Lymph Node Log-Odds Ratio Accurately Defines Prognosis in Resectable Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
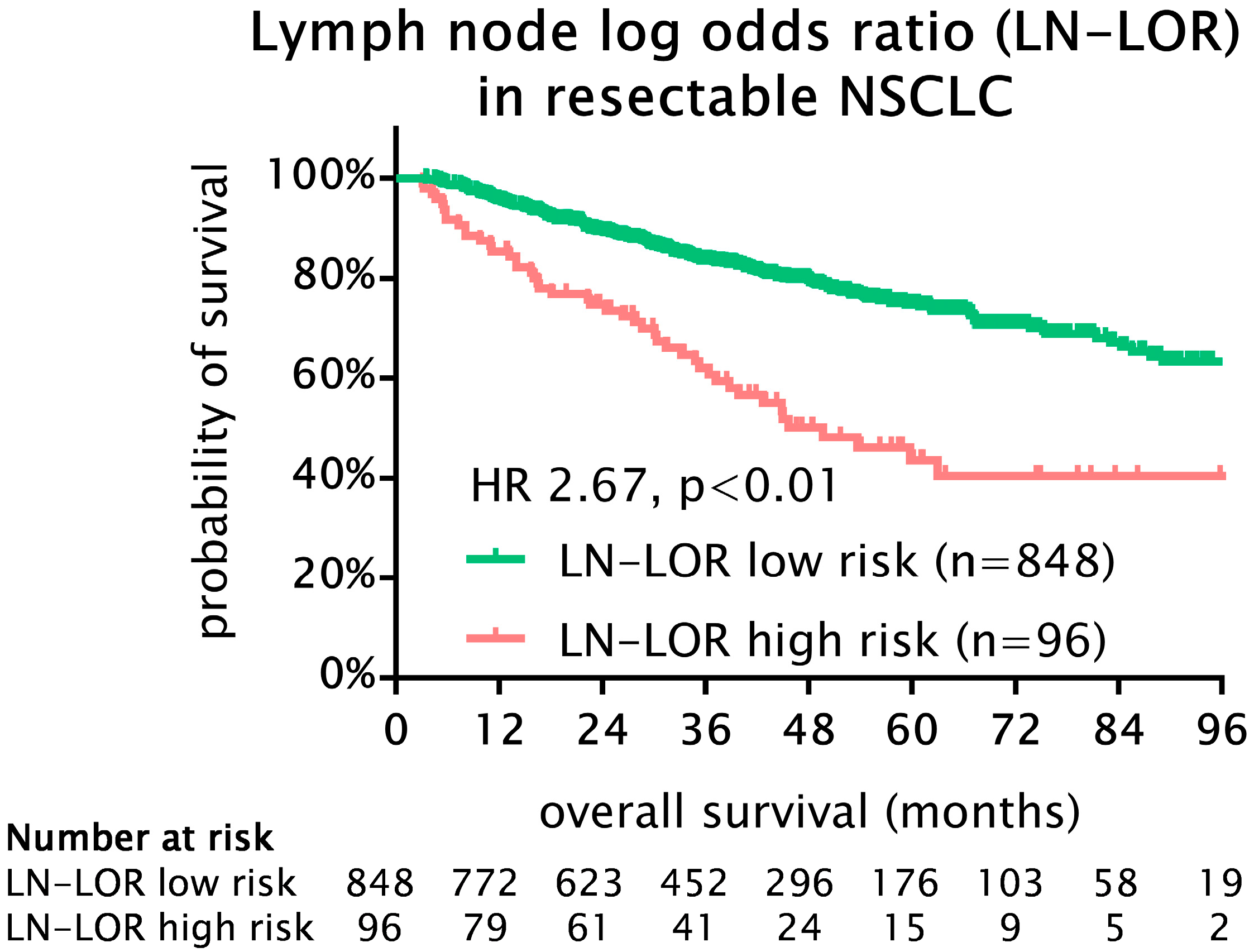
3.2. Cut-Off Analysis and Prognostic Significance of Lymph Node Log-Odds Ratio (LN-LOR)
3.3. Association of LN-LOR with Clinical and Pathological Factors
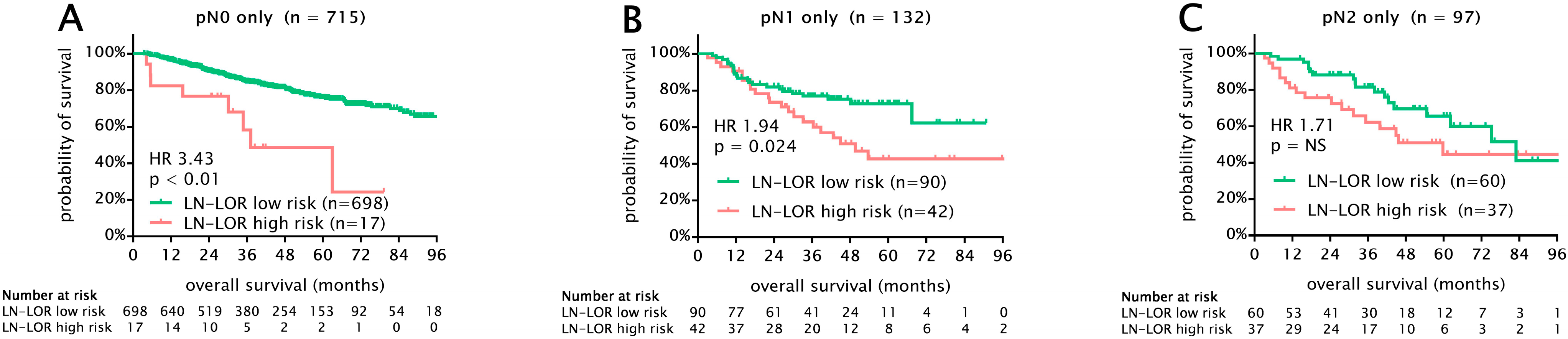
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | adenocarcinoma |
| CI | confidence interval |
| cN | clinical lymph node involvement |
| ESTS | European society of Thoracic Surgeons |
| HR | hazard ratio |
| IQR | interquartile range |
| LN | lymph node |
| LN-LOR | lymph node log-odds ratio |
| LNR | lymph node ratio |
| NSCLC | non small cell lung cancer |
| OS | overall survival |
| pN | pathological lymph node involvement |
| PORT | post operative radiotherapy |
| SD | standard deviation |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
| SQC | squamous cell carcinoma |
| TNM | tumor node metastasis |
| VAMLA | video-assisted mediastinal lymphadenectomy |
References
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P.; Members of IASLC Staging Committee. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Asamura, H.; Kawachi, R.; Sakurai, H.; Watanabe, S. Which is the better prognostic factor for resected non-small cell lung cancer: The number of metastatic lymph nodes or the currently used nodal stage classification? J. Thorac. Oncol. 2011, 6, 310–318. [Google Scholar] [CrossRef]
- Saji, H.; Tsuboi, M.; Shimada, Y.; Kato, Y.; Yoshida, K.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013, 143, 1618–1625. [Google Scholar] [CrossRef]
- Chiappetta, M.; Leuzzi, G.; Sperduti, I.; Bria, E.; Mucilli, F.; Lococo, F.; Spaggiari, L.; Ratto, G.B.; Filosso, P.L.; Facciolo, F. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: A multicentre analysis†. Eur. J. Cardiothorac. Surg. 2019, 55, 405–412. [Google Scholar] [CrossRef]
- Taylor, M.D.; LaPar, D.J.; Thomas, C.J.; Persinger, M.; Stelow, E.B.; Kozower, B.D.; Lau, C.L.; Jones, D.R. Lymph node ratio predicts recurrence and survival after R0 resection for non-small cell lung cancer. Ann. Thorac. Surg. 2013, 96, 1163–1170. [Google Scholar] [CrossRef]
- Chen, S.-B.; Weng, H.-R.; Wang, G.; Zou, X.-F.; Liu, D.-T.; Chen, Y.-P.; Zhang, H. Lymph node ratio-based staging system for esophageal squamous cell carcinoma. World J. Gastroenterol. 2015, 21, 7514–7521. [Google Scholar] [CrossRef]
- Liao, G.-S.; Chou, Y.-C.; Golshan, M.; Hsu, H.-M.; Hong, Z.-J.; Yu, J.-C.; Zhu, J.-H. Prognostic value of the lymph node ratio in breast cancer subtypes. Am. J. Surg. 2015, 210, 749–754. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Watchell, M.; Dissanaike, S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg. Oncol. 2015, 24, 84–88. [Google Scholar] [CrossRef]
- Woodward, W.A.; Vinh-Hung, V.; Ueno, N.T.; Cheng, Y.C.; Royce, M.; Tai, P.; Vlastos, G.; Wallace, A.M.; Hortobagyi, G.N.; Nieto, Y. Prognostic value of nodal ratios in node-positive breast cancer. J. Clin. Oncol. 2006, 24, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xu, Y.; Li, D.M.; Wang, Z.N.; Zhu, G.L.; Huang, B.J.; Li, K.; Xu, H.M. Log odds of positive lymph nodes: A novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010, 116, 2571–2580. [Google Scholar] [CrossRef]
- Persiani, R.; Cananzi, F.C.M.; Biondi, A.; Paliani, G.; Tufo, A.; Ferrara, F.; Vigorita, V.; D’Ugo, D. Log odds of positive lymph nodes in colon cancer: A meaningful ratio-based lymph node classification system. World J. Surg. 2012, 36, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, H.; Ma, Z.; Gong, L.; Chen, C.; Ren, P.; Shang, X.; Tang, P.; Jiang, H.; Yu, Z. Log odds of positive lymph nodes is a novel prognostic indicator for advanced ESCC after surgical resection. J. Thorac. Dis. 2017, 9, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Wang, J.; Hassett, J.M.; Dayton, M.T.; Kulaylat, M.N. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J. Gastrointest. Surg. 2008, 12, 1790–1796. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Hickey, G.L.; Dunning, J.; Seifert, B.; Sodeck, G.; Carr, M.J.; Burger, H.U.; Beyersdorf, F. Statistical and Data Reporting Guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur. J. Cardiothorac. Surg. 2015, 48, 180–193. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Mitchell, A.; Crowley, J.; Kondo, H.; Kim, Y.T.; Turrisi, A.; Goldstraw, P.; Rami-Porta, R. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2015, 10, 1515–1522. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar] [CrossRef]
- Fukui, T.; Mori, S.; Yokoi, K.; Mitsudomi, T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J. Thorac. Oncol. 2006, 1, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hanagiri, T.; Takenaka, M.; Oka, S.; Shigematsu, Y.; Nagata, Y.; Shimokawa, H.; Uramoto, H.; Tanaka, F. Clinical significance in the number of involved lymph nodes in patients that underwent surgery for pathological stage III-N2 non-small cell lung cancer. J. Cardiothorac. Surg. 2011, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, R.U.; Ogbata, O.; Yu, X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann. Thorac. Surg. 2014, 97, 385–393. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Lyu, M.; Chen, N.; Liao, H.; Wang, Z.; Hao, J.; Yan, C.; Liu, L. Prognostic value of lymph node ratio in non-small-cell lung cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2020, 50, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xue, L.; Wang, M.; Zhao, X. Lymph node ratio is a prognostic factor for non-small cell lung cancer. Oncotarget 2015, 6, 33912–33918. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, G.; Zheng, D.; Jia, M.; Dai, W.; Sun, Y.; Chen, H. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2017, 153, 702–709.e1. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Chen, G.; Zhang, P. Log odds of positive lymph nodes are superior to other measures for evaluating the prognosis of non-small cell lung cancer. Thorac. Cancer 2014, 5, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, T.; Wang, Y.; Xu, Y.; Yang, P.; Gomez, D.; Liao, Z. Log odds of positive lymph nodes may predict survival benefit in patients with node-positive non-small cell lung cancer. Lung Cancer 2018, 122, 60–66. [Google Scholar] [CrossRef]
- Dziedzic, D.; Piotr, R.; Langfort, R.; Orlowski, T.; Polish Lung Cancer Study Group (PLCSG). Log odds of positive lymph nodes as a novel prognostic indicator in NSCLC staging. Surg. Oncol. 2017, 26, 80–85. [Google Scholar] [CrossRef]
- Jin, X.; Chen, D.; Shen, Y.; Shu, J.; Sang, Y.; Yang, W.; Duan, S.; Chen, Y. Log odds of positive lymph nodes is a robust predictor of survival and benefits from postoperative radiotherapy in stage IIIA-N2 resected non-small cell lung cancer. Thorac. Cancer. 2022, 13, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Sinn, K.; Mosleh, B.; Steindl, A.; Zoechbauer-Mueller, S.; Dieckmann, K.; Widder, J.; Steiner, E.; Klepetko, W.; Hoetzenecker, K.; Laszlo, V.; et al. Neoadjuvant chemoradiotherapy is superior to chemotherapy alone in surgically treated stage III/N2 non-small-cell lung cancer: A retrospective single-center cohort study. ESMO Open 2022, 7, 100466. [Google Scholar] [CrossRef] [PubMed]
- Cackowski, M.M.; Gryszko, G.M.; Zbytniewski, M.; Dziedzic, D.A.; Orłowski, T.M. Alternative methods of lymph node staging in lung cancer: A narrative review. J. Thorac. Dis. 2020, 12, 6042–6053. [Google Scholar] [CrossRef] [PubMed]

| Parameter | n | % | |
|---|---|---|---|
| Sex | female | 415 | 44% |
| male | 529 | 56% | |
| Age | <65 | 404 | 43% |
| ≥65 | 540 | 57% | |
| Histological subtype | Adenocarcinoma | 672 | 71% |
| Squamous cell carcinoma | 211 | 22% | |
| Other | 61 | 7% | |
| Pathologic T Stage | pT1 | 487 | 52% |
| pT2 | 355 | 38% | |
| pT3 | 81 | 9% | |
| pT4 | 21 | 2% | |
| Pathologic N Stage | pN0 | 715 | 76% |
| pN1 | 132 | 14% | |
| pN2 | 97 | 10% | |
| Overall Stage | I | 599 | 63% |
| II | 212 | 22% | |
| III | 133 | 14% | |
| Total number of resected lymph nodes | median (IQR) | 12 (11) | |
| Neoadjuvant treatment | no | 870 | 92% |
| yes | 74 | 8% | |
| Adjuvant treatment | no | 754 | 80% |
| yes | 190 | 20% | |
| All Patients | LN-LOR Low Risk | LN-LOR High Risk | |||||
|---|---|---|---|---|---|---|---|
| n | n | % | n | % | p | ||
| Sex | female | 415 | 368 | 43% | 47 | 49% | 0.25 |
| male | 529 | 480 | 57% | 49 | 51% | ||
| Age | <65 | 404 | 363 | 43% | 41 | 43% | 0.138 |
| ≥65 | 540 | 485 | 57% | 55 | 57% | ||
| Histological subtype | ADC | 672 | 597 | 70% | 75 | 78% | 0.167 |
| SQC | 211 | 196 | 23% | 15 | 16% | ||
| Other | 61 | 55 | 7% | 6 | 6% | ||
| pT Stage | pT1 | 487 | 442 | 52% | 45 | 47% | 0.36 |
| pT2 | 355 | 316 | 37% | 39 | 41% | ||
| pT3 | 81 | 73 | 9% | 8 | 8% | ||
| pT4 | 21 | 17 | 2% | 4 | 4% | ||
| pN Stage | pN0 | 715 | 698 | 82% | 17 | 18% | 0.001 |
| pN1 | 132 | 90 | 11% | 42 | 44% | ||
| pN2 | 97 | 60 | 7% | 37 | 39% | ||
| Stage | I | 599 | 585 | 69% | 14 | 15% | 0.001 |
| II | 212 | 173 | 20% | 39 | 41% | ||
| III | 133 | 90 | 11% | 43 | 45% | ||
| Station 7 positive | no | 870 | 830 | 98% | 79 | 82% | 0.048 |
| yes | 74 | 18 | 2% | 17 | 18% | ||
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Lower | Upper | Lower | Upper | |||||
| Sex (male) | 0.60 | 0.49 | 0.79 | 0.001 | 1.65 | 1.25 | 2.19 | 0.001 |
| Age (continuous) | 1.01 | 1.00 | 1.03 | 0.023 | 1.02 | 1.00 | 1.03 | 0.020 |
| Histological subtype | 0.004 | 0.003 | ||||||
| ADC | - | - | - | - | - | - | ||
| SQC | 1.35 | 1.01 | 1.82 | 0.046 | 1.23 | 0.91 | 1.67 | 0.176 |
| Other | 1.94 | 1.27 | 2.96 | 0.002 | 2.11 | 1.35 | 3.28 | 0.001 |
| pT Stage | 0.001 | 0.25 | ||||||
| pT1 | - | - | - | - | - | - | ||
| pT2 | 1.52 | 1.15 | 2.01 | 0.003 | 1.33 | 0.99 | 1.78 | 0.060 |
| pT3 | 2.21 | 1.45 | 3.37 | 0.000 | 1.48 | 0.85 | 2.55 | 0.164 |
| pT4 | 2.27 | 1.14 | 4.49 | 0.019 | 1.64 | 0.62 | 4.34 | 0.31 |
| pN Stage | 0.001 | 0.68 | ||||||
| pN0 | - | - | - | - | - | - | ||
| pN1 | 1.80 | 1.29 | 2.52 | 0.010 | 0.83 | 0.49 | 1.39 | 0.47 |
| pN2 | 1.89 | 1.31 | 2.73 | 0.010 | 1.01 | 0.40 | 2.55 | 0.99 |
| Stage | 0.001 | 0.013 | ||||||
| Stage I | - | - | - | - | - | - | ||
| Stage II | 2.27 | 1.69 | 3.04 | 0.001 | 1.91 | 1.23 | 2.98 | 0.004 |
| Stage III | 2.19 | 1.56 | 3.08 | 0.001 | 1.49 | 0.60 | 3.73 | 0.39 |
| Neoadjuvant treatment (yes) | 1.37 | 0.90 | 2.07 | 0.140 | 1.33 | 0.86 | 2.07 | 0.199 |
| Adjuvant treatment (yes) | 1.39 | 1.04 | 1.87 | 0.029 | 0.98 | 0.70 | 1.37 | 0.89 |
| LN-LOR low | 0.38 | 0.28 | 0.53 | 0.001 | 0.48 | 0.32 | 0.72 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benej, M.; Klikovits, T.; Krajc, T.; Bohanes, T.; Schulte, L.; Hochmair, M.J.; Watzka, S.; Mosleh, B.; Hoetzenecker, K.; Aigner, C.; et al. Lymph Node Log-Odds Ratio Accurately Defines Prognosis in Resectable Non-Small Cell Lung Cancer. Cancers 2023, 15, 2082. https://doi.org/10.3390/cancers15072082
Benej M, Klikovits T, Krajc T, Bohanes T, Schulte L, Hochmair MJ, Watzka S, Mosleh B, Hoetzenecker K, Aigner C, et al. Lymph Node Log-Odds Ratio Accurately Defines Prognosis in Resectable Non-Small Cell Lung Cancer. Cancers. 2023; 15(7):2082. https://doi.org/10.3390/cancers15072082
Chicago/Turabian StyleBenej, Michal, Thomas Klikovits, Tibor Krajc, Tomas Bohanes, Lisa Schulte, Maximilian Johannes Hochmair, Stefan Watzka, Berta Mosleh, Konrad Hoetzenecker, Clemens Aigner, and et al. 2023. "Lymph Node Log-Odds Ratio Accurately Defines Prognosis in Resectable Non-Small Cell Lung Cancer" Cancers 15, no. 7: 2082. https://doi.org/10.3390/cancers15072082
APA StyleBenej, M., Klikovits, T., Krajc, T., Bohanes, T., Schulte, L., Hochmair, M. J., Watzka, S., Mosleh, B., Hoetzenecker, K., Aigner, C., Hoda, M. A., & Mueller, M. R. (2023). Lymph Node Log-Odds Ratio Accurately Defines Prognosis in Resectable Non-Small Cell Lung Cancer. Cancers, 15(7), 2082. https://doi.org/10.3390/cancers15072082









