The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Variables
2.4. Primary Outcomes: Survival Outcomes and Prognostic Factors
2.5. Secondary Outcomes: Prescribed Chemotherapy Regimens, Responses, and Adverse Effects
- (1)
- Neoadjuvant chemotherapy: chemotherapy given to patients before surgical resection of the primary tumor. The response to neoadjuvant chemotherapy was determined by a tumor necrosis rate (TNR) from the post-operative histopathological report. According to the Huvos grading system [20], those with a TNR between 90% and 99% (grade III) or 100% (grade IV) were considered responders, whereas those with a TNR of less than 50% (grade I) or between 50% and 89% (grade II) were considered non-responders.
- (2)
- Adjuvant chemotherapy: chemotherapy given to patients after surgical resection of the primary tumor to kill any remaining cancer cells with the aim of reducing the risk of tumor recurrence.
- (3)
- Palliative chemotherapy: chemotherapy given to patients who presented with unresectable/metastatic disease in order to relieve symptoms and lessen cancer-related suffering or in the event of disease progression or relapse after receiving multimodal treatment.
2.6. Treatment Strategy
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.1.1. Osteosarcoma
3.1.2. Ewing’s Sarcoma
3.2. Survival Outcomes and Prognostic Factors
3.2.1. Osteosarcoma
3.2.2. Ewing’s Sarcoma
3.3. Chemotherapy Prescribing Patterns and Responses
3.3.1. Osteosarcoma
3.3.2. Ewing’s Sarcoma
3.4. Self-Reported Symptoms and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. Lyon: International Agency for Research on Cancer. Cancer Incidence in Five Continents, Vol. XI (electronic version). Available online: https://ci5.iarc.fr (accessed on 8 January 2022).
- Valery, P.C.; Laversanne, M.; Bray, F. Bone cancer incidence by morphological subtype: A global assessment. Cancer Causes Control. 2015, 26, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Hung, G.Y.; Horng, J.L.; Yen, H.J.; Yen, C.C.; Chen, W.M.; Chen, P.C.; Wu, H.T.; Chiou, H.J. Incidence patterns of primary bone cancer in Taiwan (2003–2010): A population-based study. Ann. Surg. Oncol. 2014, 21, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A. Systemic therapy for osteosarcoma and Ewing sarcoma. Am. Soc. Clin. Oncol. Educ. Book. 2015, e644–e647. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef]
- Picci, P.; Mercuri, M.; Ferrari, S.; Alberghini, M.; Briccoli, A.; Ferrari, C.; Pignotti, E.; Bacci, G. Survival in high-grade osteosarcoma: Improvement over 21 years at a single institution. Ann. Oncol. 2009, 21, 1366–1373. [Google Scholar] [CrossRef]
- Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii102–iii112. [CrossRef]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Friedman, M.A.; Carter, S.K. The therapy of osteogenic sarcoma: Current status and thoughts for the future. J. Surg. Oncol. 1972, 4, 482–510. [Google Scholar] [CrossRef]
- Wang, J.J.; Cortes, E.; Sinks, L.F.; Holland, J.F. Therapeutic effect and toxicity of adriamycin in patients with neoplastic disease. Cancer 1971, 28, 837–843. [Google Scholar] [CrossRef]
- Jaffe, N. Recent advances in the chemotherapy of metastatic osteogenic sarcoma. Cancer 1972, 30, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.; Kleinerman, E.S.; Betcher, D.; Bernstein, M.L.; Conrad, E.; Ferguson, W.; Gebhardt, M.; Goorin, A.M.; et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005, 23, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Burgert, E.O., Jr.; Nesbit, M.E.; Garnsey, L.A.; Gehan, E.A.; Herrmann, J.; Vietti, T.J.; Cangir, A.; Tefft, M.; Evans, R.; Thomas, P.; et al. Multimodal therapy for the management of nonpelvic, localized Ewing’s sarcoma of bone: Intergroup study IESS-II. J. Clin. Oncol. 1990, 8, 1514–1524. [Google Scholar] [CrossRef]
- Nesbit, M.E., Jr.; Gehan, E.A.; Burgert, E.O., Jr.; Vietti, T.J.; Cangir, A.; Tefft, M.; Evans, R.; Thomas, P.; Askin, F.B.; Kissane, J.M.; et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: A long-term follow-up of the First Intergroup study. J. Clin. Oncol. 1990, 8, 1664–1674. [Google Scholar] [CrossRef]
- Grier, H.E.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.H.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Addition of Ifosfamide and Etoposide to Standard Chemotherapy for Ewing’s Sarcoma and Primitive Neuroectodermal Tumor of Bone. N. Engl. J. Med. 2003, 348, 694–701. [Google Scholar] [CrossRef]
- Lewis, I.J.; Nooij, M.A.; Whelan, J.; Sydes, M.R.; Grimer, R.; Hogendoorn, P.C.; Memon, M.A.; Weeden, S.; Uscinska, B.M.; van Glabbeke, M.; et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J. Natl. Cancer Inst. 2007, 99, 112–128. [Google Scholar] [CrossRef]
- Rosen, G.; Marcove, R.C.; Huvos, A.G.; Caparros, B.I.; Lane, J.M.; Nirenberg, A.; Cacavio, A.; Groshen, S. Primary osteogenic sarcoma: Eight-year experience with adjuvant chemotherapy. J. Cancer Res. Clin. Oncol. 1983, 106, 55–67. [Google Scholar] [CrossRef]
- Kolb, E.A.; Kushner, B.H.; Gorlick, R.; Laverdiere, C.; Healey, J.H.; LaQuaglia, M.P.; Huvos, A.G.; Qin, J.; Vu, H.T.; Wexler, L.; et al. Long-term event-free survival after intensive chemotherapy for Ewing’s family of tumors in children and young adults. J. Clin. Oncol. 2003, 21, 3423–3430. [Google Scholar] [CrossRef]
- Goorin, A.M.; Schwartzentruber, D.J.; Devidas, M.; Gebhardt, M.C.; Ayala, A.G.; Harris, M.B.; Helman, L.J.; Grier, H.E.; Link, M.P. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 2003, 21, 1574–1580. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Heise, U.; Schwenzer, D.; Winkler, K. Combined modality treatment for osteosarcoma occurring as a second malignant disease. Cooperative German-Austrian-Swiss Osteosarcoma Study Group. J. Clin. Oncol. 1999, 17, 1164. [Google Scholar] [CrossRef]
- Rebe, C.; Ghiringhelli, F. Cytotoxic effects of chemotherapy on cancer and immune cells: How can it be modulated to generate novel therapeutic strategies? Future Oncol. 2015, 11, 2645–2654. [Google Scholar] [CrossRef]
- Walczak, B.E.; Irwin, R.B. Sarcoma chemotherapy. J. Am. Acad. Orthop. Surg. 2013, 21, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Dancsok, A.R.; Asleh-Aburaya, K.; Nielsen, T.O. Advances in sarcoma diagnostics and treatment. Oncotarget 2017, 8, 7068–7093. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; Grabow, D.; Steinmann, D.; Wörmann, B.; Calaminus, G. Late Effects and Long-Term Follow-Up after Cancer in Childhood. Oncol. Res. Treat. 2017, 40, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Forni, C.; Longhi, A.; Ferrari, S.; Donati, D.; De Paolis, M.; Barbieri, E.; Pignotti, E.; Rosito, P.; Versari, M. Long-term outcome for patients with non-metastatic Ewing’s sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur. J. Cancer 2004, 40, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lim, K.M.; Noh, J.Y.; Kim, K.; Kang, S.; Chang, Y.K.; Shin, S.; Chung, J.H. Doxorubicin-induced platelet procoagulant activities: An important clue for chemotherapy-associated thrombosis. Toxicol. Sci. An. Off. J. Soc. Toxicol. 2011, 124, 215–224. [Google Scholar] [CrossRef]
- Zahir, M.N.; Masood, N.; Shabbir-Moosajee, M. Cisplatin-induced posterior reversible encephalopathy syndrome and successful re-treatment in a patient with non-seminomatous germ cell tumor: A case report. J. Med. Case Rep. 2012, 6, 409. [Google Scholar] [CrossRef]
- Cool, R.M.; Herrington, J.D.; Wong, L. Recurrent peripheral arterial thrombosis induced by cisplatin and etoposide. Pharmacotherapy 2002, 22, 1200–1204. [Google Scholar] [CrossRef]
- Sarbay, H.; Demir, Ü.F.; Yılmaz, G.; Atay, A.A.; Malbora, B. Ifosfamide induced encephalopathy in a child with osteosarcoma. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2021, 27, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Belachew, S.A.; Erku, D.A.; Mekuria, A.B.; Gebresillassie, B.M. Pattern of chemotherapy-related adverse effects among adult cancer patients treated at Gondar University Referral Hospital, Ethiopia: A cross-sectional study. Drug. Healthc. Patient Saf. 2016, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Workalemahu, G.; Abdela, O.A.; Yenit, M.K. Chemotherapy-Related Adverse Drug Reaction and Associated Factors Among Hospitalized Paediatric Cancer Patients at Hospitals in North-West Ethiopia. Drug. Healthc. Patient Saf. 2020, 12, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, S.; Feng, A.; Xu, D.; Zhu, Q.; Mao, Y.; Zhao, Y.; Lv, Y.; Han, C.; Liu, R.; et al. Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: A meta-analysis and clinical observation. Medicine 2019, 98, e15582. [Google Scholar] [CrossRef]
- Janeway, K.A.; Grier, H.E. Sequelae of osteosarcoma medical therapy: A review of rare acute toxicities and late effects. Lancet Oncol. 2010, 11, 670–678. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 5th ed.; World Health Organization: Rome, Italy, 2016. [Google Scholar]
- Janeway, K.A.; Barkauskas, D.A.; Krailo, M.D.; Meyers, P.A.; Schwartz, C.L.; Ebb, D.H.; Seibel, N.L.; Grier, H.E.; Gorlick, R.; Marina, N. Outcome for adolescent and young adult patients with osteosarcoma: A report from the Children’s Oncology Group. Cancer 2012, 118, 4597–4605. [Google Scholar] [CrossRef]
- Worch, J.; Ranft, A.; DuBois, S.G.; Paulussen, M.; Juergens, H.; Dirksen, U. Age dependency of primary tumor sites and metastases in patients with Ewing sarcoma. Pediatr. Blood Cancer 2018, 65, e27251. [Google Scholar] [CrossRef]
- Errani, C.; Longhi, A.; Rossi, G.; Rimondi, E.; Biazzo, A.; Toscano, A.; Alì, N.; Ruggieri, P.; Alberghini, M.; Picci, P.; et al. Palliative therapy for osteosarcoma. Expert Rev. Anticancer. Ther. 2011, 11, 217–227. [Google Scholar] [CrossRef]
- Nandra, R.; Hwang, N.; Matharu, G.S.; Reddy, K.; Grimer, R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ann. R. Coll. Surg. Engl. 2015, 97, 425–433. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. Survival Rates for Soft Tissue Sarcoma. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 30 April 2021).
- Najafi Moghaddam Gilani, V.; Hosseinian, S.M.; Ghasedi, M.; Nikookar, M. Data-Driven Urban Traffic Accident Analysis and Prediction Using Logit and Machine Learning-Based Pattern Recognition Models. Math. Probl. Eng. 2021, 2021, 9974219. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCA-Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Stata Corp. Stata Statistical Software: Release 16, Stata Corp LLC: College Station, TX, USA, 2019.
- Mailankody, S.; Kumar, V.S.; Khan, S.A.; Banavali, S.D.; Bajpai, J. Resource-appropriate selection of osteosarcoma treatment protocols in low- and middle-income countries. Pediatr. Blood Cancer 2022, 69, e29540. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, J.; Khanna, N.; Vora, T.; Gulia, A.; Laskar, S.; Puri, A.; Sanduptla, B.; Chinnaswamy, G.; Nayak, P.; Juvekar, S.L.; et al. Analysis of bone and soft-tissue sarcomas registered during the year 2012 at Tata Memorial Hospital, Mumbai, with clinical outcomes. Indian J. Cancer 2018, 55, 37–44. [Google Scholar] [CrossRef]
- Bajpai, J.; Panda, G.S.; Chandrasekharan, A.; Bhargava, P.; Srinivas, S.; Laskar, S.; Dandekar, S.; Mokal, S.; Rekhi, B.; Khanna, N.; et al. Adolescent–adult nonmetastatic Ewing sarcoma—Experience from a large developing country. Pediatr. Blood Cancer 2021, 68, e29081. [Google Scholar] [CrossRef]
- Brunetto, A.L.; Castillo, L.A.; Petrilli, A.S.; Macedo, C.D.; Boldrini, E.; Costa, C.; Almeida, M.T.; Kirst, D.; Rodriguez-Galindo, C.; Pereira, W.V.; et al. Carboplatin in the treatment of Ewing sarcoma: Results of the first Brazilian collaborative study group for Ewing sarcoma family tumors-EWING1. Pediatr. Blood Cancer 2015, 62, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, L.J.; Douglass, H.O., Jr.; Higby, D.J.; Mindell, E.R.; Nime, F.; Ghoorah, J.; Freeman, A.I. Adjuvant adriamycin and cis-diamminedichloroplatinum (cis-platinum) in primary osteosarcoma. Cancer 1981, 47, 248–254. [Google Scholar] [CrossRef]
- Ettinger, L.J.; Douglass, H.O., Jr.; Mindell, E.R.; Sinks, L.F.; Tebbi, C.K.; Risseeuw, D.; Freeman, A.I. Adjuvant adriamycin and cisplatin in newly diagnosed, nonmetastatic osteosarcoma of the extremity. J. Clin. Oncol. 1986, 4, 353–362. [Google Scholar] [CrossRef]
- Bramwell, V.H.; Burgers, M.; Sneath, R.; Souhami, R.; van Oosterom, A.T.; Voute, P.A.; Rouesse, J.; Spooner, D.; Craft, A.W.; Somers, R.; et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: The first study of the European Osteosarcoma Intergroup. J. Clin. Oncol. 1992, 10, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Anninga, J.K.; Gelderblom, H.; Fiocco, M.; Kroep, J.R.; Taminiau, A.H.; Hogendoorn, P.C.; Egeler, R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur. J. Cancer 2011, 47, 2431–2445. [Google Scholar] [CrossRef]
- Crom, W.R.; Pratt, C.B.; Green, A.A.; Champion, J.E.; Crom, D.B.; Stewart, C.F.; Evans, W.E. The effect of prior cisplatin therapy on the pharmacokinetics of high-dose methotrexate. J. Clin. Oncol. 1984, 2, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, N.; Bielack, S.S.; Epler, D.; Bieling, P.; Delling, G.; Körholz, D.; Graf, N.; Heise, U.; Jürgens, H.; Kotz, R.; et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann. Oncol. 1998, 9, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Smeland, S.; Mercuri, M.; Bertoni, F.; Longhi, A.; Ruggieri, P.; Alvegard, T.A.; Picci, P.; Capanna, R.; Bernini, G.; et al. Neoadjuvant Chemotherapy with High-Dose Ifosfamide, High-Dose Methotrexate, Cisplatin, and Doxorubicin for Patients with Localized Osteosarcoma of the Extremity: A Joint Study by the Italian and Scandinavian Sarcoma Groups. J. Clin. Oncol. 2005, 23, 8845–8852. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ruggieri, P.; Cefalo, G.; Tamburini, A.; Capanna, R.; Fagioli, F.; Comandone, A.; Bertulli, R.; Bisogno, G.; Palmerini, E.; et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: An Italian sarcoma group trial ISG/OS-1. J. Clin. Oncol. 2012, 30, 2112–2118. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Longhi, A.; Forni, C.; Loro, L.; Beghelli, C.; Tremosini, M.; Versari, M. Delayed methotrexate clearance in osteosarcoma patients treated with multiagent regimens of neoadjuvant chemotherapy. Oncol. Rep. 2003, 10, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wippel, B.; Gundle, K.R.; Dang, T.; Paxton, J.; Bubalo, J.; Stork, L.; Fu, R.; Ryan, C.W.; Davis, L.E. Safety and efficacy of high-dose methotrexate for osteosarcoma in adolescents compared with young adults. Cancer Med. 2019, 8, 111–116. [Google Scholar] [CrossRef]
- Wexler, L.H.; DeLaney, T.F.; Tsokos, M.; Avila, N.; Steinberg, S.M.; Weaver-McClure, L.; Jacobson, J.; Jarosinski, P.; Hijazi, Y.M.; Balis, F.M.; et al. Ifosfamide and etoposide plus vincristine, doxorubicin, and cyclophosphamide for newly diagnosed Ewing’s sarcoma family of tumors. Cancer 1996, 78, 901–911. [Google Scholar] [CrossRef]
- Huvos, A.G.; Rosen, G.; Marcove, R.C. Primary osteogenic sarcoma: Pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch. Pathol. Lab. Med. 1977, 101, 14–18. [Google Scholar]
- Xin, S.; Wei, G. Prognostic factors in osteosarcoma: A study level meta-analysis and systematic review of current practice. J. Bone Oncol. 2020, 21, 100281. [Google Scholar] [CrossRef]
- Friebele, J.C.; Peck, J.; Pan, X.; Abdel-Rasoul, M.; Mayerson, J.L. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am. J. Orthop. (Belle Mead NJ) 2015, 44, 547–553. [Google Scholar]
- Ayala, A.G.; Raymond, A.K.; Jaffe, N. The pathologist’s role in the diagnosis and treatment of osteosarcoma in children. Hum. Pathol. 1984, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Smeland, S.; Whelan, J.S.; Marina, N.; Jovic, G.; Hook, J.M.; Krailo, M.D.; Gebhardt, M.; Pápai, Z.; Meyer, J.; et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Aznab, M.; Hematti, M. Evaluation of clinical process in osteosarcoma patients treated with chemotherapy including cisplatin, adriamycin, ifosfamide, and etoposide and determination of the treatment sequels in a long-term 11-year follow-up. J. Cancer Res. Ther. 2017, 13, 291–296. [Google Scholar] [CrossRef]
- Wagner, M.J.; Amodu, L.I.; Duh, M.S.; Korves, C.; Solleza, F.; Manson, S.C.; Diaz, J.; Neary, M.P.; Demetri, G.D. A retrospective chart review of drug treatment patterns and clinical outcomes among patients with metastatic or recurrent soft tissue sarcoma refractory to one or more prior chemotherapy treatments. BMC cancer 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Karavasilis, V.; Seddon, B.M.; Ashley, S.; Al-Muderis, O.; Fisher, C.; Judson, I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer 2008, 112, 1585–1591. [Google Scholar] [CrossRef]
- Balamuth, N.J.; Womer, R.B. Ewing’s sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.L.; Devidas, M.; Lafreniere, D.; Souid, A.K.; Meyers, P.A.; Gebhardt, M.; Stine, K.; Nicholas, R.; Perlman, E.J.; Dubowy, R.; et al. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457—A report from the Children’s Oncology Group. J. Clin. Oncol. 2006, 24, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, M.; Ahrens, S.; Craft, A.W.; Dunst, J.; Fröhlich, B.; Jabar, S.; Rübe, C.; Winkelmann, W.; Wissing, S.; Zoubek, A.; et al. Ewing’s tumors with primary lung metastases: Survival analysis of 114 (European Intergroup) Cooperative Ewing’s Sarcoma Studies patients. J. Clin. Oncol. 1998, 16, 3044–3052. [Google Scholar] [CrossRef]
- Paulussen, M.; Bielack, S.; Jürgens, H.; Casali, P.G. Ewing’s sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20, 140–142. [Google Scholar] [CrossRef]
- Benjamin, R.S. Adjuvant and Neoadjuvant Chemotherapy for Osteosarcoma: A Historical Perspective. In Current Advances in Osteosarcoma: Clinical Perspectives: Past, Present and Future; Kleinerman, E.S., Gorlick, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Paulussen, M.; Ahrens, S.; Dunst, J.; Winkelmann, W.; Exner, G.U.; Kotz, R.; Amann, G.; Dockhorn-Dworniczak, B.; Harms, D.; Müller-Weihrich, S.; et al. Localized Ewing tumor of bone: Final results of the cooperative Ewing’s Sarcoma Study CESS 86. J. Clin. Oncol. 2001, 19, 1818–1829. [Google Scholar] [CrossRef]
- Wagle, S.; Park, S.-H.; Kim, K.M.; Moon, Y.J.; Bae, J.S.; Kwon, K.S.; Park, H.S.; Lee, H.; Moon, W.S.; Kim, J.R.; et al. DBC1/CCAR2 is involved in the stabilization of androgen receptor and the progression of osteosarcoma. Sci. Rep. 2015, 5, 13144. [Google Scholar] [CrossRef]
- Fang, D.; Yang, H.; Lin, J.; Teng, Y.; Jiang, Y.; Chen, J.; Li, Y. 17β-estradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating miR-9 and thus degrades MALAT-1 in osteosarcoma cell MG-63 in an estrogen receptor-independent manner. Biochem. Biophys. Res. Commun. 2015, 457, 500–506. [Google Scholar] [CrossRef]
- Collins, M.; Wilhelm, M.; Conyers, R.; Herschtal, A.; Whelan, J.; Bielack, S.; Kager, L.; Kühne, T.; Sydes, M.; Gelderblom, H.; et al. Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: Findings from a meta-analysis. J. Clin. Oncol. 2013, 31, 2303–2312. [Google Scholar] [CrossRef]
- Cotterill, S.J.; Ahrens, S.; Paulussen, M.; Jürgens, H.F.; Voûte, P.A.; Gadner, H.; Craft, A.W. Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef]
- Bacci, G.; Longhi, A.; Ferrari, S.; Mercuri, M.; Versari, M.; Bertoni, F. Prognostic factors in non-metastatic Ewing’s sarcoma tumor of bone: An analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol. 2006, 45, 469–475. [Google Scholar] [CrossRef]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef]
- Roguin, A.; Kasis, I.; Ben-Arush, M.W.; Sharon, R.; Berant, M. Fever and Neutropenia in Children with Malignant Disease. Pediatr. Hematol. Oncol. 1996, 13, 503–510. [Google Scholar] [CrossRef]
- Paulussen, M.; Craft, A.W.; Lewis, I.; Hackshaw, A.; Douglas, C.; Dunst, J.; Schuck, A.; Winkelmann, W.; Köhler, G.; Poremba, C.; et al. Results of the EICESS-92 Study: Two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J. Clin. Oncol. 2008, 26, 4385–4393. [Google Scholar] [CrossRef]
- Szabatura, A.H.; Cirrone, F.; Harris, C.; McDonnell, A.M.; Feng, Y.; Voit, D.; Neuberg, D.; Butrynski, J.; Fisher, D.C. An assessment of risk factors associated with ifosfamide-induced encephalopathy in a large academic cancer center. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2015, 21, 188–193. [Google Scholar] [CrossRef]
- Küpfer, A.; Aeschlimann, C.; Cerny, T. Methylene blue and the neurotoxic mechanisms of ifosfamide encephalopathy. Eur. J. Clin. Pharmacol. 1996, 50, 249–252. [Google Scholar] [CrossRef]
- Khanal, P.; Awan, F.; Nguyen, V. Etoposide-induced posterior reversible encephalopathy syndrome. Ann. Hematol. 2013, 92, 561–562. [Google Scholar] [CrossRef]
- Longhi, A.; Ferrari, S.; Bacci, G.; Specchia, S. Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anticancer. Drugs 2007, 18, 737–744. [Google Scholar] [CrossRef]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
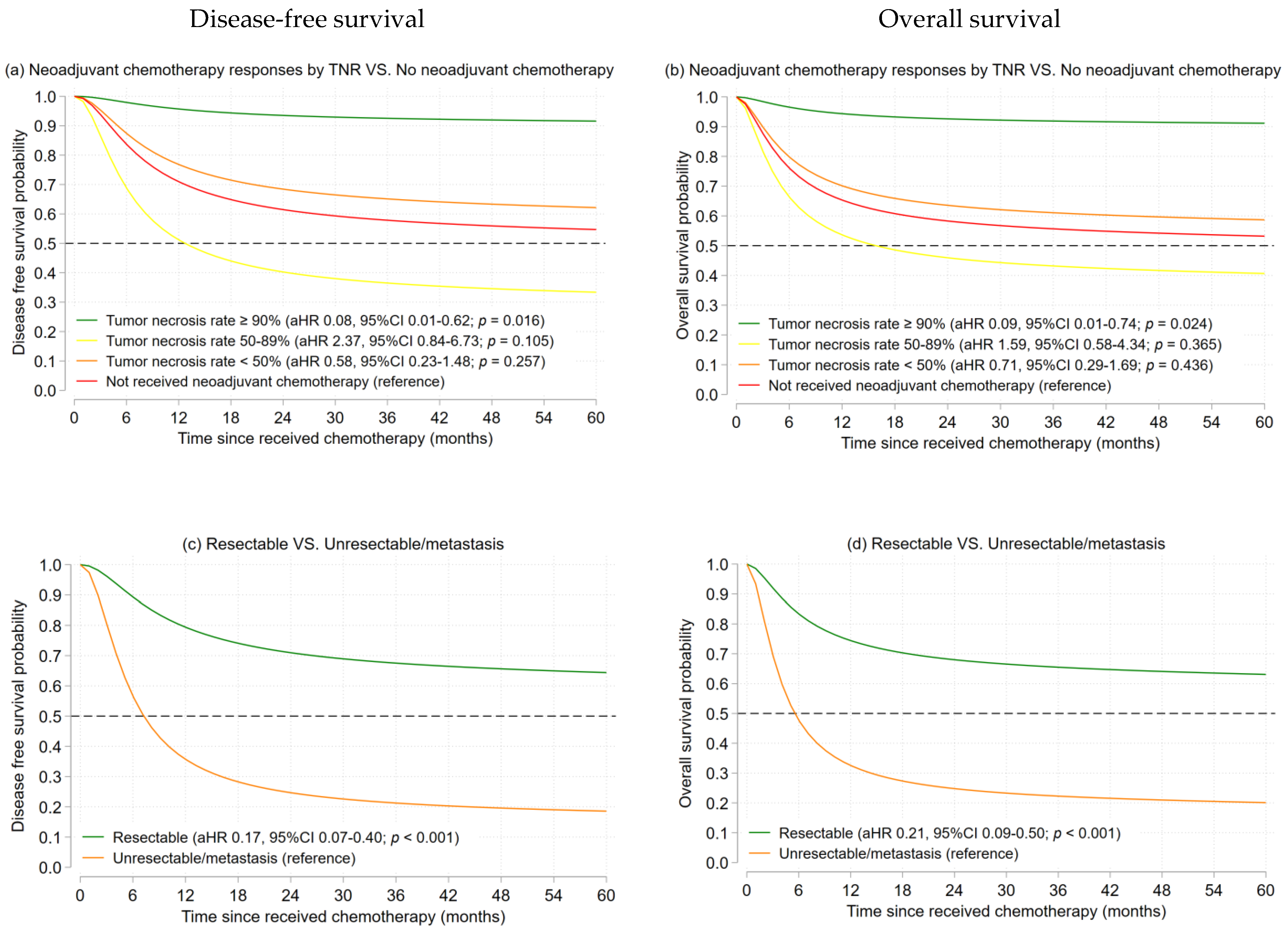
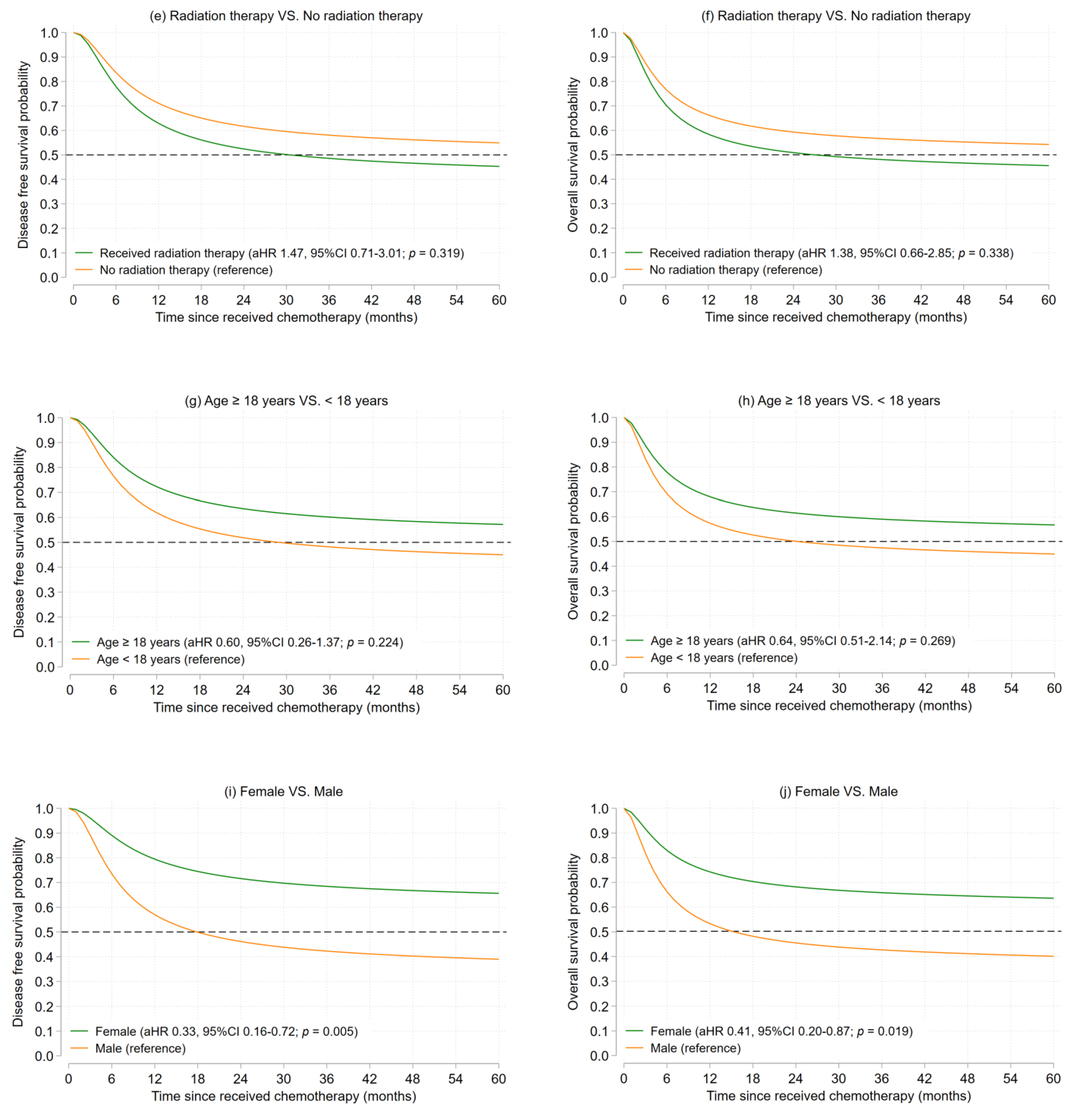
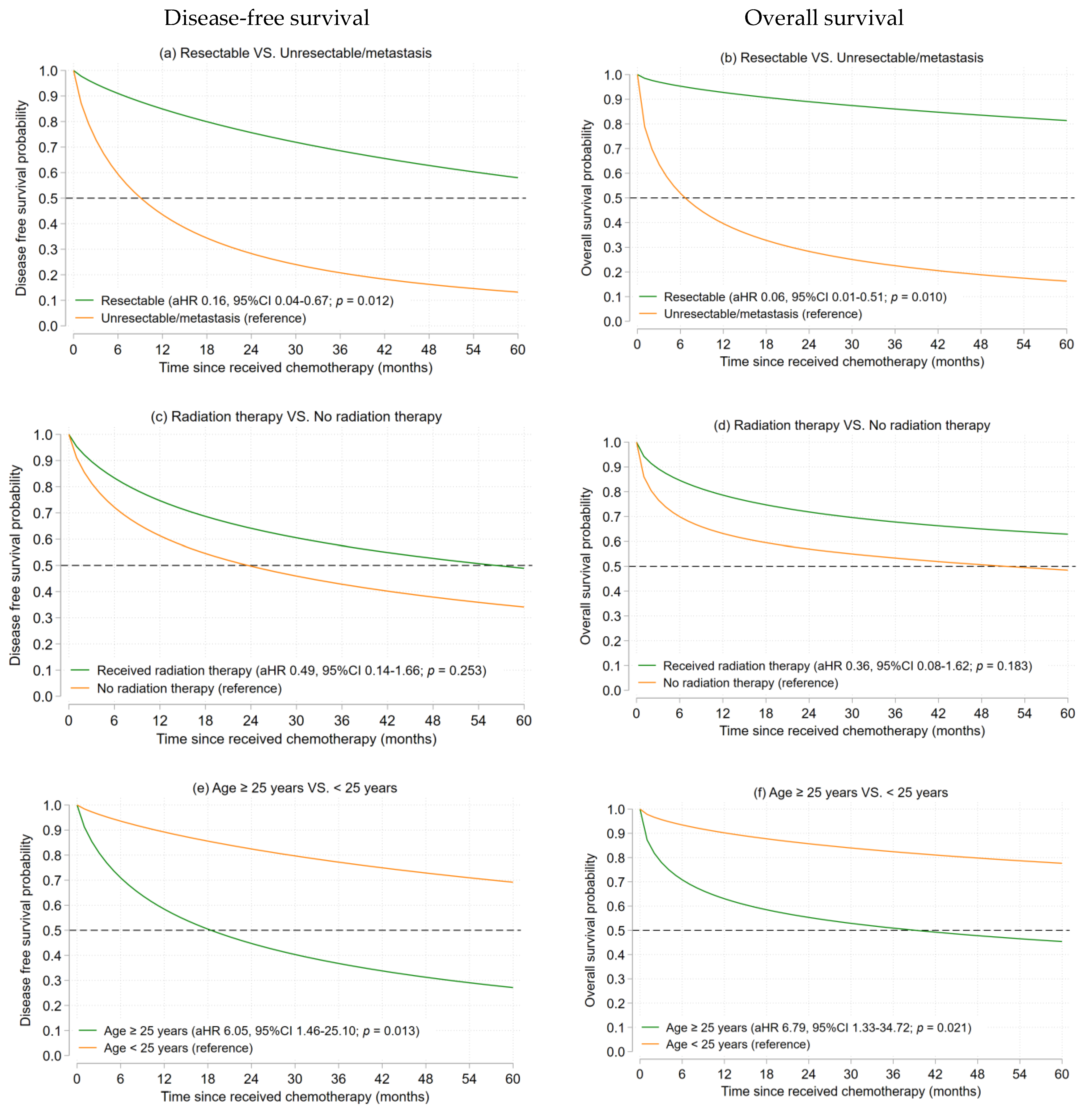
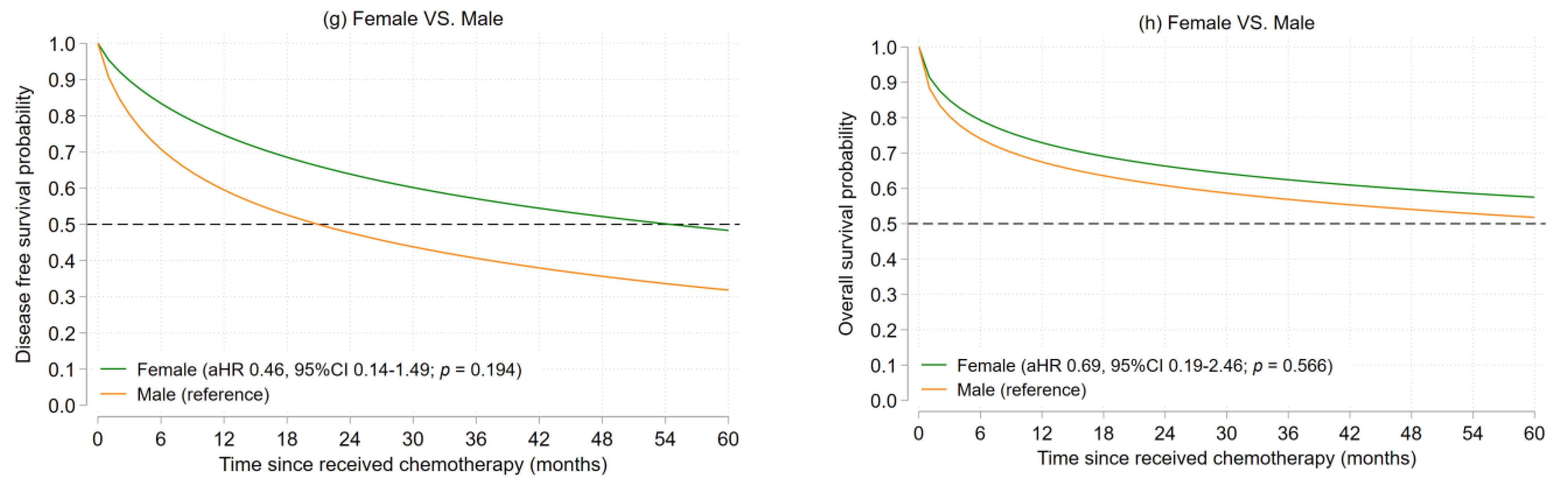
| Characteristics | Osteosarcoma (n = 79) | Ewing’s Sarcoma (n = 23) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 41 | (51.9) | 15 | (65.2) |
| Female | 38 | (48.1) | 8 | (34.8) |
| Median age, year (IQR) | 20 | (16–48) | 23 | (18–30) |
| Location of the primary tumor | ||||
| Head and neck | 3 | (3.8) | 2 | (8.7) |
| Trunk | 9 | (11.4) | 6 | (26.1) |
| Intraperitoneal | 2 | (2.5) | 3 | (13.0) |
| Extremities | 62 | (78.5) | 9 | (39.1) |
| Others | 3 | (3.8) | 3 | (13.1) |
| Localized tumor at diagnosis | 72 | (91.1) | 18 | (78.3) |
| Resectable tumor with curative surgery | 56 | (77.8) | 9 | (50.0) |
| Unresectable tumor | 4 | (5.5) | 6 | (33.3) |
| Metastasis after prior treatment | 12 | (16.7) | 3 | (16.7) |
| Metastasis at diagnosis | 7 | (8.9) | 5 | (21.7) |
| Palliative surgery | 15 | (78.9) | 2 | (25.0) |
| Radiation therapy | 23 | (29.1) | 8 | (34.8) |
| Chemotherapy for resectable disease | ||||
| Neoadjuvant only | 21 | (26.6) | 7 | (30.4) |
| Adjuvant only | 27 | (34.2) | 3 | (13.0) |
| Neoadjuvant followed by adjuvant | 20 | (25.3) | 2 | (8.7) |
| Chemotherapy for unresectable/metastatic disease | ||||
| First-line treatment | 20 | (25.3) | 12 | (52.2) |
| Second-line treatment | 11 | (13.9) | 7 | (30.4) |
| Third-line treatment | 1 | (1.3) | 5 | (21.7) |
| Chemotherapy Regimens | Osteosarcoma (n = 79) | Chemotherapy Regimens | Ewing’s Sarcoma (n = 23) | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Neoadjuvant only | 21 | Neoadjuvant only | 7 | ||
| AP | 17 | (81.0) | IE | 3 | (42.8) |
| AI | 2 | (9.5) | VAC + IE | 2 | (28.6) |
| A | 2 | (9.5) | VAC | 1 | (14.3) |
| AI | 1 | (14.3) | |||
| Adjuvant only | 27 | Adjuvant only | 3 | ||
| AP | 13 | (48.2) | VAC + IE | 2 | (66.7) |
| AI | 4 | (14.8) | PE | 1 | (33.3) |
| IE | 3 | (11.1) | |||
| AP + IE | 2 | (7.4) | |||
| MAP | 1 | (3.7) | |||
| P + IE | 1 | (3.7) | |||
| MP + IE | 1 | (3.7) | |||
| MAP + IE | 1 | (3.7) | |||
| PIE + Cy | 1 | (3.7) | |||
| Neoadjuvant followed by adjuvant | 20 | Neoadjuvant followed by adjuvant | 2 | ||
| AP/AP | 5 | (25.0) | VAC + IE/VAC | 2 | (100.0) |
| AP/IE | 4 | (20.0) | |||
| MAP/AP | 2 | (10.0) | |||
| AP/P + IE | 2 | (10.0) | |||
| AP/AI | 1 | (5.0) | |||
| AP/AP + IE | 1 | (5.0) | |||
| MAP/M | 1 | (5.0) | |||
| AP + IE/G + D | 1 | (5.0) | |||
| AI/AI | 1 | (5.0) | |||
| AI/M | 1 | (5.0) | |||
| Ir + P/I | 1 | (5.0) | |||
| Chemotherapy regimen for unresectable/metastatic disease | |||||
| First-line | 20 | First-line | 12 | ||
| AP | 11 | (55.0) | PE | 6 | (50.0) |
| IE | 4 | (20.0) | IE | 4 | (33.4) |
| I | 3 | (15.0) | VAC | 1 | (8.3) |
| AI | 1 | (5.0) | A | 1 | (8.3) |
| Cy + E | 1 | (5.0) | |||
| Second-line | 11 | Second-line | 7 | ||
| I | 3 | (27.3) | IE | 3 | (42.8) |
| G | 3 | (27.3) | VAC | 2 | (28.6) |
| AP | 2 | (18.1) | V + IE | 1 | (14.3) |
| PE | 1 | (9.1) | I | 1 | (14.3) |
| IE | 1 | (9.1) | |||
| G + DTX | 1 | (9.1) | |||
| Third-line | 1 | Third-line | 5 | ||
| Tr | 1 | (100.0) | IE | 2 | (40.0) |
| I | 2 | (40.0) | |||
| AP | 1 | (20.0) | |||
| Chemotherapy Regimens | (n = 32) n (%) | Tumor Necrosis Rate after Neoadjuvant Chemotherapy, n (%) | ||
|---|---|---|---|---|
| <50% | 50–89% | ≥90% | ||
| AP | 25 (78.1) | 15 (60.0) | 7 (28.0) | 3 (12.0) |
| AI | 3 (9.4) | 1 (33.3) | 2 (66.7) | |
| AP + IE | 2 (6.3) | 1 (50.0) | 1 (50.0) | |
| MAP | 1 (3.1) | 1 (100.0) | ||
| A | 1 (3.1) | 1 (100.0) | ||
| Self-Reported Adverse Symptoms | Osteosarcoma | Ewing’s Sarcoma | |||||
|---|---|---|---|---|---|---|---|
| 1 Drug (n = 22) | 2 Drugs (n = 105) | 3 Drugs (n = 7) | 1 Drug (n = 4) | 2 Drugs (n = 27) | 3 Drugs (n = 16) | ||
| Alteration of consciousness | - | - | - | 1 (25.0%) | - | - | |
| Anorexia | - | 1 (1.0%) | - | - | - | - | |
| Diarrhea | - | - | - | 1 (25.0%) | - | - | |
| Dyspnea | 2 (9.1%) | 6 (5.7%) | 2 (28.6%) | - | 1 (3.7%) | - | |
| Fatigue | 3 (13.6%) | 10 (9.5%) | - | - | 3 (11.1%) | - | |
| Fever | 3 (13.6%) | 5 (4.8%) | 4 (57.1%) | - | 4 (14.8%) | 5 (31.3%) | |
| Nausea | 2 (9.1%) | 4 (3.8%) | - | - | 2 (7.4%) | 1 (6.3%) | |
| Oral ulcer | - | 1 (1.0%) | - | - | - | - | |
| Rash | - | - | 1 (14.3%) | - | - | - | |
| Tumor pain | 5 (22.7%) | 21 (20.0%) | 2 (28.6%) | - | 1 (3.7%) | 3 (18.8%) | |
| Weakness | - | - | - | - | 3 (11.1%) | - | |
| Wound | 1 (4.5%) | 2 (1.9%) | - | - | 1 (3.7%) | - | |
| Adverse Drug Reaction with Grade II–IV | Grade | Osteosarcoma | Ewing’s Sarcoma | ||||
|---|---|---|---|---|---|---|---|
| 1 Drug (n = 22) | 2 Drugs (n = 105) | 3 Drugs (n = 7) | 1 Drug (n = 4) | 2 Drugs (n = 27) | 3 Drugs (n = 16) | ||
| Vascular and hematologic system | |||||||
| Anemia | II | - | 1 (0.9%) | - | 1 (25.0%) | - | - |
| III | 1 (4.5%) | - | - | - | - | - | |
| Neutropenia | III | - | 5 (4.8%) | - | - | 3 (11.1%) | 1 (6.3%) |
| Thrombocytopenia | III | - | 1 (0.9%) | - | - | - | - |
| Pancytopenia | III | - | 2 (1.9%) | - | - | 1 (3.7%) | - |
| Thrombosis | III | - | 1 (0.9%) | - | - | - | - |
| IV | - | 1 (0.9%) | - | - | - | - | |
| Infection | |||||||
| Febrile neutropenia | III | - | 6 (5.7%) | 1 (14.3%) | - | 2 (7.4%) | 4 (25.0%) |
| Unspecified infection | II | 2 (9.1%) | 10 (9.5%) | 3 (42.9%) | 1 (25.0%) | 3 (11.1%) | 3 (18.8%) |
| III | - | 1 (0.9%) | - | - | - | - | |
| Nephrology | |||||||
| Acute kidney injury | III | - | - | - | - | 1 (3.7%) | - |
| Hypokalemia | III | - | 1 (0.9%) | - | - | - | - |
| Neuropsychology | |||||||
| Encephalitis | II | - | - | - | 1 (25.0%) | - | - |
| III | - | - | - | - | 1 (3.7%) | - | |
| Gastrointestinal system | |||||||
| Mucositis | II | - | 1 (0.9%) | - | - | - | - |
| Peptic ulcer | II | - | 1 (0.9%) | - | - | - | - |
| Integument | |||||||
| Oral ulcer | II | - | 1 (0.9%) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirikul, W.; Buawangpong, N.; Pruksakorn, D.; Charoentum, C.; Teeyakasem, P.; Koonrungsesomboon, N. The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand. Cancers 2023, 15, 1979. https://doi.org/10.3390/cancers15071979
Sirikul W, Buawangpong N, Pruksakorn D, Charoentum C, Teeyakasem P, Koonrungsesomboon N. The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand. Cancers. 2023; 15(7):1979. https://doi.org/10.3390/cancers15071979
Chicago/Turabian StyleSirikul, Wachiranun, Nida Buawangpong, Dumnoensun Pruksakorn, Chaiyut Charoentum, Pimpisa Teeyakasem, and Nut Koonrungsesomboon. 2023. "The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand" Cancers 15, no. 7: 1979. https://doi.org/10.3390/cancers15071979
APA StyleSirikul, W., Buawangpong, N., Pruksakorn, D., Charoentum, C., Teeyakasem, P., & Koonrungsesomboon, N. (2023). The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand. Cancers, 15(7), 1979. https://doi.org/10.3390/cancers15071979







