Association between Posttreatment Serum Platelet-to-Lymphocyte Ratio and Distant Metastases in Patients with Hepatocellular Carcinoma Receiving Curative Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Serum Indices and Data Collection
2.3. Treatments
2.4. Study End Points
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Treatment Characteristics
3.3. Distribution of Serum Indices
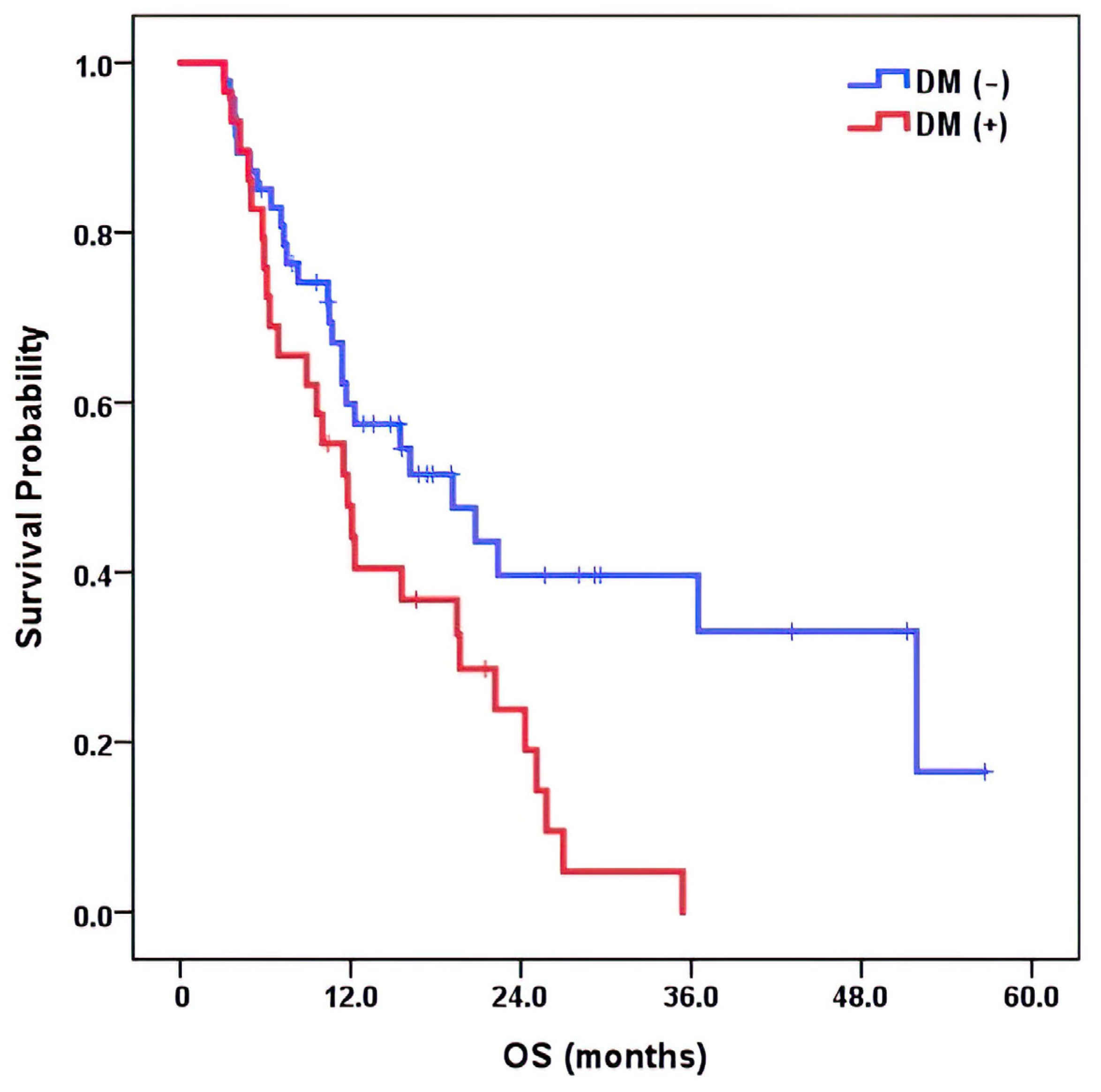
3.4. Survival Analysis and Effect on Overall Survival
3.5. Determination of the Optimal Cutoff Points for Variables
3.6. Multivariate Prognostic Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Melkonian, S.C.; Jim, M.A.; Reilley, B.; Erdrich, J.; Berkowitz, Z.; Wiggins, C.L.; Haverkamp, D.; White, M.C. Incidence of primary liver cancer in American Indians and Alaska Natives, US, 1999-2009. Cancer Causes Control. 2018, 29, 833–844. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G.; The Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Seong, J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer 2014, 3, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Huo, T.I.; Miksad, R.A. Hepatocellular Carcinoma with Portal Vein Tumor Involvement: Best Management Strategies. Semin. Liver Dis. 2018, 38, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Rim, C.H.; Cheng, J.; Huang, W.Y.; Kimura, T.; Lee, V.; Zeng, Z.C.; Seong, J. An evaluation of hepatocellular carcinoma practice guidelines from a radiation oncology perspective. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 148, 73–81. [Google Scholar] [CrossRef]
- Su, T.S.; Li, L.Q.; Meng, W.W.; Wang, Y.D.; Chen, Y.T.; Li, J.X.; Du, Y.Q.; Qu, S.; Zhao, C.; Huang, D.J.; et al. Long-Term Survival Analysis of Transarterial Chemoembolization Plus Radiotherapy vs. Radiotherapy for Hepatocellular Carcinoma With Macroscopic Vascular Invasion. Front. Oncol. 2020, 10, 1205. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, L.; Wu, Q.; Wang, L.; Lei, J.; Luo, H.; Yi, F.; Wei, Y.; Yu, J.; Zhang, W. Stereotactic Body Radiotherapy Combined with Transcatheter Arterial Chemoembolization versus Stereotactic Body Radiotherapy Alone as the First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Meta-Analysis and Systematic Review. Chemotherapy 2019, 64, 248–258. [Google Scholar] [CrossRef]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef]
- Ishikawa, H.; Nakai, K.; Nonaka, T.; Sakurai, H. Particle Therapy in Cancer Treatment-Current and Future Perspective. Gan to Kagaku Ryoho. Cancer Chemother. 2019, 46, 1219–1225. [Google Scholar]
- Kwon, J.H.; Bae, S.H.; Kim, J.Y.; Choi, B.O.; Jang, H.S.; Jang, J.W.; Choi, J.Y.; Yoon, S.K.; Chung, K.W. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010, 10, 475. [Google Scholar] [CrossRef]
- Noble, D.J.; Burnet, N.G. The future of image-guided radiotherapy-is image everything? Br. J. Radiol. 2018, 91, 20170894. [Google Scholar] [CrossRef]
- Rackwitz, T.; Debus, J. Clinical applications of proton and carbon ion therapy. Semin. Oncol. 2019, 46, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Mayles, W.P.; Cooper, T.; Mackay, R.; Staffurth, J.; Williams, M. Progress with Intensity-modulated radiotherapy implementation in the UK. Clin. Oncol. 2012, 24, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H.; Carr, B.I.; Bag, H.G.; Karaoğullarından, Ü.; Yalçın, K.; Ekin, N.; Özakyol, A.; Altıntaş, E.; Balaban, H.Y.; Şimşek, H.; et al. Serum levels of inflammatory markers CRP, ESR and albumin in relation to survival for patients with hepatocellular carcinoma. Int. J. Clin. Pract. 2021, 75, e13593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Hu, D.; Zhang, J.; Xu, Y.; Feng, H.; Chen, Z.; Luo, Y.; Lou, Y.; Wu, H. Predictive Value of Pretreatment Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio in the Survival of Nasopharyngeal Carcinoma Patients. Cancer Manag. Res. 2021, 13, 8767–8779. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhang, X.P.; Cai, X.R.; Xie, S.D.; Liu, M.M.; Lin, J.X.; Ma, X.K.; Chen, J.; Lin, Q.; Dong, M.; et al. The Predictive Value of Albumin-to-Alkaline Phosphatase Ratio for Overall Survival of Hepatocellular Carcinoma Patients Treated with Trans-Catheter Arterial Chemoembolization Therapy. J. Cancer 2018, 9, 3467–3478. [Google Scholar] [CrossRef]
- Jiang, C.; Lu, Y.; Zhang, S.; Huang, Y. Systemic Immune-Inflammation Index Is Superior to Neutrophil to Lymphocyte Ratio in Prognostic Assessment of Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. BioMed Res. Int. 2020, 2020, 7961568. [Google Scholar] [CrossRef]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef]
- Li, N.; Tian, G.W.; Wang, Y.; Zhang, H.; Wang, Z.H.; Li, G. Prognostic Role of the Pretreatment C-Reactive Protein/Albumin Ratio in Solid Cancers: A Meta-Analysis. Sci. Rep. 2017, 7, 41298. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Yang, Y.; An, W.; Li, S.; Wang, B.; He, Y.; Li, J.; Shao, Q.; Qin, L. Prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in patients with laryngeal squamous cell carcinoma. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2021, 46, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Mu, X.; He, K.; Wang, P.; Wang, D.; Liu, C.; Yu, J. Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol. 2020, 16, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, J.; Deng, H.; Li, H.; Su, C.; Guo, X. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Oncotarget 2016, 7, 45283–45301. [Google Scholar] [CrossRef]
- Zheng, J.; Cai, J.; Li, H.; Zeng, K.; He, L.; Fu, H.; Zhang, J.; Chen, L.; Yao, J.; Zhang, Y.; et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: A Meta-Analysis and Systematic Review. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 967–981. [Google Scholar] [CrossRef]
- Rodel, R.M.; Matthias, C.; Blomeyer, B.D.; Wolff, H.A.; Jung, K.; Christiansen, H. Impact of distant metastasis in patients with cervical lymph node metastases from cancer of an unknown primary site. Ann. Otol. Rhinol. Laryngol. 2009, 118, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Rosa Mendoza, E.S.; Moreno, E.; Caguioa, P.B. Predictors of early distant metastasis in women with breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, S.; Sawai, N.Y.; Kishino, M.; Ishihama, K.; Minami, M.; Okura, M. Risk factors for distant metastasis in squamous cell carcinoma of the oral cavity. J. Oral. Maxillofac. Surg. 2013, 71, 1291–1297. [Google Scholar] [CrossRef]
- Abdallah, E.A.; Souza, E.S.V.; Braun, A.C.; Gasparini, V.A.; Kupper, B.E.C.; Tariki, M.S.; Tarazona, J.G.R.; Takahashi, R.M.; Aguiar Junior, S.; Chinen, L.T.D. A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl. Oncol. 2021, 14, 100932. [Google Scholar] [CrossRef]
- Bae, B.K.; Park, H.C.; Yoo, G.S.; Choi, M.S.; Oh, J.H.; Yu, J.I. The Significance of Systemic Inflammation Markers in Intrahepatic Recurrence of Early-Stage Hepatocellular Carcinoma after Curative Treatment. Cancers 2022, 14, 2081. [Google Scholar] [CrossRef]
- Dharmapuri, S.; Ozbek, U.; Lin, J.Y.; Sung, M.; Schwartz, M.; Branch, A.D.; Ang, C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020, 9, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Langsenlehner, T.; Pichler, M.; Thurner, E.M.; Krenn-Pilko, S.; Stojakovic, T.; Gerger, A.; Langsenlehner, U. Evaluation of the platelet-to-lymphocyte ratio as a prognostic indicator in a European cohort of patients with prostate cancer treated with radiotherapy. Urol. Oncol. 2015, 33, 201.e209–216. [Google Scholar] [CrossRef] [PubMed]
- Riguetto, C.M.; Barreto, I.S.; Maia, F.F.R.; Assumpção, L.; Zantut-Wittmann, D.E. Usefulness of pre-thyroidectomy neutrophil-lymphocyte, platelet-lymphocyte, and monocyte-lymphocyte ratios for discriminating lymph node and distant metastases in differentiated thyroid cancer. Clinics 2021, 76, e3022. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, K.; Zhong, F.P.; Fan, Y.W.; Peng, L.; Zou, S.B. Clinicopathological and prognostic significance of platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma. Oncotarget 2016, 7, 81830–81838. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tong, J.; Tang, M.; Lu, Y.; Liang, G.; Zhang, Z.; Chen, T. Pretreatment Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Factors and Reference Markers of Treatment Options for Locally Advanced Squamous Cell Carcinoma Located in the Middle and Upper Esophagus. Cancer Manag. Res. 2021, 13, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Chen, Y.Y.; Kee, K.M.; Wang, C.C.; Tsai, M.C.; Kuo, Y.H.; Hung, C.H.; Li, W.F.; Lai, H.L.; Chen, Y.H. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Patients with Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab. Cancers 2022, 14, 343. [Google Scholar] [CrossRef]
- Wang, Y.; Attar, B.M.; Fuentes, H.E.; Jaiswal, P.; Tafur, A.J. Evaluation of the prognostic value of platelet to lymphocyte ratio in patients with hepatocellular carcinoma. J. Gastrointest. Oncol. 2017, 8, 1065–1071. [Google Scholar] [CrossRef]
- Chavaudra, J.; Bridier, A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2001, 5, 472–478. [Google Scholar] [CrossRef]
- Hodapp, N. The ICRU Report 83: Prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft 2012, 188, 97–99. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Shim, J.H.; Yoon, S.M.; Kim, P.H.; Alrashidi, I. Chemoembolization Plus Radiotherapy Versus Chemoembolization Plus Sorafenib for the Treatment of Hepatocellular Carcinoma Invading the Portal Vein: A Propensity Score Matching Analysis. Cancers 2020, 12, 1116. [Google Scholar] [CrossRef]
- Yoon, S.M.; Ryoo, B.Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef]
- Casotti, V.; D’Antiga, L. Basic principles of liver physiology. In Pediatric Hepatology and Liver Transplantation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 21–39. [Google Scholar]
- Triantafyllou, E.; Woollard, K.J.; McPhail, M.J.W.; Antoniades, C.G.; Possamai, L.A. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front. Immunol. 2018, 9, 2948. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.; Dranoff, G. Dual roles for immunity in gastrointestinal cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Toiyama, Y.; Okugawa, Y.; Oki, S.; Ide, S.; Saigusa, S.; Araki, T.; Kusunoki, M. Clinical Implications of Pretreatment: Lymphocyte-to-Monocyte Ratio in Patients With Rectal Cancer Receiving Preoperative Chemoradiotherapy. Dis. Colon Rectum 2019, 62, 171–180. [Google Scholar] [CrossRef]
- Sica, A.; Allavena, P.; Mantovani, A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008, 267, 204–215. [Google Scholar] [CrossRef]
- Brandau, S.; Dumitru, C.A.; Lang, S. Protumor and antitumor functions of neutrophil granulocytes. Semin. Immunopathol. 2013, 35, 163–176. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Kahn, M.L. Platelets and tumor cells: A new form of border control. Cancer Cell 2013, 24, 9–11. [Google Scholar] [CrossRef]
- Watanabe, K.; Yasumoto, A.; Amano, Y.; Kage, H.; Goto, Y.; Yatomi, Y.; Takai, D.; Nagase, T. Mean platelet volume and lymphocyte-to-monocyte ratio are associated with shorter progression-free survival in EGFR-mutant lung adenocarcinoma treated by EGFR tyrosine kinase inhibitor. PLoS ONE 2018, 13, e0203625. [Google Scholar] [CrossRef]
- Krenn-Pilko, S.; Langsenlehner, U.; Thurner, E.M.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer 2014, 110, 2524–2530. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, X.Z.; Song, Y.X.; Gao, P.; Sun, J.X.; Wang, Z.N. High Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Breast Cancer: A Meta-Analysis. BioMed Res. Int. 2017, 2017, 9503025. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yin, H.; Xia, Y.; Wu, J.Z.; Liang, J.H.; Zhu, H.Y.; Fan, L.; Li, J.Y.; Wang, L.; Xu, W. Prognostic nutritional index, a novel biomarker which predicts worse prognosis in diffuse large B cell lymphoma. Leuk. Res. 2021, 110, 106664. [Google Scholar] [CrossRef]
- Kubota, K.; Ito, R.; Narita, N.; Tanaka, Y.; Furudate, K.; Akiyama, N.; Chih, C.H.; Komatsu, S.; Kobayashi, W. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer 2022, 22, 368. [Google Scholar] [CrossRef]
- Okadome, K.; Baba, Y.; Yagi, T.; Kiyozumi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 2020, 271, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; Fletcher, C.D.; O’Reilly, D.S.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur. J. Cancer 2011, 47, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Cao, W.; Gao, X.; Tang, M.; Zhu, D.; Liu, W. Pretreatment “prognostic nutritional index” as an indicator of outcome in lung cancer patients receiving ICI-based treatment: Systematic review and meta-analysis. Medicine 2022, 101, e31113. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y. The prognostic nutritional index is prognostic factor of gynecological cancer: A systematic review and meta-analysis. Int. J. Surg. 2019, 67, 79–86. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.M.; Wu, H.M.; Wen, X.M.; Qiu, D.Q.; Yang, Y.Y.; Wang, L.Z.; Zhu, W.B.; He, L.S.; Li, J.J. Prognostic significance of prognostic nutritional index and systemic immune-inflammation index in patients after curative breast cancer resection: A retrospective cohort study. BMC Cancer 2022, 22, 1128. [Google Scholar] [CrossRef]
- Demirelli, B.; Babacan, N.A.; Ercelep, Ö.; Öztürk, M.A.; Kaya, S.; Tanrıkulu, E.; Khalil, S.; Hasanov, R.; Alan, Ö.; Telli, T.A.; et al. Modified Glasgow Prognostic Score, Prognostic Nutritional Index and ECOG Performance Score Predicts Survival Better than Sarcopenia, Cachexia and Some Inflammatory Indices in Metastatic Gastric Cancer. Nutr. Cancer 2021, 73, 230–238. [Google Scholar] [CrossRef]
- Pinato, D.; North, B.; Sharma, R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: The prognostic nutritional index (PNI). Br. J. Cancer 2012, 106, 1439–1445. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Huang, R.; Pan, H.; Zuo, Y.; Zhao, R.; Xue, Y.; Song, H. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front. Nutr. 2022, 9, 1038118. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, X.; Xiao, L.; Long, G.; Yao, L.; Wang, Z.; Zhou, L. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2021, 25, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Struggling with nutrition in patients with advanced cancer: Nutrition and nourishment-focusing on metabolism and supportive care. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, ii27–ii34. [Google Scholar] [CrossRef]
- Laviano, A.; Di Lazzaro, L.; Koverech, A. Nutrition support and clinical outcome in advanced cancer patients. Proc. Nutr. Soc. 2018, 77, 388–393. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [PubMed]
- Carleo, G.; Cirac, I.; Cranmer, K.; Daudet, L.; Schuld, M.; Tishby, N.; Vogt-Maranto, L.; Zdeborová, L. Machine learning and the physical sciences. Rev. Mod. Phys. 2019, 91, 045002. [Google Scholar] [CrossRef]
| Characteristics | n (%) | |
|---|---|---|
| Age | ||
| Mean ± SD | 61.4 ± 10.6 | |
| Gender | ||
| Male | 62 (81.6) | |
| Female | 14 (18.4) | |
| ECOG PS | ||
| 0 | 30 (39.5) | |
| 1 | 43 (56.6) | |
| 2 | 3 (3.9) | |
| Comorbidity | ||
| No | 36 (47.4) | |
| Yes | 40 (52.6) | |
| HCC etiology | ||
| Alcoholic | 10 (13.2) | |
| HBV | 43 (56.6) | |
| HCV | 4 (5.3) | |
| NBNC | 4 (5.3) | |
| Mixed | 12 (15.8) | |
| Unknown | 3 (3.9) | |
| Combined PVT/IVT | ||
| No | 44 (57.9) | |
| Yes | 32 (42.1) | |
| C-T-P score | ||
| 5 (A) | 37 (48.7) | |
| 6 (A) | 18 (23.7) | |
| 7 (B) | 12 (15.8) | |
| 8 (B) | 5 (6.6) | |
| 9 (B) | 3 (3.9) | |
| 10 (C) | 1 (1.3) | |
| AFP (ng/mL) | ||
| Mean ± SD | 709.5 ± 1808.9 | |
| PIVKA-II (mAU/mL) | ||
| Mean ± SD | 3996.2 ± 18160 |
| Characteristics | n (%) | |
|---|---|---|
| Pre-RT TACE/HAIC | ||
| No | 2 (2.6) | |
| Yes | 74 (97.4) | |
| RT method | ||
| 3-dimensional CRT | 14 (18.4) | |
| Gating SBRT/IMRT | 26 (34.2) | |
| Arc | 25 (32.9) | |
| sIMRT | 11 (14.5) | |
| RT fraction number | ||
| Median (range) | 12 (4–30) | |
| RT fractional dose (Gy) | ||
| Median (range) | 5 (2–12) | |
| ≤5 Gy | 53 (69.7) | |
| >5 Gy | 23 (30.3) | |
| Total BED10 (Gy) | ||
| Median (range) | 72.6 (51.5–119) | |
| GTV sum (cc) | ||
| Median (range) | 55.3 (2.8–1288) | |
| Total liver volume (cc) | ||
| Median (range) | 1260.6 (551–2559.8) | |
| Mean total liver dose (cGy) | ||
| Median (range) | 2150.2 (327.5–4142.9) | |
| Mean liver dose (TL-PTV) (cGy) | ||
| Median (range) | 1615 (253.4–2993.9) |
| Indices | Pre-SII | Pre-NLR | Pre-PLR | Pre-PNI | Pre-ALC | Pre-LMR | Pre-A | Pre-AAR | Pre-P | Pre-H |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 374 ± 347.8 | 2.6 ± 1.6 | 119 ± 69 | 41 ± 8.1 | 1.3 ± 0.7 | 2.8 ± 1.1 | 35.3 ± 5.2 | 0.1 ± 0.05 | 71.3 ± 9.3 | 12.7 ± 1.8 |
| Indices | Post-SII-H | Post-SII-L | Post-NLR-H | Post-NLR-L | Post-PLR-H | Post-PLR-L | Post-PNI-H | Post-PNI-L | Post-ALC-L | |
| Mean ± SD | 1251.4 ± 1501.3 | 201.8 ± 194.7 | 14.8 ± 30.5 | 3.4 ± 10.1 | 320 ± 340 | 90.7 ± 36.6 | 40.5 ± 5.6 | 31.4 ± 6.3 | 0.4 ± 0.2 | |
| Indices | Post-LMR-H | Post-LMR-L | Post-A-H | Post-A-L | Post-AAR-H | Post-AAR-L | Post-P-H | Post-P-L | Post-H-H | Post-H-L |
| Mean ± SD | 3.1 ± 2.2 | 0.8 ± 0.4 | 36.7 ± 4.9 | 28.2 ± 5.8 | 1 ± 7.8 | 0.7 ± 5.3 | 73.3 ± 9.1 | 59.7 ± 9.7 | 13.4 ± 1.8 | 10.7 ± 2.1 |
| Indices | Pre-SII | Pre-NLR | Pre-PLR | Pre-PNI | Pre-ALC | Pre-LMR | Pre-A | Pre-AAR | Pre-P | Pre-H | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DC | |||||||||||
| p-value | 0.756 | 0.910 | 0.874 | 0.596 | 0.390 | 0.690 | 0.511 | 0.841 | 1 | 0.885 | |
| Cut-point | 391.4 | 1.6 | 68.9 | 37.7 | 1.8 | 1.8 | 34 | 0.06 | 71 | 12.4 | |
| OS | |||||||||||
| p-value | 0.968 | 0.552 | 0.896 | 0.075 | 0.514 | 0.187 | 0.283 | 0.285 | 0.737 | 0.522 | |
| Cut-point | 391.4 | 3.2 | 118.9 | 36.9 | 0.7 | 1.8 | 36 | 0.1 | 74 | 13.6 | |
| Indices | Post-SII-H | Post-SII-L | Post-NLR-H | Post-NLR-L | Post-PLR-H | Post-PLR-L | Post-PNI-H | Post-PNI-L | Post-ALC-L | ||
| DC | |||||||||||
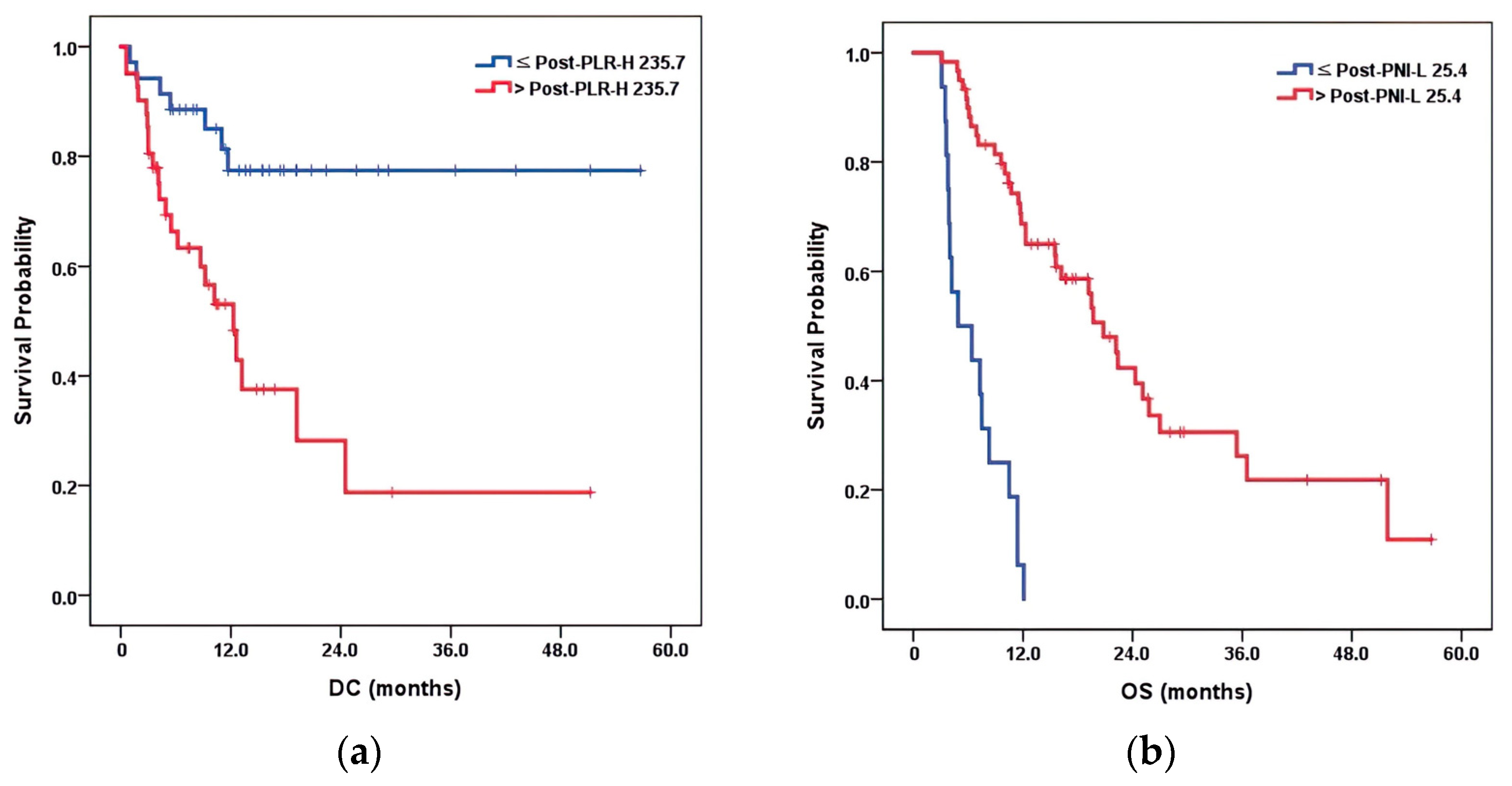
| p-value | 0.277 | 0.080 | 0.105 | 0.082 | 0.014 | 0.358 | 0.489 | 0.180 | 0.176 | ||
| Cut-point | 804.8 | 288.3 | 4 | 1.9 | 235.7 | 94.7 | 37.2 | 38.6 | 0.6 | ||
| OS | |||||||||||
| p-value | 0.047 | 0.570 | 0.213 | 0.825 | 0.017 | 0.898 | 0.060 | 0.001 | 0.495 | ||
| Cut-point | 426.9 | 277.9 | 3.8 | 3.1 | 235.7 | 68.2 | 41.2 | 25.4 | 0.6 | ||
| Indices | Post-LMR-H | Post-LMR-L | Post-A-H | Post-A-L | Post-AAR-H | Post-AAR-L | Post-P-H | Post-P-L | Post-H-H | Post H-L | |
| DC | |||||||||||
| p-value | 0.114 | 0.0857 | 0.479 | 0.699 | 0.618 | 0.900 | 0.806 | 0.975 | 0.134 | 0.846 | |
| Cut-point | 4 | 0.9 | 34 | 25 | 0.1 | 0.04 | 78 | 59 | 13.4 | 10.8 | |
| OS | |||||||||||
| p-value | 0.196 | 0.065 | 0.069 | 0.002 | 0.419 | 0.102 | 0.916 | 0.006 | 0.844 | 0.024 | |
| Cut-point | 4.2 | 0.7 | 39 | 32 | 0.08 | 0.07 | 68 | 66 | 14.8 | 12.3 | |
| Indices | AFP | PIVKA-II | Pre-CPTS | GTV sum | Total BED10 | MLD* | MLD** | ||||
| DC | |||||||||||
| p-value | 0.101 | 0.165 | 0.790 | 0.035 | 0.808 | 0.272 | 0.700 | ||||
| Cut-point | 47.4 | 24 | 5 | 504.7 | 67.5 | 2071.9 | 950.2 | ||||
| OS | |||||||||||
| p-value | 0.214 | 0.288 | 0.473 | 0.712 | 0.975 | 0.975 | 0.963 | ||||
| Cut-point | 3.5 | 15 | 6 | 77.2 | 63.5 | 3458.8 | 1953.1 | ||||
| End-Points | Variables | p-Value | Group | HR (95% CI) | 1-Year Probability (%) |
|---|---|---|---|---|---|
| DC | |||||
| Post-PLR-H | 0.006 | ≤235.7 | 0.286 (0.117–0.700) | 77.5 | |
| >235.7 | 1 | 53.1 | |||
| GTV sum | 0.068 | ≤504.7 | 0.413 (0.160–1.067) | 68.1 | |
| >504.7 | 1 | 28.6 | |||
| OS | |||||
| Post-SII-H | 0.875 | ≤426.9 | 1 | 73 | |
| >426.9 | 1.076 (0.435–2.662) | 47.2 | |||
| Post-PLR-H | 0.096 | ≤235.7 | 0.552 (0.275–1.110) | 72.9 | |
| >235.7 | 1 | 40.2 | |||
| Post-PNI-L | <0.001 | ≤25.4 | 4.790 (2.253–10.184) | 0.63 | |
| >25.4 | 1 | 68.7 | |||
| Post-A-L | 0.136 | ≤32 | 1.963 (0.809–4.759) | 45.1 | |
| >32 | 1 | 80.7 | |||
| Post-H-L | 0.735 | ≤12.3 | 1.188 (0.438–3.226) | 45 | |
| >12.3 | 1 | 92.9 | |||
| Post-P-L | 0.256 | ≤66 | 1.981 (0.609–6.444) | 49.1 | |
| >66 | 1 | 79.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.S.; Kim, C.W.; Kim, H.Y.; Ku, Y.-M.; Won, Y.D.; Lee, S.-L.; Sun, D.S. Association between Posttreatment Serum Platelet-to-Lymphocyte Ratio and Distant Metastases in Patients with Hepatocellular Carcinoma Receiving Curative Radiation Therapy. Cancers 2023, 15, 1978. https://doi.org/10.3390/cancers15071978
Lee DS, Kim CW, Kim HY, Ku Y-M, Won YD, Lee S-L, Sun DS. Association between Posttreatment Serum Platelet-to-Lymphocyte Ratio and Distant Metastases in Patients with Hepatocellular Carcinoma Receiving Curative Radiation Therapy. Cancers. 2023; 15(7):1978. https://doi.org/10.3390/cancers15071978
Chicago/Turabian StyleLee, Dong Soo, Chang Wook Kim, Hee Yeon Kim, Young-Mi Ku, Yoo Dong Won, Su-Lim Lee, and Der Sheng Sun. 2023. "Association between Posttreatment Serum Platelet-to-Lymphocyte Ratio and Distant Metastases in Patients with Hepatocellular Carcinoma Receiving Curative Radiation Therapy" Cancers 15, no. 7: 1978. https://doi.org/10.3390/cancers15071978
APA StyleLee, D. S., Kim, C. W., Kim, H. Y., Ku, Y.-M., Won, Y. D., Lee, S.-L., & Sun, D. S. (2023). Association between Posttreatment Serum Platelet-to-Lymphocyte Ratio and Distant Metastases in Patients with Hepatocellular Carcinoma Receiving Curative Radiation Therapy. Cancers, 15(7), 1978. https://doi.org/10.3390/cancers15071978






