Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Knowledge Gaps
- The number of liver metastases that is considered safe to resect;
- The number of liver resections or liver segments that is considered safe to resect;
- The size of the future liver remnant that is considered safe (for simultaneous surgery);
- The timing of staged resection (liver or primary tumor first);
- Whether chemotherapy should be administered before or after surgery (or if at all);
- Oncological endpoints (recurrence, time to recurrence, survival, etc.);
- Quality of life;
- Health economics.
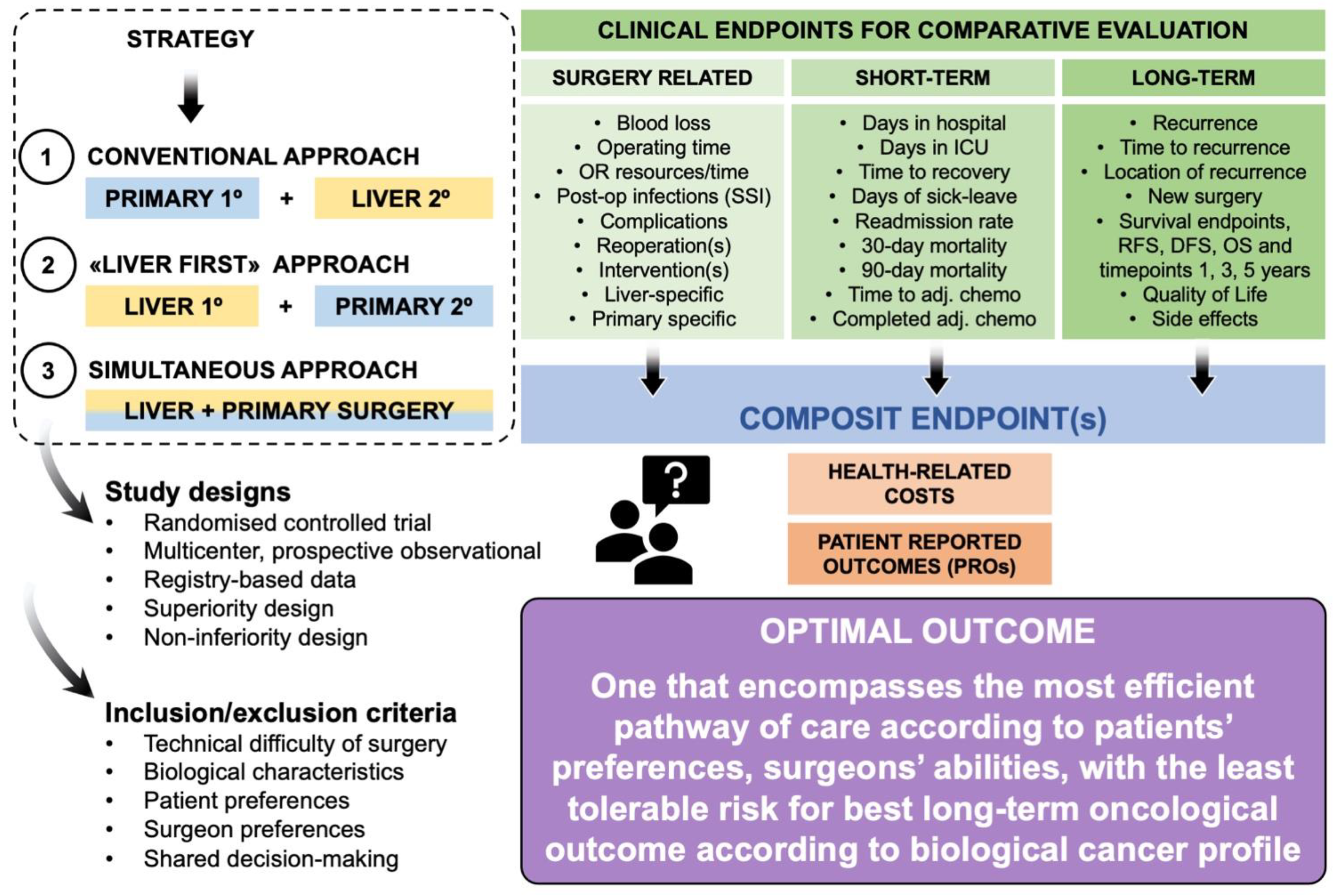
3. Endpoints—Which One and for What Trial Design?
4. Surgery-Related Endpoints and Complications
5. Short-Term Outcomes
6. Long-Term Outcomes and Survival
7. Why a Composite Endpoint?
8. Other Related Endpoints to Consider
- EORTC-QLQ-30 (designed to measure cancer patients physical, psychological, and social functions);
- QLQ-CR29 (QoL measurement disease-specific for colorectal cancer);
- QLQ-LM21 (QoL measurement disease-specific for CRLM);
- EQ-5D-5L (measuring health-related QoL).
9. Who Should Be Included in a Surgical Trial?
9.1. Staged or Simultaneous Surgery?
9.2. Tumor Biology and Molecular Markers—Any Role?
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, A.K.; Mason, J.M.; Mullamitha, S.; Hancock, H.C.; Jegatheeswaran, S. Management of colorectal cancer presenting with synchronous liver metastases. Nat. Rev. Clin. Oncol. 2014, 11, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A. Outcomes after surgical treatment of colorectal cancer liver metastases. Semin. Oncol. 2005, 32 (Suppl. S9), S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Scherman, P.; Syk, I.; Holmberg, E.; Naredi, P.; Rizell, M. Impact of patient, primary tumor and metastatic pattern including tumor location on survival in patients undergoing ablation or resection for colorectal liver metastases: A population-based national cohort study. Eur. J. Surg. Oncol. 2021, 47, 375–383. [Google Scholar] [CrossRef]
- Adam, R.; De Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Version 3. Available online: https://www.nccn.org (accessed on 8 February 2023).
- Zeyara, A.; Toren, W.; Soreide, K.; Andersson, R. The liver-first approach for synchronous colorectal liver metastases: A systematic review and meta-analysis of completion rates and effects on survival. Scand. J. Surg. 2022, 111, 14574969211030131. [Google Scholar] [CrossRef]
- Labori, K.J.; Guren, M.G.; Brudvik, K.W.; Rosok, B.I.; Waage, A.; Nesbakken, A.; Larsen, S.; Dueland, S.; Edwin, B.; Bjornbeth, B.A. Resection of synchronous liver metastases between radiotherapy and definitive surgery for locally advanced rectal cancer: Short-term surgical outcomes, overall survival and recurrence-free survival. Colorectal. Dis. 2017, 19, 731–738. [Google Scholar] [CrossRef]
- Giuliante, F.; Vigano, L.; De Rose, A.M.; Mirza, D.F.; Lapointe, R.; Kaiser, G.; Barroso, E.; Ferrero, A.; Isoniemi, H.; Lopez-Ben, S.; et al. Liver-First Approach for Synchronous Colorectal Metastases: Analysis of 7360 Patients from the LiverMetSurvey Registry. Ann. Surg. Oncol. 2021, 28, 8198–8208. [Google Scholar] [CrossRef]
- Gumiero, J.L.; Oliveira, B.M.S.; Neto, P.A.O.; Pandini, R.V.; Gerbasi, L.S.; Figueiredo, M.N.; Kruger, J.A.P.; Seid, V.E.; Araujo, S.E.A.; Tustumi, F. Timing of resection of synchronous colorectal liver metastasis: A systematic review and meta-analysis. J. Surg. Oncol. 2022, 126, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, A.K. Synchronous resection of primary colorectal cancer with liver metastases: Two birds with one stone? Br. J. Surg. 2022, 109, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, L.; Li, X.; Wang, M.; Zhao, Y.; Zhang, N.; Wang, L.; Zhang, T.; Mao, A.; Xu, Y.; et al. The Value of Laparoscopic Simultaneous Colorectal and Hepatic Resection for Synchronous Colorectal Cancer Liver Metastasis: A Propensity Score Matching Study. Front. Oncol. 2022, 12, 916455. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, A.; Wang, H.; Fang, G.; Zhou, J.; He, X.; Cai, S.; Wang, L. Association of Simultaneous vs Delayed Resection of Liver Metastasis with Complications and Survival among Adults with Colorectal Cancer. JAMA Netw. Open 2022, 5, e2231956. [Google Scholar] [CrossRef]
- Kazi, M.; Patkar, S.; Patel, P.; Kunte, A.; Desouza, A.; Saklani, A.; Goel, M. Simultaneous resection of synchronous colorectal liver metastasis: Feasibility and development of a prediction model. Ann. Hepatobiliary Pancreat. Surg. 2022, 27, 40–48. [Google Scholar] [CrossRef]
- Boudjema, K.; Locher, C.; Sabbagh, C.; Ortega-Deballon, P.; Heyd, B.; Bachellier, P.; Metairie, S.; Paye, F.; Bourlier, P.; Adam, R.; et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann. Surg. 2021, 273, 49–56. [Google Scholar] [CrossRef]
- Chan, A.K.C.; Mason, J.M.; Baltatzis, M.; Siriwardena, A.K.; Co, S.C. Management of Colorectal Cancer with Synchronous Liver Metastases: An Inception Cohort Study (CoSMIC). Ann. Surg. Oncol. 2022, 29, 1939–1951. [Google Scholar] [CrossRef]
- Carbone, F.; Chee, Y.; Rasheed, S.; Cunningham, D.; Bhogal, R.H.; Jiao, L.; Tekkis, P.; Kontovounisios, C. Which surgical strategy for colorectal cancer with synchronous hepatic metastases provides the best outcome? A comparison between primary first, liver first and simultaneous approach. Updates Surg. 2022, 74, 451–465. [Google Scholar] [CrossRef]
- Kleive, D.; Aas, E.; Angelsen, J.H.; Bringeland, E.A.; Nesbakken, A.; Nymo, L.S.; Schultz, J.K.; Soreide, K.; Yaqub, S. Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps. Oncol. Ther. 2021, 9, 111–120. [Google Scholar] [CrossRef]
- Krul, M.F.; Elfrink, A.K.E.; Buis, C.I.; Swijnenburg, R.J.; Te Riele, W.W.; Verhoef, C.; Gobardhan, P.D.; Dulk, M.D.; Liem, M.S.L.; Tanis, P.J.; et al. Hospital variation and outcomes of simultaneous resection of primary colorectal tumour and liver metastases: A population-based study. HPB 2022, 24, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shubert, C.R.; Habermann, E.B.; Bergquist, J.R.; Thiels, C.A.; Thomsen, K.M.; Kremers, W.K.; Kendrick, M.L.; Cima, R.R.; Nagorney, D.M. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J. Gastrointest. Surg. 2015, 19, 1982–1994. [Google Scholar] [CrossRef]
- Driedger, M.R.; Yamashita, T.S.; Starlinger, P.; Mathis, K.L.; Smoot, R.L.; Cleary, S.P.; Nagorney, D.M. Synchronous resection of colorectal cancer primary and liver metastases: An outcomes analysis. HPB 2021, 23, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
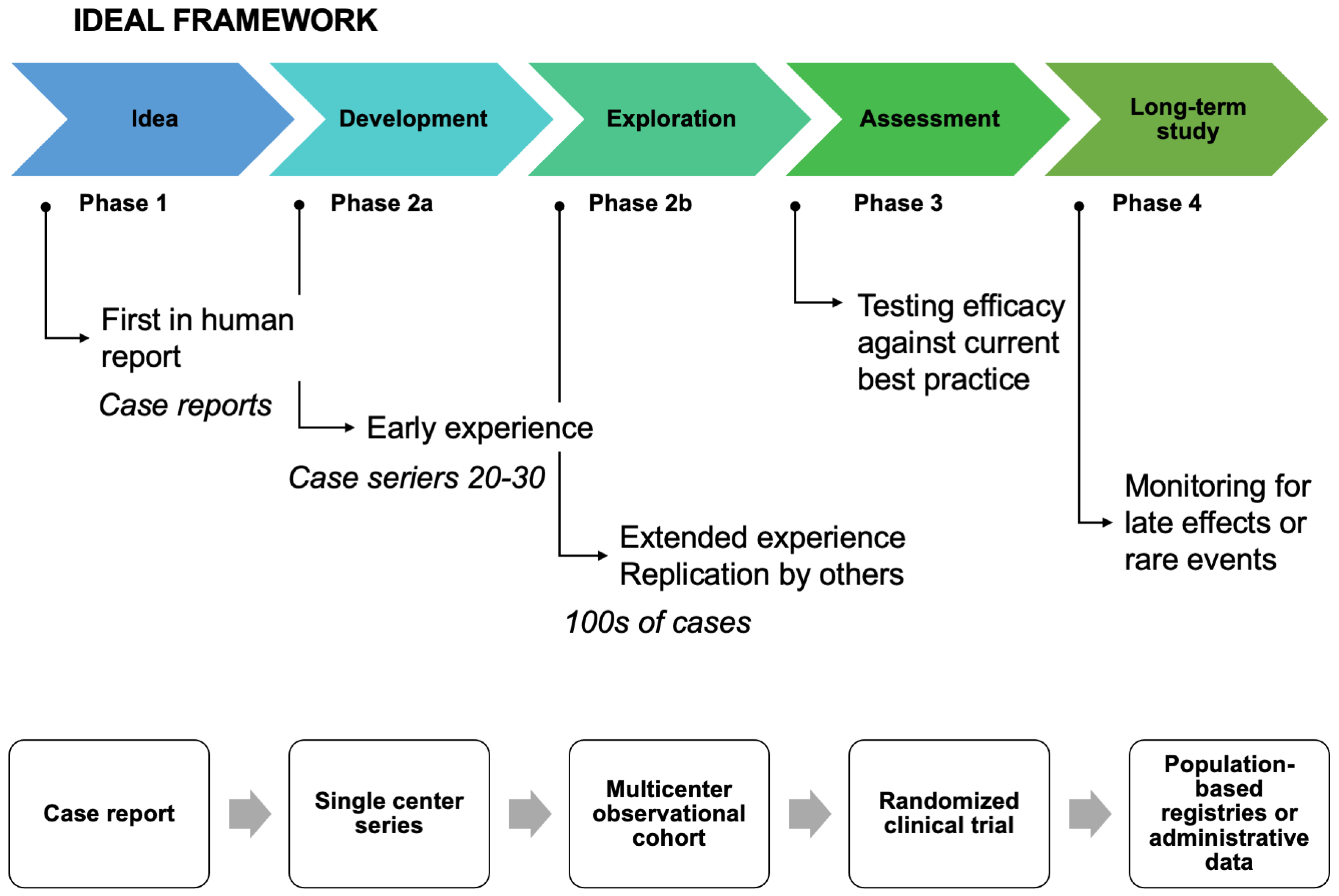
- Dimick, J.B.; Sedrakyan, A.; McCulloch, P. The IDEAL Framework for Evaluating Surgical Innovation: How It Can Be Used to Improve the Quality of Evidence. JAMA Surg. 2019, 154, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Kazaryan, A.M.; Rosok, B.I.; Edwin, B. Morbidity assessment in surgery: Refinement proposal based on a concept of perioperative adverse events. ISRN Surg. 2013, 2013, 625093. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Hayashi, H.; Miyamoto, Y.; Imai, K.; Kitano, Y.; Kaida, T.; Sawayama, H.; Beppu, T.; Yamashita, Y.I.; Baba, H. Clinical Impact of Operative Order in Laparoscopic Simultaneous Resection for Synchronous Colorectal Liver Metastases. Cancer Diagn. Progn. 2021, 1, 151–156. [Google Scholar] [CrossRef]
- Ono, Y.; Saiura, A.; Arita, J.; Takahashi, Y.; Takahashi, M.; Inoue, Y. Short-Term Outcomes after Simultaneous Colorectal and Major Hepatic Resection for Synchronous Colorectal Liver Metastases. Dig. Surg. 2017, 34, 447–454. [Google Scholar] [CrossRef]
- De Graaff, M.R.; Elfrink, A.K.E.; Buis, C.I.; Swijnenburg, R.J.; Erdmann, J.I.; Kazemier, G.; Verhoef, C.; Mieog, J.S.D.; Derksen, W.J.M.; Van den Boezem, P.B.; et al. Defining Textbook Outcome in liver surgery and assessment of hospital variation: A nationwide population-based study. Eur. J. Surg. Oncol. 2022, 48, 2414–2423. [Google Scholar] [CrossRef]
- Van den Broek, M.A.; Van Dam, R.M.; Van Breukelen, G.J.; Bemelmans, M.H.; Oussoultzoglou, E.; Pessaux, P.; Dejong, C.H.; Freemantle, N.; Olde Damink, S.W. Development of a composite endpoint for randomized controlled trials in liver surgery. Br. J. Surg. 2011, 98, 1138–1145. [Google Scholar] [CrossRef]
- Le Souder, E.B.; Azin, A.; Hirpara, D.H.; Walker, R.; Cleary, S.; Quereshy, F. Considering the cost of a simultaneous versus staged approach to resection of colorectal cancer with synchronous liver metastases in a publicly funded healthcare model. J. Surg. Oncol. 2018, 117, 1376–1385. [Google Scholar] [CrossRef]
- Ejaz, A.; Semenov, E.; Spolverato, G.; Kim, Y.; Tanner, D.; Hundt, J.; Pawlik, T.M. Synchronous primary colorectal and liver metastasis: Impact of operative approach on clinical outcomes and hospital charges. HPB 2014, 16, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Lee, J.; Saadat, L.V.; Aparicio, T.; Buisman, F.E.; Balachandran, V.P.; Drebin, J.A.; Hasegawa, K.; Jarnagin, W.R.; Kemeny, N.E.; et al. Recurrence-free survival versus overall survival as a primary endpoint for studies of resected colorectal liver metastasis: A retrospective study and meta-analysis. Lancet Oncol. 2022, 23, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Ignatowicz, A.M.; Mason, J.M.; Siriwardena, A.K. Colorectal cancer and synchronous liver metastases: An individual case-based qualitative study (CoSMIC-Q). Eur. J. Surg. Oncol. 2022, 48, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K. Time to halt perioperative chemotherapy for resectable colorectal liver metastasis? Br. J. Surg. 2022, 109, 242–243. [Google Scholar] [CrossRef]
- Soreide, K.; Watson, M.M.; Hagland, H.R. Deciphering the Molecular Code to Colorectal Liver Metastasis Biology Through Microsatellite Alterations and Allelic Loss: The Good, the Bad, and the Ugly. Gastroenterology 2016, 150, 811–814. [Google Scholar] [CrossRef]
- PelvEx Collaborative. Management strategies for patients with advanced rectal cancer and liver metastases using modified Delphi methodology: Results from the PelvEx Collaborative. Colorectal. Dis. 2020, 22, 1184–1188. [Google Scholar] [CrossRef]
- PelvEx Collaborative. Simultaneous pelvic exenteration and liver resection for primary rectal cancer with synchronous liver metastases: Results from the PelvEx Collaborative. Colorectal. Dis. 2020, 22, 1258–1262. [Google Scholar] [CrossRef]
- Fossum, C.C.; Alabbad, J.Y.; Romak, L.B.; Hallemeier, C.L.; Haddock, M.G.; Huebner, M.; Dozois, E.J.; Larson, D.W. The role of neoadjuvant radiotherapy for locally-advanced rectal cancer with resectable synchronous metastasis. J. Gastrointest. Oncol. 2017, 8, 650–658. [Google Scholar] [CrossRef]
- Silberhumer, G.R.; Paty, P.B.; Temple, L.K.; Araujo, R.L.; Denton, B.; Gonen, M.; Nash, G.M.; Allen, P.J.; DeMatteo, R.P.; Guillem, J.; et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am. J. Surg. 2015, 209, 935–942. [Google Scholar] [CrossRef]
- Chan, A.K.C.; Siriwardena, A.K. Practical Implications of KRAS Mutation Status and Sidedness of Primary Tumour in Patients with Colorectal Cancer and Synchronous Liver Metastases: A Subset Analysis of the CoSMIC Study. Cancers 2022, 14, 4833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqub, S.; Margonis, G.A.; Søreide, K. Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints. Cancers 2023, 15, 2177. https://doi.org/10.3390/cancers15072177
Yaqub S, Margonis GA, Søreide K. Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints. Cancers. 2023; 15(7):2177. https://doi.org/10.3390/cancers15072177
Chicago/Turabian StyleYaqub, Sheraz, Georgios Antonios Margonis, and Kjetil Søreide. 2023. "Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints" Cancers 15, no. 7: 2177. https://doi.org/10.3390/cancers15072177
APA StyleYaqub, S., Margonis, G. A., & Søreide, K. (2023). Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints. Cancers, 15(7), 2177. https://doi.org/10.3390/cancers15072177






