TrkA Co-Receptors: The Janus Face of TrkA?
Abstract
Simple Summary
Abstract
1. Introduction
2. State of the Art: Therapeutic Potential of the NGF/TrkA Axis
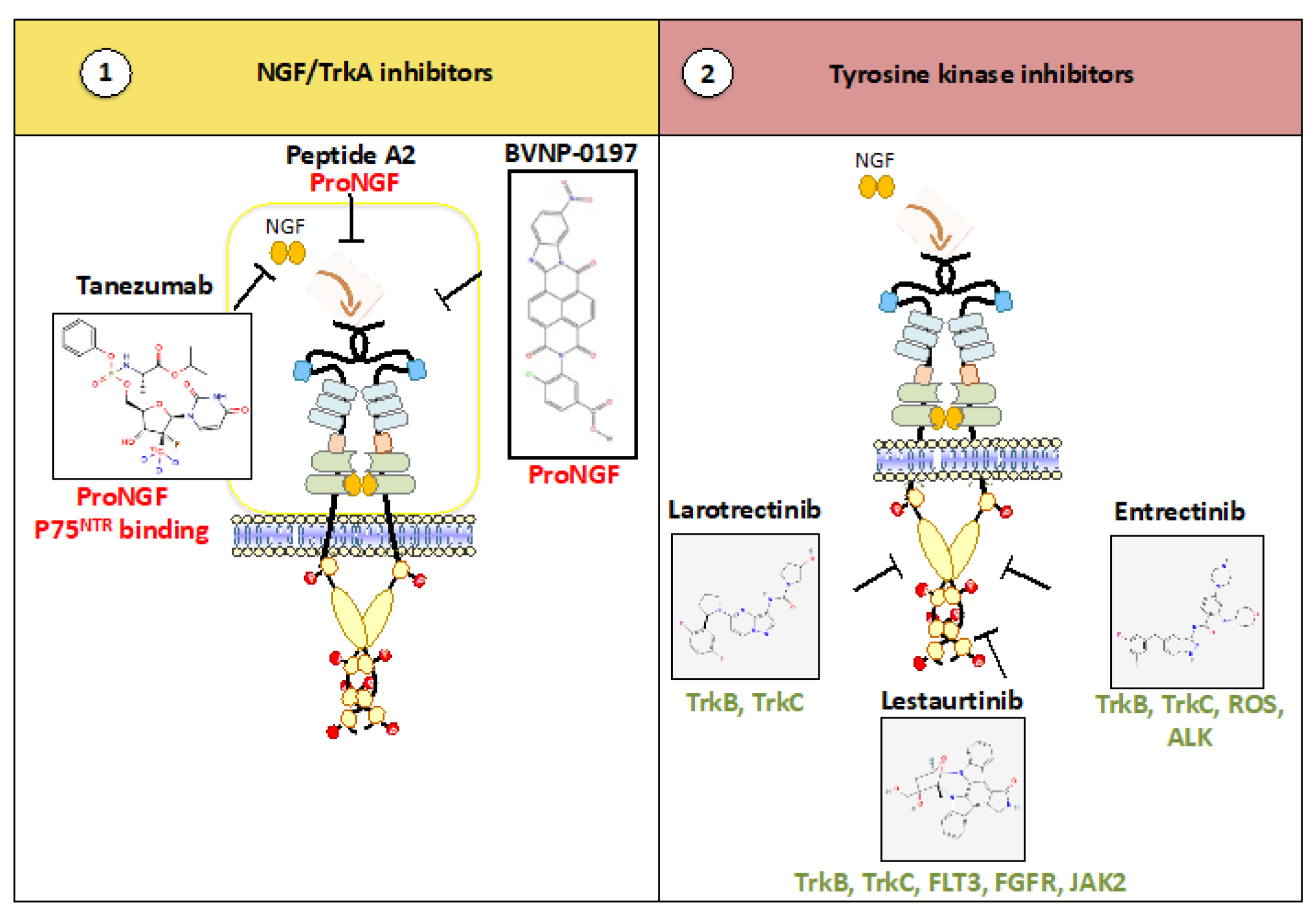
2.1. Anti-(Pro)NGF: The Next Generation of Anti-Pain?
2.2. Kinase Inhibitors: The Next Generation of Targeted Therapies?
2.3. Second-Generation Trk Inhibitors for Cancers with Point Mutation(s)
3. Genomic Alterations Cannot Recapitulate All Resistance Mechanisms
3.1. Is TrkA TKI Resistance Related to Co-Alterations of Other Oncogenic Pathways?
3.2. Can TrkA and Their Co-Receptors Elicit TKI Lack of Efficiency?
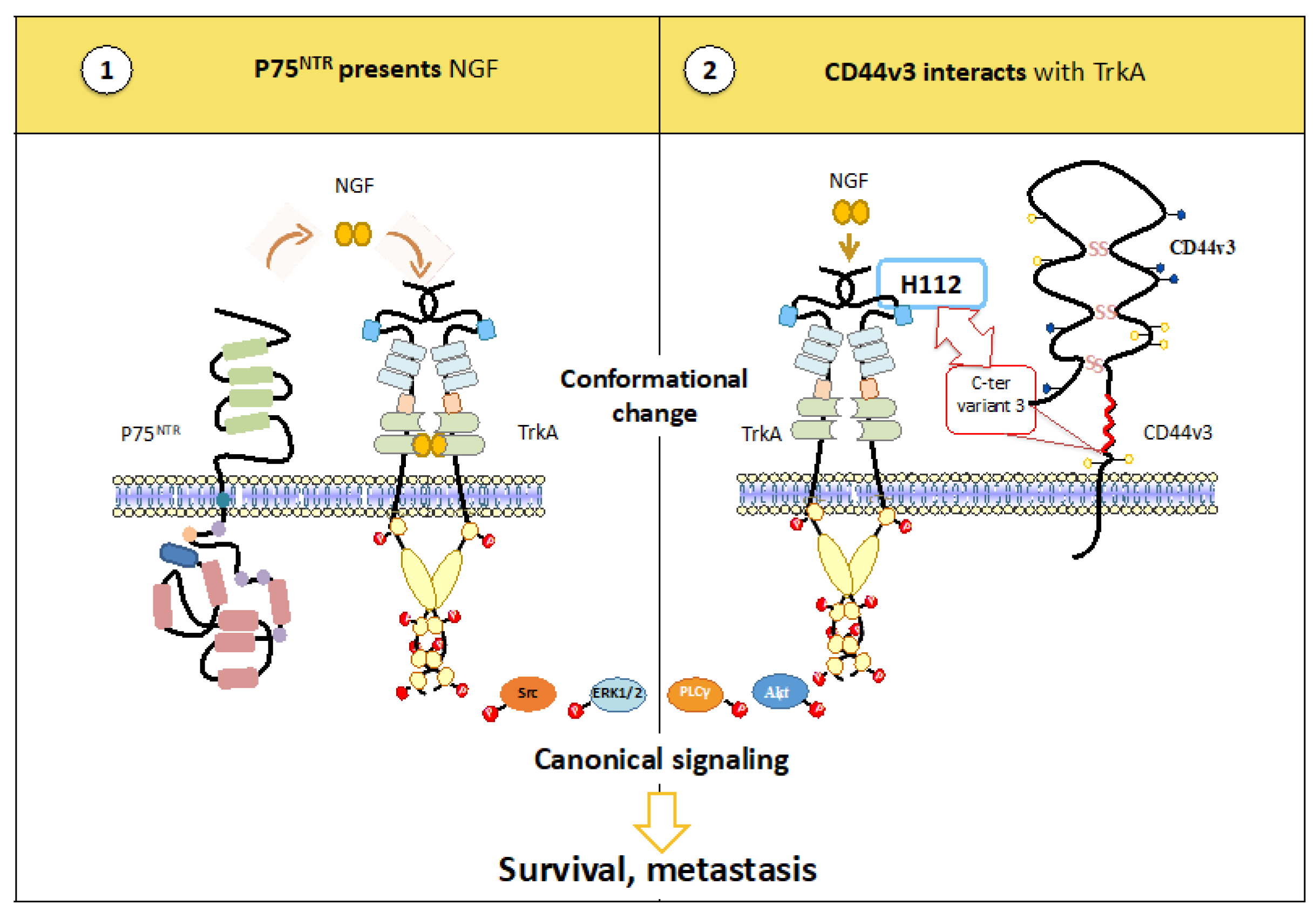
3.2.1. TrkA Heterocomplexes May Favor Ligand Binding
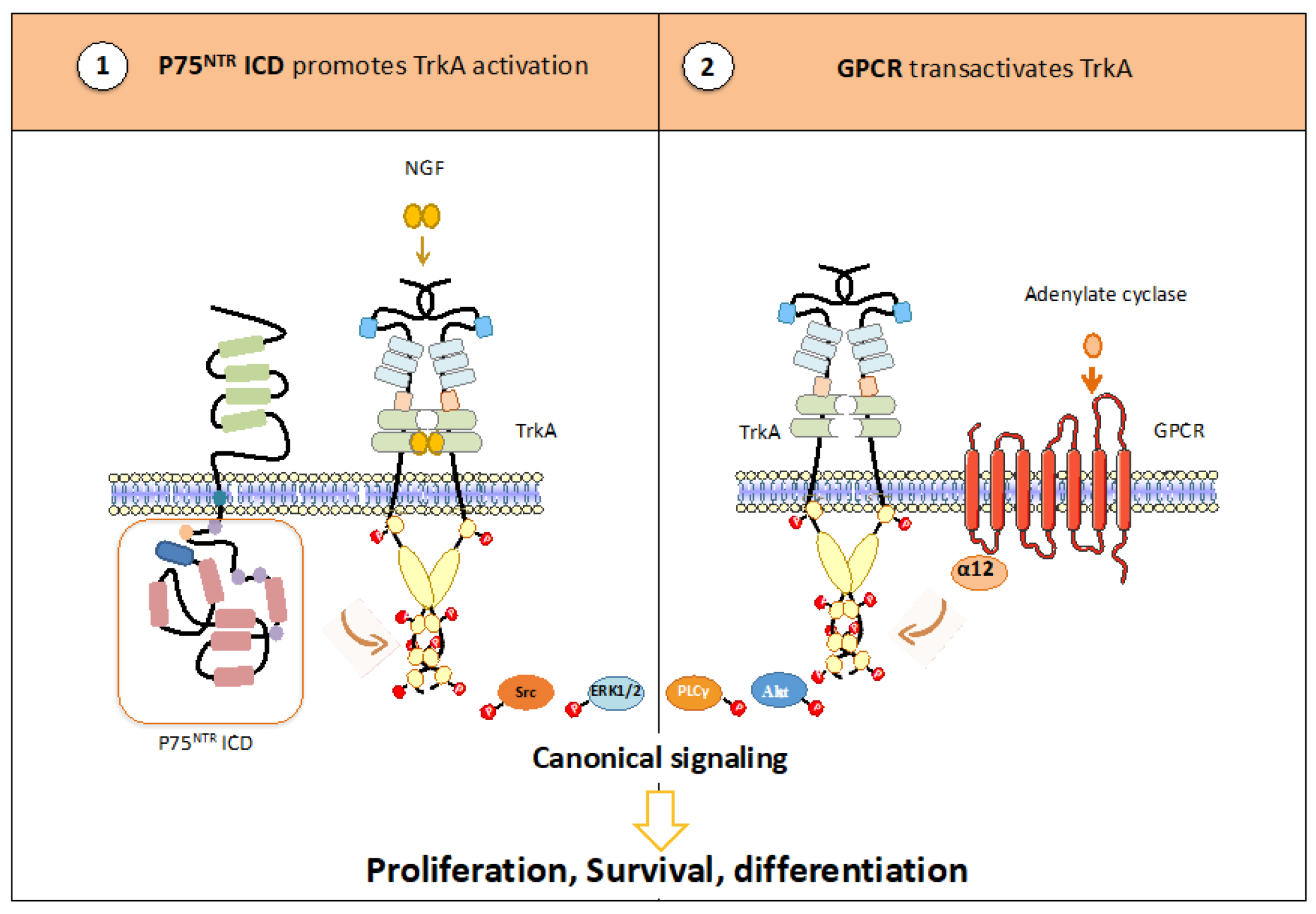
3.2.2. TrkA Heterocomplexes Regulate Its Phosphorylation
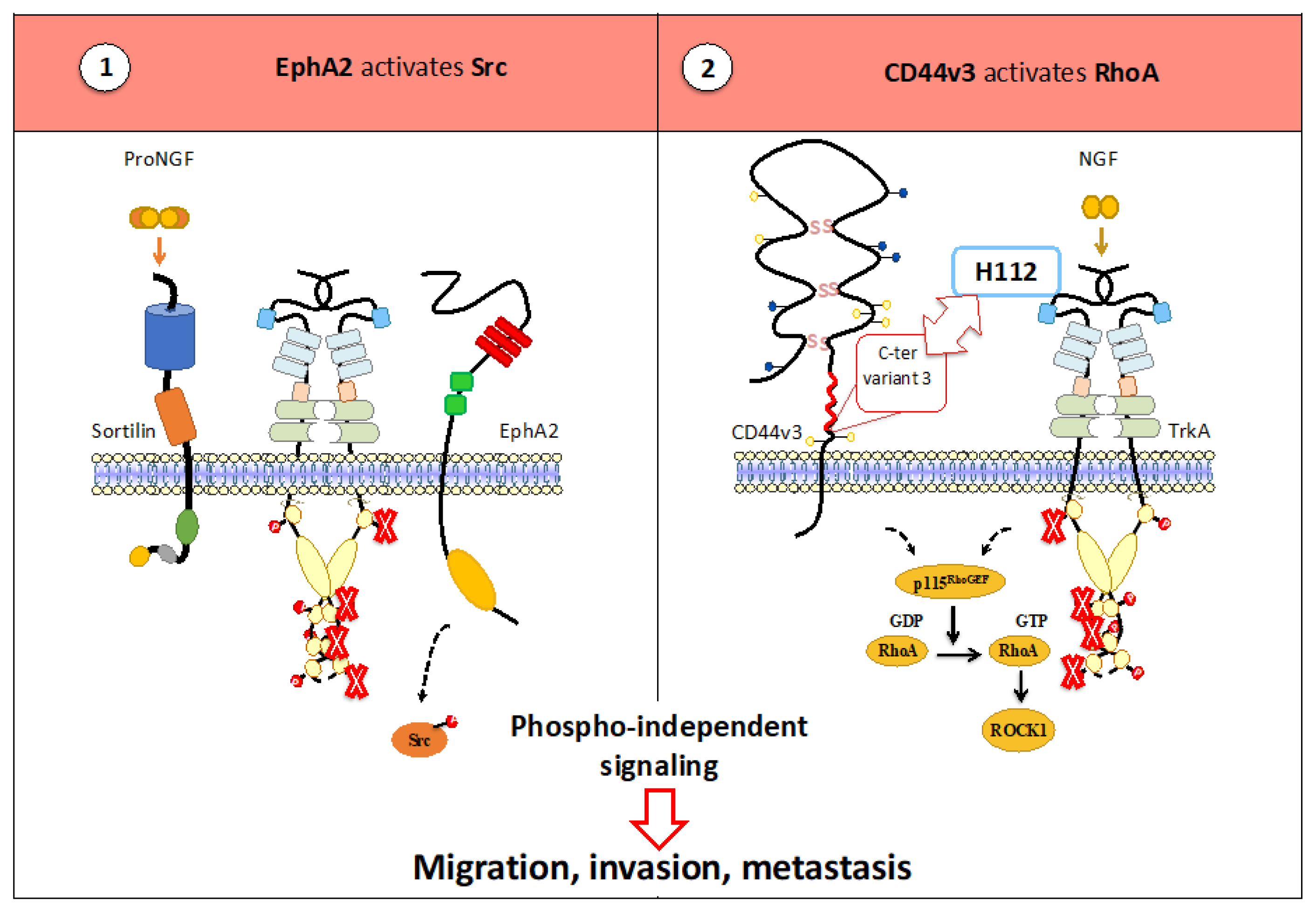
3.2.3. TrkA Heterocomplexes Permit the Activation of a Phospho-Independent Pathway
4. What Could Be Done to Counteract the Oncogenic Effects of TrkA?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martin-Zanca, D.; Hughes, S.H.; Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986, 319, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Butti, M.G.; Bongarzone, I.; Ferraresi, G.; Mondellini, P.; Borrello, M.G.; Pierotti, M.A. A Sequence Analysis of the Genomic Regions Involved in the Rearrangements between TPM3 and NTRK1 Genes Producing TRK Oncogenes in Papillary Thyroid Carcinomas. Genomics 1995, 28, 15–24. [Google Scholar] [CrossRef]
- Greco, A.; Mariani, C.; Miranda, C.; Lupas, A.; Pagliardini, S.; Pomati, M.; Pierotti, M.A. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Mol. Cell. Biol. 1995, 15, 6118–6127. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019, 30, VIII23–VIII30. [Google Scholar] [CrossRef]
- Arevalo, J.C.; Conde, B.; Hempstead, B.I.; Chao, M.V.; Martín-Zanca, D.; Pérez, P. A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. Oncogene 2001, 20, 1229–1234. [Google Scholar] [CrossRef]
- Reuther, G.W.; Lambert, Q.T.; Caligiuri, M.A.; Der, C.J. Identification and Characterization of an Activating TrkA Deletion Mutation in Acute Myeloid Leukemia. Mol. Cell. Biol. 2000, 20, 8655–8666. [Google Scholar] [CrossRef]
- Tive, L.; Bello, A.E.; Radin, D.; Schnitzer, T.J.; Nguyen, H.; Brown, M.T.; West, C.R. Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase iii clinical trials in patients with osteoarthritis pain of the knee or hip. J. Pain Res. 2019, 12, 975–995. [Google Scholar] [CrossRef]
- Kivitz, A.J.; Gimbel, J.S.; Bramson, C.; Nemeth, M.A.; Keller, D.S.; Brown, M.T.; West, C.R.; Verburg, K.M. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013, 154, 1009–1021. [Google Scholar] [CrossRef]
- Nalley, C. Study Explores Efficacy & Safety of Tanezumab for Cancer Pain. Oncol. Times 2021, 43, 24. [Google Scholar] [CrossRef]
- Chao, M.V.; Bothwell, M.A.; Ross, A.H.; Koprowski, H.; Lanahan, A.A.; Buck, C.R.; Sehgal, A. Gene transfer and molecular cloning of the human NGF receptor. Science 1986, 232, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Ohtori, S.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Suzuki, M.; Orita, S.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Low affinity NGF receptor (p75 neurotrophin receptor) inhibitory antibody reduces pain behavior and CGRP expression in DRG in the mouse sciatic nerve crush model. J. Orthop. Res. 2010, 28, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Boldin, M.P.; Varfolomeev, E.E.; Pancer, Z.; Mett, I.L.; Camonis, J.H.; Wallach, D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995, 270, 7795–7798. [Google Scholar] [CrossRef]
- Conroy, J.N.; Coulson, E.J. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J. Neurosci. 2006, 26, 11974–11986. [Google Scholar] [CrossRef]
- Poole, A.J.; Frigotto, L.; Smith, M.E.; Baar, C.; Ivanova-Berndt, G.; Jaulent, A.; Stace, C.; Ullman, C.G.; Hine, A.V. A C-terminal cysteine residue is required for peptide-based inhibition of the NGF/TrkA interaction at nM concentrations: Implications for peptide-based analgesics. Sci. Rep. 2019, 9, 930. [Google Scholar] [CrossRef]
- Kennedy, A.E.; Laamanen, C.A.; Ross, M.S.; Vohra, R.; Boreham, D.R.; Scott, J.A.; Ross, G.M. Nerve growth factor inhibitor with novel-binding domain demonstrates nanomolar efficacy in both cell-based and cell-free assay systems. Pharmacol. Res. Perspect. 2017, 5, e00339. [Google Scholar] [CrossRef]
- Somwar, R.; Hofmann, N.E.; Smith, B.; Odintsov, I.; Vojnic, M.; Linkov, I.; Tam, A.; Khodos, I.; Mattar, M.S.; de Stanchina, E.; et al. NTRK kinase domain mutations in cancer variably impact sensitivity to type I and type II inhibitors. Commun. Biol. 2020, 3, 776. [Google Scholar] [CrossRef]
- George, D.J.; Dionne, C.A.; Jani, J.; Angeles, T.; Murakata, C.; Lamb, J.; Isaacs, J.T. Sustained in Vivo Regression of Dunning H Rat Prostate Cancers Treated with Combinations of Androgen Ablation and Trk Tyrosine Kinase Inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555)1. Cancer Res. 1999, 59, 2395–2401. [Google Scholar]
- Miknyoczki, S.J.; Chang, H.; Klein-Szanto, A.; Dionne, C.A.; Ruggeri, B.A. The Trk tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin. Cancer Res. 1999, 5, 2205–2212. [Google Scholar]
- Dollé, L.; El Yazidi-Belkoura, I.; Adriaenssens, E.; Nurcombe, V.; Hondermarck, H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 2003, 22, 5592–5601. [Google Scholar] [CrossRef]
- Collins, C.; Carducci, M.A.; Eisenberger, M.A.; Partin, J.T.; Partin, A.W.; Pili, R.; Sinibaldi, V.; Walczak, J.S.; Denmeade, S.R. Preclinical and Clinical Studies with the Multi-Kinase Inhibitor CEP-701 as Treatment for Prostate Cancer Demonstrate the Inadequacy of PSA Response as a Primary Endpoint. Cancer Biol. Ther. 2007, 23, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Mulkerin, D.; Rothenberg, M.; Holen, K.D.; Locckhart, A.C.; Thomas, J.; Berlin, J. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Investig. New Drugs 2008, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.S.; Janning, A.; Renné, C.; Gattenlöhner, S.; Spieker, T.; Bräuninger, A. Induction of Endoplasmic Reticulum Stress by Sorafenib and Activation of NF- kappaB by Lestaurtinib as a Novel Resistance Mechanism in Hodgkin Lymphoma Cell Lines. Mol. Cancer Ther. 2013, 12, 173–183. [Google Scholar] [CrossRef]
- Wilson, T.; Sokol, E.S.; Ross, J.S.; Maund, S.L. 131PNTRK1/2/3 fusions in secretory versus non-secretory breast cancers. Ann. Oncol. 2020, 31, S292. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Kummar, S.; Berlin, J.; Mascarenhas, L.; van Tilburg, C.M.; Geoerger, B.; Lassen, U.N.; Schilder, R.J.; Turpin, B.; Nanda, S.; Keating, K.; et al. Quality of Life in Adult and Pediatric Patients with Tropomyosin Receptor Kinase Fusion Cancer Receiving Larotrectinib. Curr. Probl. Cancer 2021, 45, 100734. [Google Scholar] [CrossRef]
- Roth, J.A.; Carlson, J.J.; Xia, F.; Williamson, T.; Sullivan, S.D. The potential long-term comparative effectiveness of Larotrectinib and Entrectinib for second-line treatment of trk fusion-positive metastatic lung cancer. J. Manag. Care Spec. Pharm. 2020, 26, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Fuse, M.J.; Okada, K.; Oh-hara, T.; Ogura, H.; Fujita, N. Mechanisms of resistance to NTRK inhibitors and therapeutic strategies in NTRK1-rearranged cancers. Mol. Cancer Ther. 2017, 16, 2130–2143. [Google Scholar] [CrossRef]
- Drilon, A.; Nagasubramanian, R.; Blake, J.F.; Ku, N.; Tuch, B.B.; Ebata, K.; Smith, S.; Lauriault, V.; Kolakowski, G.R.; Brandhuber, B.J.; et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior trk kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017, 7, 963–972. [Google Scholar] [CrossRef]
- Descamps, S.; Toillon, R.-A.; Adriaenssens, E.; Pawlowski, V.; Cool, S.M.; Nurcombe, V.; Le Bourhis, X.; Boilly, B.; Peyrat, J.-P.; Hondermarck, H. Nerve Growth Factor Stimulates Proliferation and Survival of Human Breast Cancer Cells through Two Distinct Signaling Pathways. J. Biol. Chem. 2001, 276, 17864–17870. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.M.; Wang, F.; Mortensen, M.; Wertz, R.; Chen, G. Targeting an oncogenic kinase/phosphatase signaling network for cancer therapy. Acta Pharm. Sin. B 2018, 8, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Schram, A.M.; Kulick, A.; Misale, S.; Won, H.H.; Yaeger, R.; Razavi, P.; Ptashkin, R.; Hechtman, J.F.; Toska, E.; et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat. Med. 2019, 25, 1422–1427. [Google Scholar] [CrossRef]
- Kyker-Snowman, K.; Hughes, R.M.; Yankaskas, C.L.; Cravero, K.; Karthikeyan, S.; Button, B.; Waters, I.; Rosen, D.M.; Dennison, L.; Hunter, N.; et al. TrkA overexpression in non-tumorigenic human breast cell lines confers oncogenic and metastatic properties. Breast Cancer Res. Treat. 2020, 179, 631–642. [Google Scholar] [CrossRef]
- Barker, P.A.; Shooter, E.M. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron 1994, 13, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Wehrman, T.; He, X.; Raab, B.; Dukipatti, A.; Blau, H.; Garcia, K.C. Structural and Mechanistic Insights into Nerve Growth Factor Interactions with the TrkA and p75 Receptors. Neuron 2007, 53, 25–38. [Google Scholar] [CrossRef]
- Aubert, L.; Guilbert, M.; Corbet, C.; Génot, E.; Adriaenssens, E.; Chassat, T.; Bertucci, F.; Daubon, T.; Magné, N.; Le Bourhis, X.; et al. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget 2015, 6, 9807–9819. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Wainwright, D.; Herrlich, P. The CD44 protein family. Int. J. Biochem. Cell Biol. 1998, 30, 299–305. [Google Scholar] [CrossRef]
- Tremmel, M.; Matzke, A.; Albrecht, I.; Laib, A.M.; Olaku, V.; Ballmer-Hofer, K.; Christofori, G.; Héroult, M.; Augustin, H.G.; Ponta, H.; et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood 2009, 114, 5236–5244. [Google Scholar] [CrossRef]
- Matzke-Ogi, A.; Jannasch, K.; Shatirishvili, M.; Fuchs, B.; Chiblak, S.; Morton, J.; Tawk, B.; Lindner, T.; Sansom, O.; Alves, F.; et al. Inhibition of Tumor Growth and Metastasis in Pancreatic Cancer Models by Interference with CD44v6 Signaling. Gastroenterology 2016, 150, 513–525.e10. [Google Scholar] [CrossRef]
- Morath, I.; Jung, C.; Lévêque, R.; Linfeng, C.; Toillon, R.-A.; Warth, A.; Orian-Rousseau, V. Differential recruitment of CD44 isoforms by ERBB ligands reveals an involvement of CD44 in breast cancer. Oncogene 2018, 37, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- Trouvilliez, S.; Cicero, J.; Lévêque, R.; Aubert, L.; Corbet, C.; Van Outryve, A.; Streule, K.; Angrand, P.-O.; Völkel, P.; Magnez, R.; et al. Direct interaction of TrkA / CD44v3 is essential for NGF-promoted aggressiveness of breast cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 110. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Arevalo, J.C.; Chao, M.V. Ternary complex with Trk, p75, and an ankyrin-rich membrane spanning protein. J. Neurosci. Res. 2004, 78, 186–192. [Google Scholar] [CrossRef]
- Geetha, T.; Jiang, J.; Wooten, M.W. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol. Cell 2005, 20, 301–312. [Google Scholar] [CrossRef]
- Makkerh, J.P.; Ceni, C.; Auld, D.S.; Vaillancourt, F.; Dorval, G.; Barker, P.A. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005, 6, 936–941. [Google Scholar] [CrossRef]
- Matusica, D.; Skeldal, S.; Sykes, A.M.; Palstra, N.; Sharma, A.; Coulson, E.J. An intracellular domain fragment of the p75 neurotrophin receptor (p75 NTR) enhances tropomyosin receptor kinase A(TrkA) receptor function. J. Biol. Chem. 2013, 288, 11144–11154. [Google Scholar] [CrossRef]
- Urra, S.; Escudero, C.A.; Ramos, P.; Lisbona, F.; Allende, E.; Covarrubias, P.; Parraguez, J.I.; Zampieri, N.; Chao, M.V.; Annaert, W.; et al. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal γ-secretase-mediated release of the p75 intracellular domain. J. Biol. Chem. 2007, 282, 7606–7615. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.S.; Chao, M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA 2001, 98, 3555–3560. [Google Scholar] [CrossRef]
- Malek, R.L.; Nie, Z.; Ramkumar, V.; Lee, N.H. Adenosine A(2A) receptor mRNA regulation by nerve growth factor is TrkA-, Src-, and Ras-dependent via extracellular regulated kinase and stress- activated protein kinase/c-Jun NH2-terminal kinase. J. Biol. Chem. 1999, 274, 35499–35504. [Google Scholar] [CrossRef]
- Plouffe, B.; Guimond, M.O.; Beaudry, H.; Gallo-Payet, N. Role of tyrosine kinase receptors in angiotensin II AT2 receptor signaling: Involvement in neurite outgrowth and in p42/p44mapk activation in NG108-15 cells. Endocrinology 2006, 147, 4646–4654. [Google Scholar] [CrossRef]
- Guimond, M.-O.; Roberge, C.; Gallo-Payet, N. Fyn is involved in angiotensin II type 2 receptor-induced neurite outgrowth, but not in p42/p44mapk in NG108-15 cells. Mol. Cell. Neurosci. 2010, 45, 201–212. [Google Scholar] [CrossRef]
- Castaldo, M.; Zollo, C.; Esposito, G.; Ammendola, R.; Cattaneo, F. NOX2-Dependent Reactive Oxygen Species Regulate Formyl-Peptide Receptor 1-Mediated TrkA Transactivation in SH-SY5Y Cells. Oxid. Med. Cell. Longev. 2019, 2019, 2051235. [Google Scholar] [CrossRef] [PubMed]
- El Zein, N.; D’Hondt, S.; Sariban, E. Crosstalks between the receptors tyrosine kinase EGFR and TrkA and the GPCR, FPR, in human monocytes are essential for receptors-mediated cell activation. Cell. Signal. 2010, 22, 1437–1447. [Google Scholar] [CrossRef]
- Lin, C.; Ren, Z.; Yang, X.; Yang, R.; Chen, Y.; Liu, Z.; Dai, Z.; Zhang, Y.; He, Y.; Zhang, C.; et al. Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett. 2020, 472, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Castiglioni, F.; Ghirelli, C.; Modugno, M.; Asnaghi, L.; Somenzi, G.; Melani, C.; Ménard, S. Nerve growth factor cooperates with p185(HER2) in activating growth of human breast carcinoma cells. J. Biol. Chem. 2000, 275, 5388–5394. [Google Scholar] [CrossRef]
- Blöchl, A.; Blumenstein, L.; Ahmadian, M.R. Inactivation and activation of Ras by the neurotrophin receptor p75. Eur. J. Neurosci. 2004, 20, 2321–2335. [Google Scholar] [CrossRef]
- Deponti, D.; Buono, R.; Catanzaro, G.; De Palma, C.; Longhi, R.; Meneveri, R.; Bresolin, N.; Bassi, M.T.; Cossu, G.; Clementi, E.; et al. The Low-Affinity Receptor for Neurotrophins p75NTR Plays a Key Role for Satellite Cell Function in Muscle Repair Acting via RhoA. Mol. Biol. Cell 2009, 20, 3620–3627. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef]
- Lévêque, R.; Corbet, C.; Aubert, L.; Guilbert, M.; Lagadec, C.; Adriaenssens, E.; Duval, J.; Finetti, P.; Birnbaum, D.; Magné, N.; et al. ProNGF increases breast tumor aggressiveness through functional association of TrkA with EphA2. Cancer Lett. 2019, 449, 196–206. [Google Scholar] [CrossRef]
- Davis, S.; Gale, N.W.; Aldrich, T.H.; Maisonpierre, P.C.; Lhotak, V.; Pawson, T.; Goldfarb, M.; Yancopoulos, G.D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 1994, 266, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, B.L.; Torres, A.M.; Woodward, W.A.; Krishnamurthy, S.; Meric-Bernstam, F.; Ueno, N.T. Abstract P3-07-04: EphA2: An emerging target in triple-negative breast cancer. Cancer Res. 2018, 78, P3-07-04. [Google Scholar] [CrossRef]
- Zelinski, D.P.; Zantek, N.D.; Stewart, J.C.; Irizarry, A.R.; Kinch, M.S. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001, 61, 2301–2306. [Google Scholar] [PubMed]
- Kikawa, K.D.; Vidale, D.R.; Van Etten, R.L.; Kinch, M.S. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J. Biol. Chem. 2002, 277, 39274–39279. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, D.Q.; Mukherjee, A.; Guo, H.; Petty, A.; Cutter, J.; Basilion, J.P.; Sedor, J.; Wu, J.; Danielpour, D.; et al. EphA2 Mediates Ligand-Dependent Inhibition and Ligand-Independent Promotion of Cell Migration and Invasion via a Reciprocal Regulatory Loop with Akt. Cancer Cell 2009, 16, 9–20. [Google Scholar] [CrossRef]
- Thomas, R.; Weihua, Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front. Oncol. 2019, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Su, H.P.; Rickert, K.; Burlein, C.; Narayan, K.; Bukhtiyarova, M.; Hurzy, D.M.; Stump, C.A.; Zhang, X.; Reid, J.; Krasowska-Zoladek, A.; et al. Structural characterization of nonactive site, TrkA-selective kinase inhibitors. Proc. Natl. Acad. Sci. USA 2017, 114, E297–E306. [Google Scholar] [CrossRef]
- Furuya, N.; Momose, T.; Katsuno, K.; Fushimi, N.; Muranaka, H.; Handa, C.; Ozawa, T.; Kinoshita, T. The juxtamembrane region of TrkA kinase is critical for inhibitor selectivity. Bioorg. Med. Chem. Lett. 2017, 27, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Bagal, S.K.; Omoto, K.; Blakemore, D.C.; Bungay, P.J.; Bilsland, J.G.; Clarke, P.J.; Corbett, M.S.; Cronin, C.N.; Cui, J.J.; Dias, R.; et al. Discovery of Allosteric, Potent, Subtype Selective, and Peripherally Restricted TrkA Kinase Inhibitors. J. Med. Chem. 2019, 62, 247–265. [Google Scholar] [CrossRef]
- Furuya, N.; Momose, T.; Katsuno, K.; Fushimi, N.; Muranaka, H.; Handa, C.; Sawa, M.; Ozawa, T.; Kinoshita, T. An isoform-selective inhibitor of tropomyosin receptor kinase A behaves as molecular glue. Bioorg. Med. Chem. Lett. 2020, 30, 126775. [Google Scholar] [CrossRef]
- Chung, V.; Wang, L.; Fletcher, M.S.; Massarelli, E.; Cristea, M.C.; Kamaraju, S.; Alistar, A.T.; Feng, C.; Li, Y.; Whiting, R.L.; et al. First-time in-human study of VMD-928, an oral allosteric TrkA selective inhibitor targeting TrkA protein overexpression, in patients with solid tumors or lymphoma. J. Clin. Oncol. 2021, 39, 3081. [Google Scholar] [CrossRef]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouvilliez, S.; Lagadec, C.; Toillon, R.-A. TrkA Co-Receptors: The Janus Face of TrkA? Cancers 2023, 15, 1943. https://doi.org/10.3390/cancers15071943
Trouvilliez S, Lagadec C, Toillon R-A. TrkA Co-Receptors: The Janus Face of TrkA? Cancers. 2023; 15(7):1943. https://doi.org/10.3390/cancers15071943
Chicago/Turabian StyleTrouvilliez, Sarah, Chann Lagadec, and Robert-Alain Toillon. 2023. "TrkA Co-Receptors: The Janus Face of TrkA?" Cancers 15, no. 7: 1943. https://doi.org/10.3390/cancers15071943
APA StyleTrouvilliez, S., Lagadec, C., & Toillon, R.-A. (2023). TrkA Co-Receptors: The Janus Face of TrkA? Cancers, 15(7), 1943. https://doi.org/10.3390/cancers15071943






