choi et al. [18] | 1.23 (0.64–2.39) | NR | 0.52 | 1.41 (0.86–2.32) | NR | 0.17 |
| Kawai et al. [19] | NR | NR | 0.23 | 0.42 (0.25–0.72) | NR | 0.001 |
| Joechle et al. [20] | NR | 16 vs. 17.3 | 0.4 | NR | 22.9 vs. 14.3 | 0.88 |
| Margonis et al. [16] | 0.37 (0.21–0.64) | 13 vs. 0 | <0.001 | 0.63 (0.42–0.94) | 15.9 vs. 4.3 | 0.02 |
| mutRAS | wtRAS | ||||
|---|---|---|---|---|---|
| Study | Total Patients | R1, n (%) | Total Patients | R1, n (%) | p-Value |
| Brudvik et al. [14] | 229 | 26 (11.4) | 404 | 22 (5.4) | 0.007 |
| Choi et al. [18] | 94 | 3 (3.2) | 156 | 4 (2.6) | 0.55 |
| Hatta et al. [21] | 146 | 42 (28.8) | 284 | 84 (29.6) | 0.88 |
| Margonis et al. [16] | 178 | 35 (19.7) | 307 | 70 (22.8) | 0.49 |
| Xu et al. [22] | 100 | 41 (41) | 114 | 26 (22.8) | 0.005 |
| Zhang et al. [15] | 117 | 26 (21.5) | 130 | 12 (9.2) | 0.007 |
| mutRAS | wtRAS | mutRAS | wtRAS | |||||
|---|---|---|---|---|---|---|---|---|
| Study | 5-Year OS (%) | p-Value | 5-Year LS-DFS (%) | p-Value | OS Hazard Ratio | LS-DFS Hazard Ratio | ||
| Procopio et al. [23] | 6.8 | 26.9 | NR | 1.7 | 4.8 | NR | NR | NR |
| Xu et al. [22] | 4 | 27.8 | 0.02 | NR | NR | 0.12 | 1.77 (1.08–2.88) | NR |
| Hatta et al. [21] | 55.5 | 56.3 | 0.57 | 26.8 | 35.8 | 0.15 | 1.08 (0.77–1.52) | 1.21 (0.93–1.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaconstantinou, D.; Pararas, N.; Pikouli, A.; Nastos, C.; Charalampopoulos, A.; Dellaportas, D.; Bagias, G.; Pikoulis, E. Precision Surgery of Colorectal Liver Metastases in the Current Era: A Systematic Review. Cancers 2023, 15, 2083. https://doi.org/10.3390/cancers15072083
Papaconstantinou D, Pararas N, Pikouli A, Nastos C, Charalampopoulos A, Dellaportas D, Bagias G, Pikoulis E. Precision Surgery of Colorectal Liver Metastases in the Current Era: A Systematic Review. Cancers. 2023; 15(7):2083. https://doi.org/10.3390/cancers15072083
Chicago/Turabian StylePapaconstantinou, Dimitrios, Nikolaos Pararas, Anastasia Pikouli, Constantinos Nastos, Anestis Charalampopoulos, Dionysios Dellaportas, George Bagias, and Emmanouil Pikoulis. 2023. "Precision Surgery of Colorectal Liver Metastases in the Current Era: A Systematic Review" Cancers 15, no. 7: 2083. https://doi.org/10.3390/cancers15072083
APA StylePapaconstantinou, D., Pararas, N., Pikouli, A., Nastos, C., Charalampopoulos, A., Dellaportas, D., Bagias, G., & Pikoulis, E. (2023). Precision Surgery of Colorectal Liver Metastases in the Current Era: A Systematic Review. Cancers, 15(7), 2083. https://doi.org/10.3390/cancers15072083







