The Association between Early-Onset Diagnosis and Clinical Outcomes in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
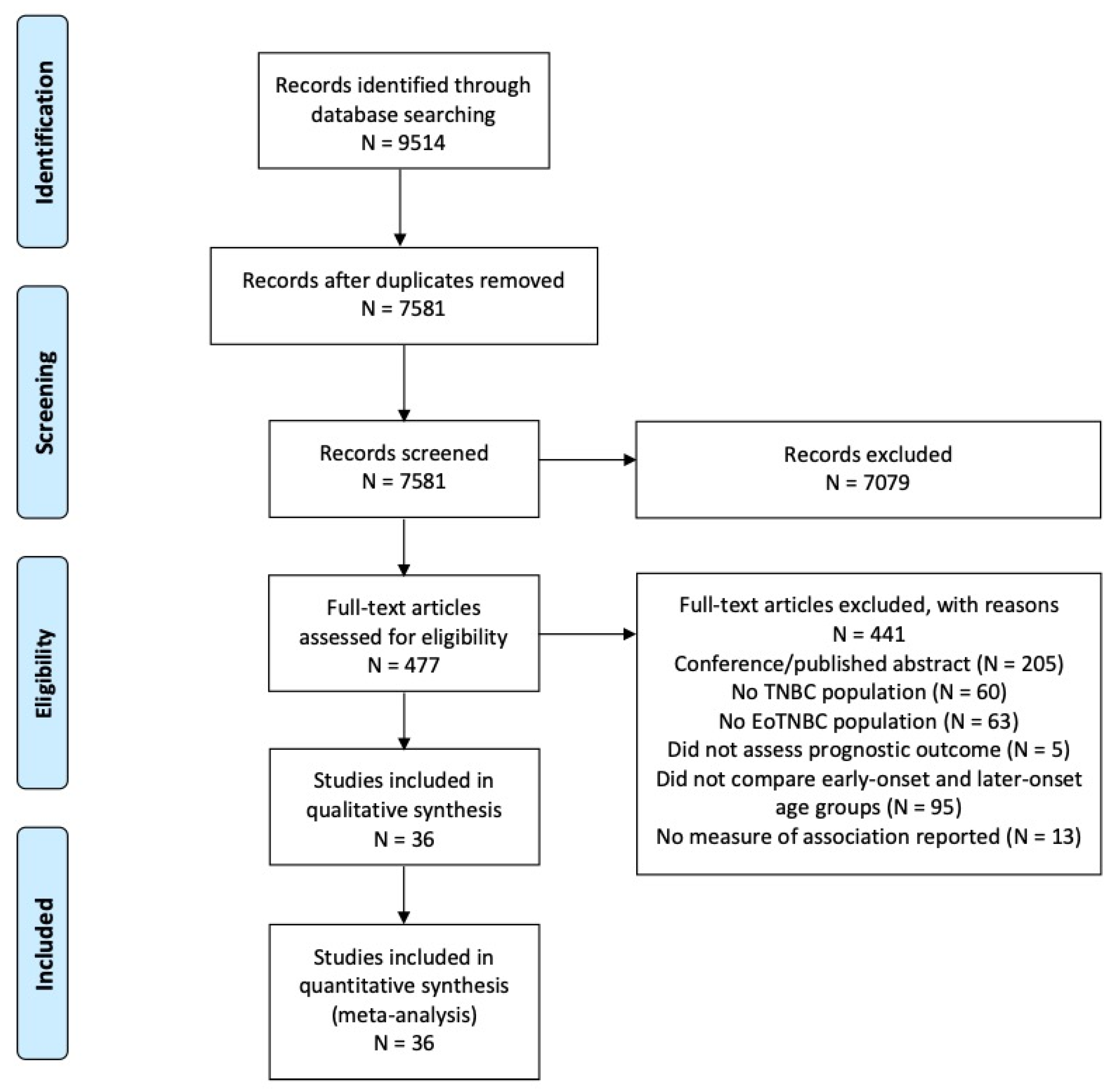
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
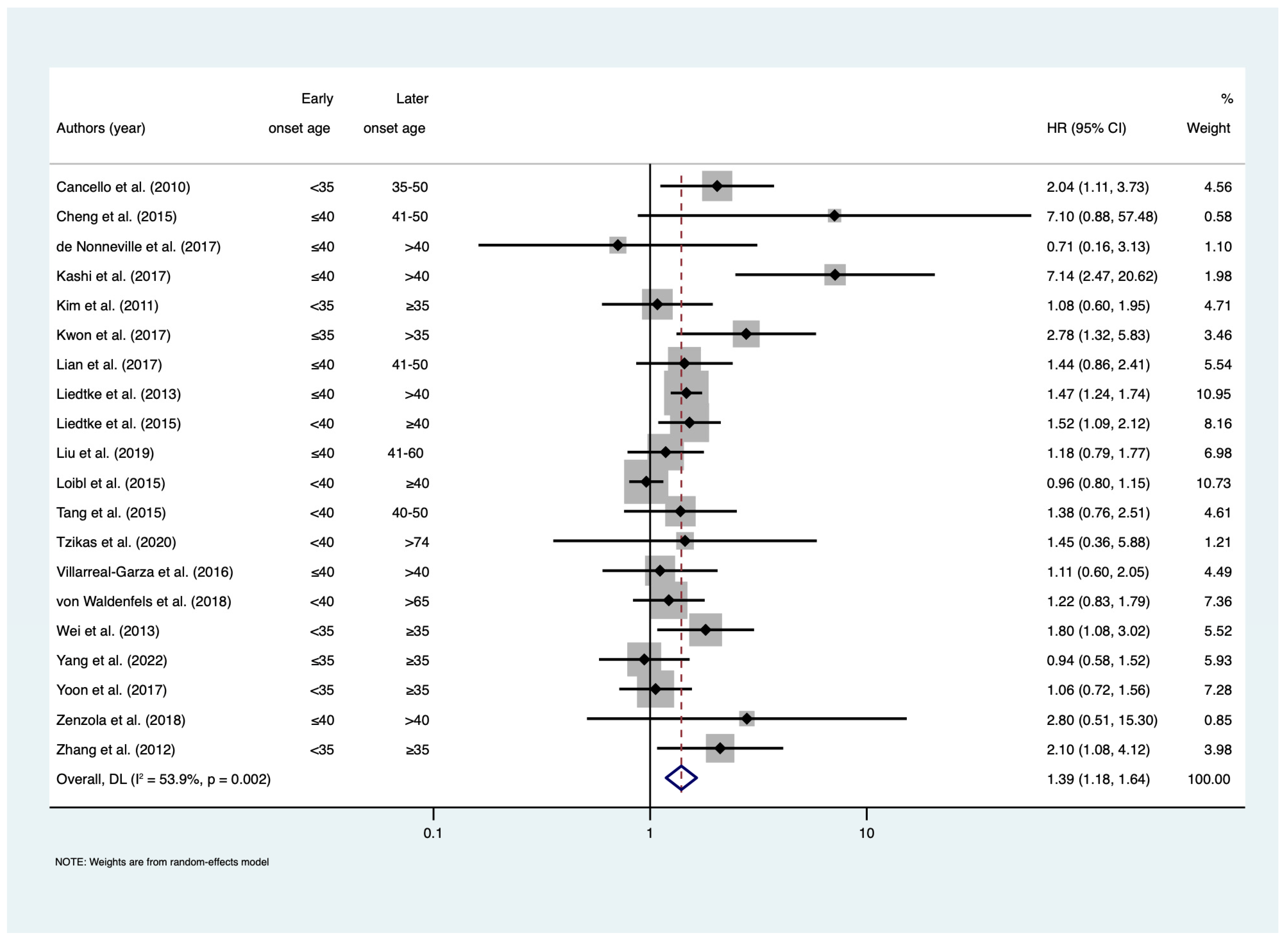
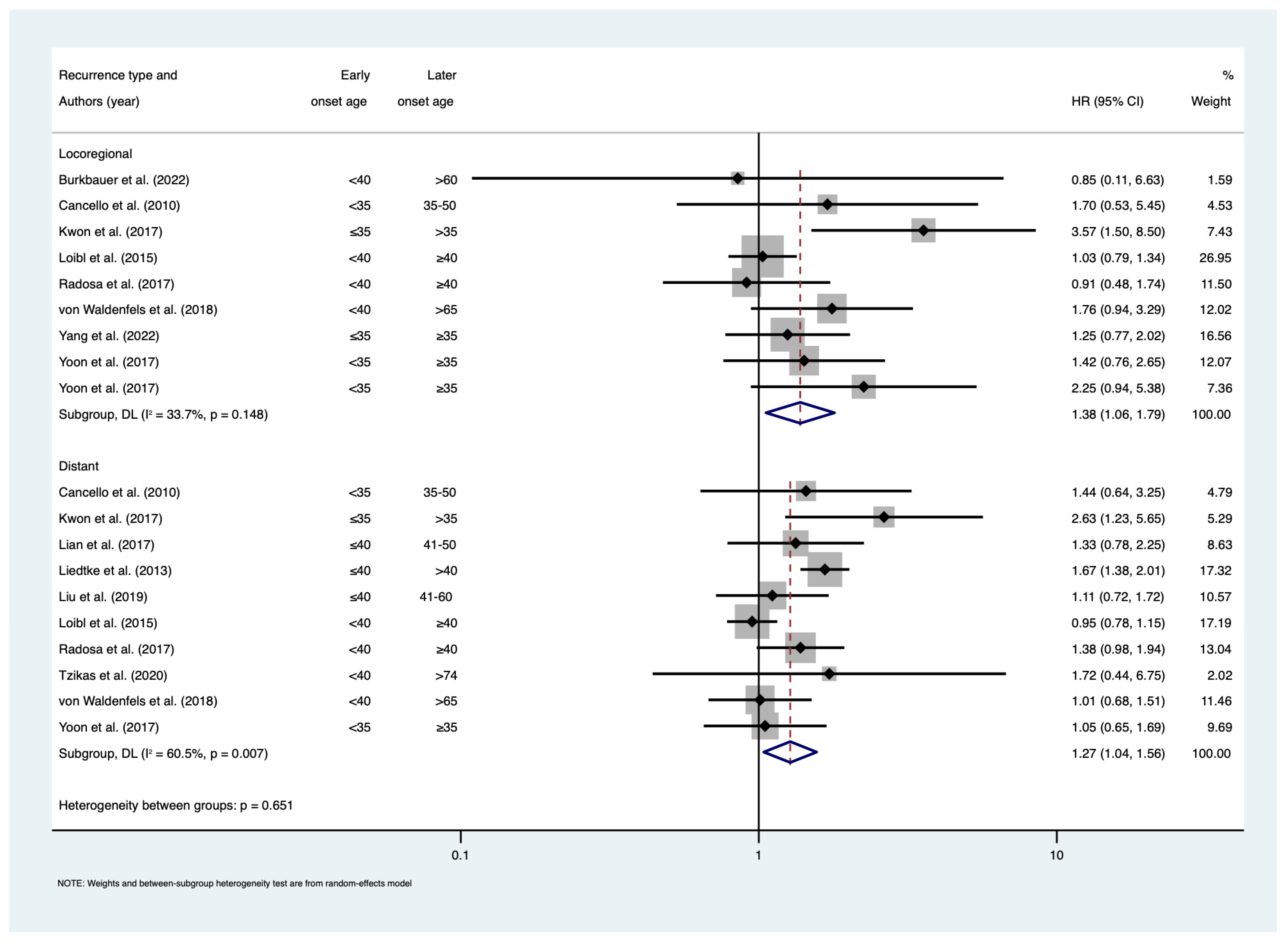
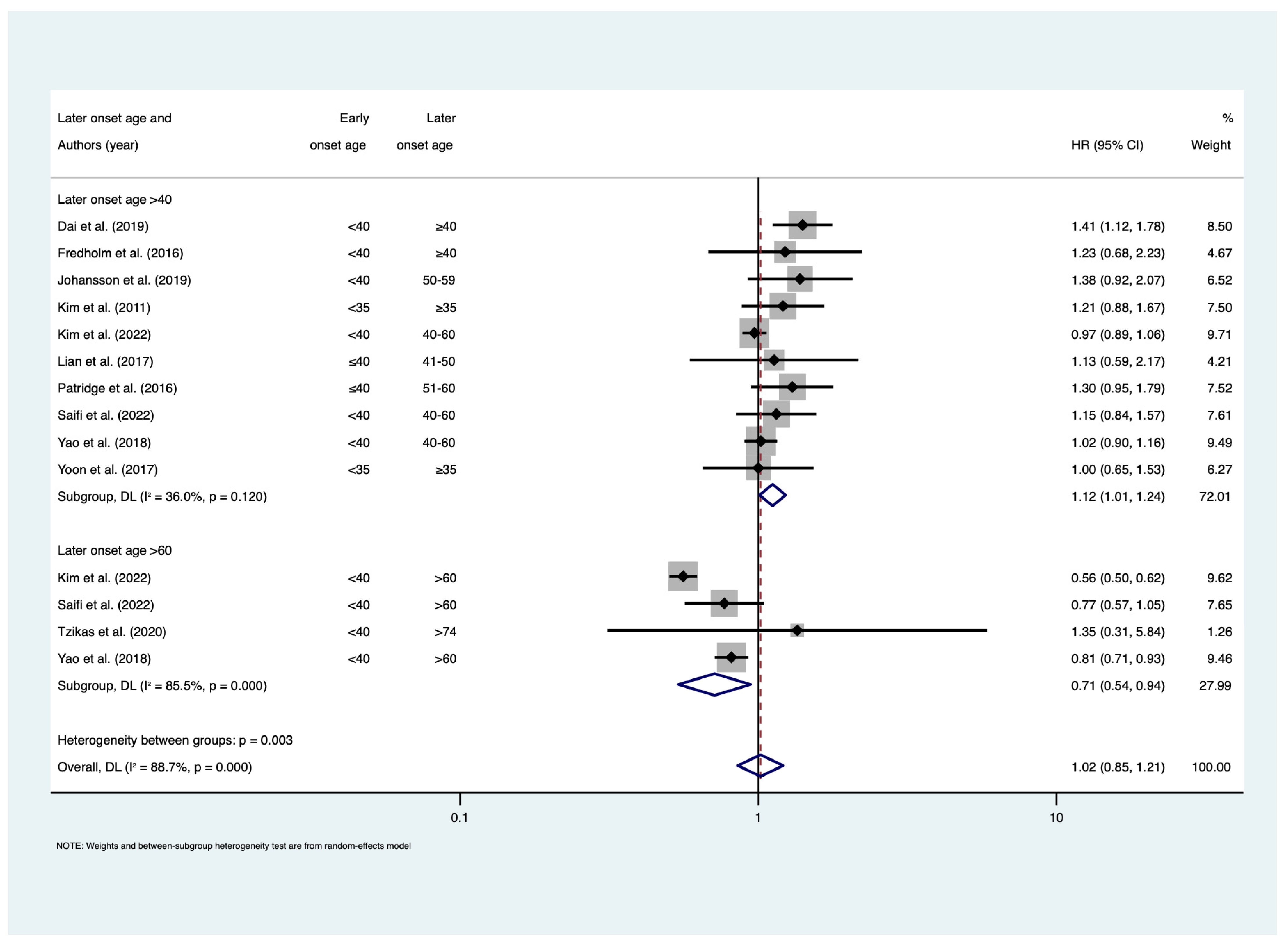
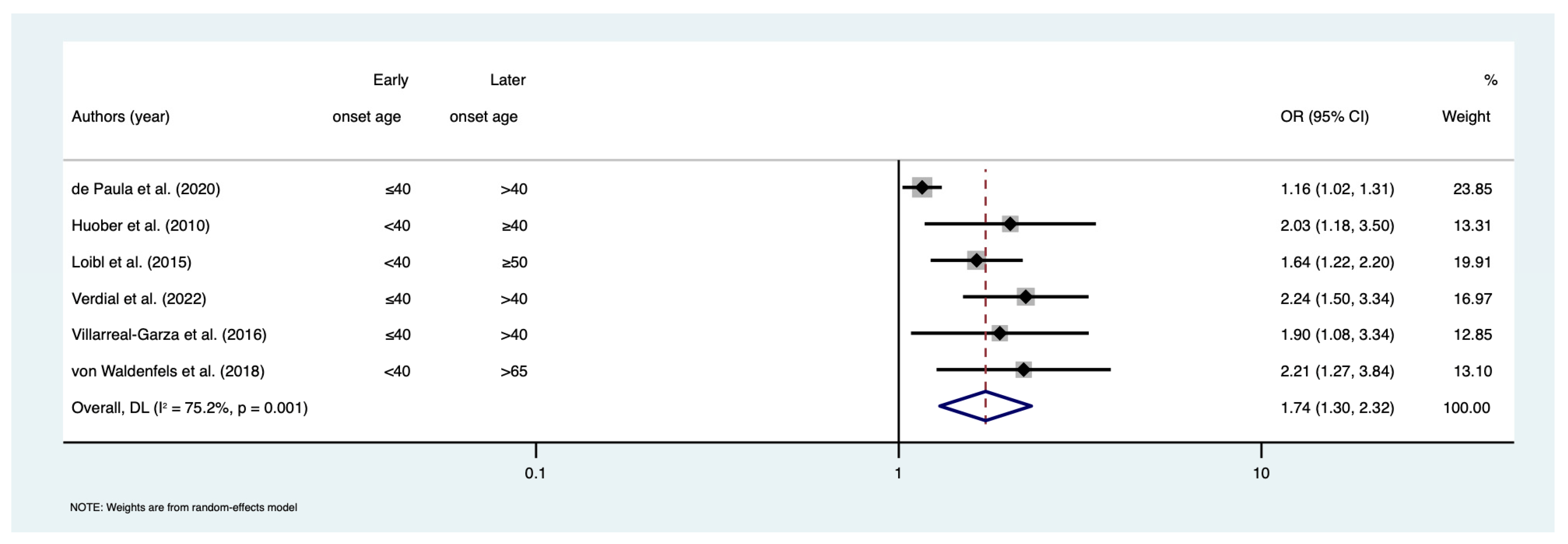
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Ruan, Y.; Mealey, N.; Quan, M.L.; Brenner, D.R. The incidence of breast cancer in Canada 1971–2015: Trends in screening-eligible and young-onset age groups. Can. J. Public Health 2020, 111, 787–793. [Google Scholar] [CrossRef]
- Bouchardy, C.; Fioretta, G.; Verkooijen, H.M.; Vlastos, G.; Schaefer, P.; Delaloye, J.F.; Neyroud-Caspar, I.; Balmer Majno, S.; Wespi, Y.; Forni, M.; et al. Recent increase of breast cancer incidence among women under the age of forty. Br. J. Cancer 2007, 96, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9 (Suppl. S2), S73–S81. [Google Scholar] [CrossRef]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Guo, Y.; Shi, X.; Chen, X.; Feng, W.; Wu, L.L.; Zhang, J.; Yu, S.; Wang, Y.; et al. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int. J. Biol. Sci. 2023, 19, 897–915. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A., Jr.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Harbeck, N.; et al. ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann. Oncol. 2020, 31, 674–696. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Johnson, R.; Litton, J.; Phillips, M.; Bleyer, A. Breast cancer before age 40 years. Semin. Oncol. 2009, 36, 237–249. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Partridge, A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014, 16, 427. [Google Scholar] [CrossRef]
- Narod, S.A. Breast cancer in young women. Nat. Rev. Clin. Oncol. 2012, 9, 460–470. [Google Scholar] [CrossRef]
- Colleoni, M.; Anders, C.K. Debate: The biology of breast cancer in young women is unique. Oncologist 2013, 18, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Partridge, A.H.; Hughes, M.E.; Warner, E.T.; Ottesen, R.A.; Wong, Y.-N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J. Clin. Oncol. 2016, 34, 3308–3314. [Google Scholar] [CrossRef]
- Sheridan, W.; Scott, T.; Caroline, S.; Yvonne, Z.; Vanessa, B.; David, V.; Karen, G.; Stephen, C. Breast cancer in young women: Have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res. Treat. 2014, 147, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in meta-Analyses; Oxford. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 February 2022).
- Abulkhair, O.; Moghraby, J.S.; Badri, M.; Alkushi, A. Clinicopathologic features and prognosis of triple-negative breast cancer in patients 40 years of age and younger in Saudi Arabia. Hematol. Oncol. Stem Cell Ther. 2012, 5, 101–106. [Google Scholar] [CrossRef]
- Burkbauer, L.; Goldbach, M.; Hoffman, D.I.; Giannakou, A.; Dultz, R.; Brooks, A.D.; Sataloff, D.M.; Keele, L.; Tchou, J. Preoperative MRI and Its Impact on Surgical Outcomes in Patients with Triple Negative Breast Cancer Treated with Primary Surgery: Did New Margin Guidelines or Cavity Shave Margins Practice Diminish the Role of Preoperative MRI? Ann. Surg. Oncol. 2022, 29, 4079–4088. [Google Scholar] [CrossRef]
- Cancello, G.; Maisonneuve, P.; Rotmensz, N.; Viale, G.; Mastropasqua, M.; Pruneri, G.; Veronesi, P.; Torrisi, R.; Montagna, E.; Luini, A. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann. Oncol. 2010, 21, 1974–1981. [Google Scholar] [PubMed]
- Cheng, S.H.-C.; Yu, B.-L.; Horng, C.-F.; Tsai, S.Y.; Chen, C.-M.; Chu, N.-M.; Tsou, M.-H.; Lin, C.K.; Shih, L.-S.; Liu, M.-C. Long-term survival and stage I breast cancer subtypes. J. Cancer Res. Pract. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Dai, D.; Zhong, Y.; Wang, Z.; Yousafzai, N.A.; Jin, H.; Wang, X. The prognostic impact of age in different molecular subtypes of breast cancer: A population-based study. PeerJ 2019, 7, e7252. [Google Scholar] [CrossRef]
- De Nonneville, A.; Gonçalves, A.; Zemmour, C.; Cohen, M.; Classe, J.; Reyal, F.; Colombo, P.; Jouve, E.; Giard, S.; Barranger, E. Adjuvant chemotherapy in pT1ab node-negative triple-negative breast carcinomas: Results of a national multi-institutional retrospective study. Eur. J. Cancer 2017, 84, 34–43. [Google Scholar] [CrossRef] [PubMed]
- de Paula, B.H.R.; Kumar, S.; Morosini, F.M.; Cardoso, D.E.M.C.; de Sousa, C.A.M.; Crocamo, S. Real-world assessment of the effect of impact of tumor size on pathological complete response rates in triple negative breast cancer after neoadjuvant chemotherapy. Chin. Clin. Oncol. 2020, 9, 78. [Google Scholar] [CrossRef]
- Fredholm, H.; Magnusson, K.; Lindström, L.S.; Garmo, H.; Fält, S.E.; Lindman, H.; Bergh, J.; Holmberg, L.; Pontén, F.; Frisell, J. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res. Treat. 2016, 160, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Huober, J.; von Minckwitz, G.; Denkert, C.; Tesch, H.; Weiss, E.; Zahm, D.M.; Belau, A.; Khandan, F.; Hauschild, M.; Thomssen, C. Effect of neoadjuvant anthracycline–taxane-based chemotherapy in different biological breast cancer phenotypes: Overall results from the GeparTrio study. Breast Cancer Res. Treat. 2010, 124, 133–140. [Google Scholar] [CrossRef]
- Johansson, A.L.; Trewin, C.B.; Hjerkind, K.V.; Ellingjord-Dale, M.; Johannesen, T.B.; Ursin, G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int. J. Cancer 2019, 144, 1251–1261. [Google Scholar] [CrossRef]
- Kashi, A.S.Y.; Yazdanfar, S.; Akbari, M.-E.; Rakhsha, A. Triple negative breast cancer in iranian women: Clinical profile and survival study. Int. J. Cancer Manag. 2017, 10, e10471. [Google Scholar] [CrossRef]
- Kim, E.-K.; Noh, W.C.; Han, W.; Noh, D.-Y. Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: A nationwide registry-based study. World J. Surg. 2011, 35, 1244–1253. [Google Scholar] [PubMed]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age < 40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar]
- Kwon, J.; Eom, K.-Y.; Koo, T.R.; Kim, B.H.; Kang, E.; Kim, S.-W.; Kim, Y.J.; Park, S.Y.; Kim, I.A. A prognostic model for patients with triple-negative breast cancer: Importance of the modified nottingham prognostic index and age. J. Breast Cancer 2017, 20, 65–73. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, K.-I.; Bae, J.W.; Jung, Y.-H.; An, H.; Lee, E.S. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res. Treat. 2010, 123, 177–187. [Google Scholar] [CrossRef]
- Lian, W.; Fu, F.; Lin, Y.; Lu, M.; Chen, B.; Yang, P.; Zeng, B.; Huang, M.; Wang, C. The impact of young age for prognosis by subtype in women with early breast cancer. Sci. Rep. 2017, 7, 11625. [Google Scholar] [CrossRef]
- Liedtke, C.; Hess, K.; Karn, T.; Rody, A.; Kiesel, L.; Hortobagyi, G.; Pusztai, L.; Gonzalez-Angulo, A. The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2013, 138, 591–599. [Google Scholar] [CrossRef]
- Liedtke, C.; Rody, A.; Gluz, O.; Baumann, K.; Beyer, D.; Kohls, E.-B.; Lausen, K.; Hanker, L.; Holtrich, U.; Becker, S. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res. Treat. 2015, 152, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sahli, Z.; Wang, Y.; Wolff, A.C.; Cope, L.M.; Umbricht, C.B. Young age at diagnosis is associated with worse prognosis in the Luminal A breast cancer subtype: A retrospective institutional cohort study. Breast Cancer Res. Treat. 2018, 172, 689–702. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Lederer, B.; Untch, M.; Paepke, S.; Kümmel, S.; Schneeweiss, A.; Huober, J.; Hilfrich, J.; Hanusch, C. Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res. Treat. 2015, 152, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.C.; Eaton, A.; Stempel, M.; Khander, A.; Liedtke, C.; Solomayer, E.-F.; Karsten, M.; Pilewskie, M.; Morrow, M.; King, T.A. Evaluation of local and distant recurrence patterns in patients with triple-negative breast cancer according to age. Ann. Surg. Oncol. 2017, 24, 698–704. [Google Scholar] [CrossRef]
- Ryu, J.M.; Yu, J.; Kim, S.I.; Kim, K.S.; Moon, H.-G.; Choi, J.E.; Jeong, J.; Do Byun, K.; Nam, S.J.; Lee, J.E. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. Treat. 2017, 166, 833–842. [Google Scholar] [CrossRef]
- Saifi, O.; Chahrour, M.A.; Li, Z.; Hoballah, J.; Panoff, J.; Vallow, L.A.; Zeidan, Y.H. Is breast conservation superior to mastectomy in early stage triple negative breast cancer? Breast 2022, 62, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-C.; Jin, X.; Yang, H.-Y.; He, M.; Chang, H.; Shao, Z.-M.; Di, G.-H. Luminal B subtype: A key factor for the worse prognosis of young breast cancer patients in China. Bmc Cancer 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.-K.; Nemes, S.; Linderholm, B.K. A comparison between young and old patients with triple-negative breast cancer: Biology, survival and metastatic patterns. Breast Cancer Res. Treat. 2020, 182, 643–654. [Google Scholar] [CrossRef]
- Verdial, F.C.; Mamtani, A.; Pawloski, K.R.; Sevilimedu, V.; D’Alfonso, T.M.; Zhang, H.; Gemignani, M.L.; Barrio, A.V.; Morrow, M.; Tadros, A.B. The Effect of Age on Outcomes After Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. Oncol. 2022, 29, 3810–3819. [Google Scholar] [CrossRef]
- Villarreal-Garza, C.; Bargallo-Rocha, J.E.; Soto-Perez-de-Celis, E.; Lasa-Gonsebatt, F.; Arce-Salinas, C.; Lara-Medina, F.; Reynoso-Noverón, N.; Matus-Santos, J.; Cabrera, P.; Alvarado-Miranda, A. Real-world outcomes in young women with breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2016, 157, 385–394. [Google Scholar] [CrossRef]
- von Waldenfels, G.; Loibl, S.; Furlanetto, J.; Machleidt, A.; Lederer, B.; Denkert, C.; Hanusch, C.; Kümmel, S.; von Minckwitz, G.; Schneeweiss, A. Outcome after neoadjuvant chemotherapy in elderly breast cancer patients–a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Oncotarget 2018, 9, 15168. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Li, X.; Xin, X.-J.; Tong, Z.-S.; Zhang, S. Clinical features and survival analysis of very young (age < 35) breast cancer patients. Asian Pac. J. Cancer Prev. 2013, 14, 5949–5952. [Google Scholar] [PubMed]
- Yang, Y.; Wei, W.; Jin, L.; He, H.; Wei, M.; Shen, S.; Pi, H.; Liu, Z.; Li, H.; Liu, J. Comparison of the Characteristics and Prognosis Between Very Young Women and Older Women With Breast Cancer: A Multi-Institutional Report From China. Front. Oncol. 2022, 12, 783487. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Radiotherapy after surgery has significant survival benefits for patients with triple-negative breast cancer. Cancer Med. 2019, 8, 554–563. [Google Scholar] [CrossRef]
- Yoon, T.I.; Hwang, U.-K.; Kim, E.T.; Lee, S.; Sohn, G.; Ko, B.S.; Lee, J.W.; Son, B.H.; Kim, S.; Ahn, S.H. Survival improvement in hormone-responsive young breast cancer patients with endocrine therapy. Breast Cancer Res. Treat. 2017, 165, 311–320. [Google Scholar] [CrossRef]
- Yoon, T.I.; Kwak, B.S.; Yi, O.V.; Kim, S.; Um, E.; Yun, K.W.; Shin, H.-n.; Lee, S.; Sohn, G.; Chung, I.Y. Age-related risk factors associated with primary contralateral breast cancer among younger women versus older women. Breast Cancer Res. Treat. 2019, 173, 657–665. [Google Scholar] [CrossRef]
- Zenzola, V.; Cabezas-Quintario, M.; Arguelles, M.; Pérez-Fernández, E.; Izarzugaza, Y.; Correa, A.; Garcia-Foncillas, J. Prognostic value of Ki-67 according to age in patients with triple-negative breast cancer. Clin. Transl. Oncol. 2018, 20, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, C.; Dong, G.; Tong, Z. Analysis of clinical features and outcome of 356 triple-negative breast cancer patients in China. Breast Care 2012, 7, 13–17. [Google Scholar] [CrossRef]
- Wang, J.; Xie, X.; Wang, X.; Tang, J.; Pan, Q.; Zhang, Y.; Di, M. Locoregional and distant recurrences after breast conserving therapy in patients with triple-negative breast cancer: A meta-analysis. Surg. Oncol. 2013, 22, 247–255. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Murray, L.J.; Brand, J.S.; Bhoo-Pathy, N. The value of adjuvant radiotherapy on survival and recurrence in triple-negative breast cancer: A systematic review and meta-analysis of 5507 patients. Cancer Treat. Rev. 2016, 47, 12–21. [Google Scholar] [CrossRef]
- Sio, T.T.; Chang, K.; Jayakrishnan, R.; Wu, D.; Politi, M.; Malacarne, D.; Saletnik, J.; Chung, M. Patient age is related to decision-making, treatment selection, and perceived quality of life in breast cancer survivors. World J. Surg. Oncol. 2014, 12, 230. [Google Scholar] [CrossRef]
- Blackmore, T.; Lawrenson, R.; Lao, C.; Edwards, M.; Kuper-Hommel, M.; Elwood, M.; Campbell, I. The characteristics, management and outcomes of older women with breast cancer in New Zealand. Maturitas 2018, 112, 64–70. [Google Scholar] [CrossRef]
- Cancello, G.; Maisonneuve, P.; Rotmensz, N.; Viale, G.; Mastropasqua, M.G.; Pruneri, G.; Montagna, E.; Dellapasqua, S.; Iorfida, M.; Cardillo, A.; et al. Prognosis in women with small (T1mic, T1a, T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res. Treat. 2011, 127, 713–720. [Google Scholar] [CrossRef]
- Yoon, J.; Knapp, G.; Quan, M.L.; Bouchard-Fortier, A. Cancer-Specific Outcomes in the Elderly with Triple-Negative Breast Cancer: A Systematic Review. Curr. Oncol. 2021, 28, 2337–2345. [Google Scholar] [CrossRef]
- Frank, S.; Carton, M.; Dubot, C.; Campone, M.; Pistilli, B.; Dalenc, F.; Mailliez, A.; Levy, C.; D’Hondt, V.; Debled, M.; et al. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast 2020, 52, 50–57. [Google Scholar] [CrossRef]
- Xie, Y.; Gou, Q.; Zhang, Y.; Xie, K.; Zheng, D.; Luo, C.; Suo, J.; Zhong, X.; Luo, T. Association between age at initial diagnosis and post-metastasis mortality among women with recurrent metastatic breast cancer in China. BMC Cancer 2022, 22, 385. [Google Scholar] [CrossRef]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathological features and sites of recurrence according to breast cancer subtype in the National Comprehensive Cancer Network (NCCN). J. Clin. Oncol. 2009, 27, 543. [Google Scholar] [CrossRef]
- Pogoda, K.; Niwińska, A.; Murawska, M.; Pieńkowski, T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med. Oncol. 2013, 30, 388. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.H.; Liu, C.Y.; Shiau, C.Y.; Hsu, C.Y.; Tsai, Y.F.; Wang, Y.L.; Tai, L.C.; King, K.L.; Chao, T.C.; Chiu, J.H.; et al. Effect of age and biological subtype on the risk and timing of brain metastasis in breast cancer patients. PLoS ONE 2014, 9, e89389. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Tesch, M.E.; Partridge, A.H. Treatment of Breast Cancer in Young Adults. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.-S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Ludmir, E.B.; Mainwaring, W.; Lin, T.A.; Miller, A.B.; Jethanandani, A.; Espinoza, A.F.; Mandel, J.J.; Lin, S.H.; Smith, B.D.; Smith, G.L.; et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol. 2019, 5, 1769–1773. [Google Scholar] [CrossRef]
- Patel, M.A.; Shah, J.L.; Abrahamse, P.H.; Jagsi, R.; Katz, S.J.; Hawley, S.T.; Veenstra, C.M. A population-based study of invitation to and participation in clinical trials among women with early-stage breast cancer. Breast Cancer Res. Treat 2020, 184, 507–518. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gulbahce, H.E.; Bernard, P.S.; Weltzien, E.K.; Factor, R.E.; Kushi, L.H.; Caan, B.J.; Sweeney, C. Differences in molecular features of triple-negative breast cancers based on the age at diagnosis. Cancer 2018, 124, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.M.; Bacchi, L.M.; Santos, P.P.; Bacchi, C.E. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics 2010, 65, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.M.; Green, A.R.; Nolan, C.C.; Morgan, D.A.; Ellis, I.O.; Cheung, K.L. Biological characteristics and clinical outcome of triple negative primary breast cancer in older women—Comparison with their younger counterparts. PLoS ONE 2014, 9, e100573. [Google Scholar] [CrossRef]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]

| Authors | Publication Year | Study Start–End | Location | TNBC Size | EoTNBC Age Group(s) | LoTNBC Age Group(s) | Outcome Type(s) | Effect Estimate (95% CI) | Newcastle–Ottawa Scale Assessments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | ||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q1 | Q2 | Q3 | |||||||||
| Abulkhair et al. [24] | 2012 | 2001–2008 | Saudi Arabia | 26 | ≤40 | >40 | OS | 5.43 (1.94–15.2) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Burkbauer et al. [25] | 2022 | 2009–2018 | United States | 648 | <40 | >60 | LRFS | 0.85 (0.11–6.69) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cancello et al. [26] | 2010 | 1997–2004 | Italy | 251 | <35 | 35–50 | OS | 2.2 (1.1–4.41) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| DFS | 2.04 (1.11–3.72) | |||||||||||||||
| LRRFS | 1.7 (0.53–5.44) | |||||||||||||||
| DMFS | 1.44 (0.64–3.27) | |||||||||||||||
| Cheng et al. [27] | 2015 | 1990–2008 | Taiwan | 171 | ≤40 | 41–50 | RFS | 7.1 (0.90–59) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Dai et al. [28] | 2019 | 2010–NR | China | 378 | <40 | ≥40 | OS | 1.15 (0.93–1.43) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| BCSS | 1.41 (1.12–1.79) | |||||||||||||||
| de Nonneville et al. [29] | 2017 | 1987–2013 | France | 284 | ≤40 | >40 | DFS | 0.71 (0.16–3.10) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| de Paula et al. [30] | 2020 | 2010–2013 | Brazil | NR | ≤40 | >40 | pCR | 1.16 (1.02–1.31) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Fredholm et al. [31] | 2016 | 1992–2005 | Sweden | 152 | <40 | ≥40 | BCSS | 1.23 (0.68–2.23) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Huober et al. [32] | 2010 | 2002–2005 | Europe | 378 | <40 | ≥40 | pCR | 2.03 (1.18–3.5) | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Johansson et al. [33] | 2019 | 2005–2015 | Norway | 2030 | <40 | 50–59 | BCSS | 1.38 (0.92–2.07) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Kashi et al. [34] | 2017 | 2002–2014 | Iran | 180 | ≤40 | >40 | DFS | 7.14 (4.00–33.33) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| OS | 5.88 (1.06–33.33) | |||||||||||||||
| Kim et al. [35] | 2011 | 2000–2005 | Korea | 513 5806 | <35 | ≥35 | RFS | 1.08 (0.6–1.95) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| 1993–2006 | <35 | ≥35 | BCSS | 1.21 (0.88–1.67) | ||||||||||||
| Kim et al. [36] | 2022 | 2010–2015 | United States | 29,893 | <40 | 40–60 | BCSS | 0.97 (0.88–1.06) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 61–75 | BCSS | 0.55 (0.49–0.62) | ||||||||||||||
| >75 | BCSS | 0.58 (0.47–0.71) | ||||||||||||||
| Kwon et al. [37] | 2017 | 2003–2012 | Korea | 233 | ≤35 | >35 | DFS | 2.78 (1.33–5.88) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| LRFS | 3.57 (1.47–8.33) | |||||||||||||||
| DMFS | 2.63 (1.20–5.56) | |||||||||||||||
| Lee et al. [38] | 2010 | 1993–2008 | Korea | 5586 | <35 | 35–50 | OS | 1.03 (0.54–1.96) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| >50 | OS | 1.26 (0.65–2.45) | ||||||||||||||
| Lian et al. [39] | 2017 | 2004–2007 | China | 82 | ≤40 | 41–50 | DFS | 1.44 (0.86–2.40) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| DRFS | 1.33 (0.78–2.24) | |||||||||||||||
| BCSS | 1.13 (0.59–2.17) | |||||||||||||||
| Liedtke et al. [40] | 2013 | 1982–2008 | United States | 1732 | ≤40 | >40 | OS | 1.43 (1.16–1.72) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| DDFS | 1.67 (1.37–2.00) | |||||||||||||||
| DFS | 1.47 (1.23–1.72) | |||||||||||||||
| Liedtke et al. [41] | 2015 | Varying Periods | International | 783 | <40 | ≥40 | EFS | 1.52 (1.09–2.12) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Liu et al. [42] | 2019 | 2000–2016 | United States | 94 | ≤40 | 41–60 | DFS | 1.18 (0.79–1.78) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| DMFS | 1.11 (0.72–1.72) | |||||||||||||||
| Loibl et al. [43] | 2015 | 1998–2010 | Germany | 1645 | <40 | ≥50 | pCR | 1.64 (1.22–2.19) | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 40–49 | DDFS | 0.87 (0.66–1.15) | ||||||||||||||
| ≥50 | DDFS | 1.03 (0.79–1.35) | ||||||||||||||
| 40–49 | LRFS | 1.03 (0.70–1.52) | ||||||||||||||
| ≥50 | LRFS | 1.03 (0.71–1.49) | ||||||||||||||
| 40–49 | DFS | 0.93 (0.72–1.20) | ||||||||||||||
| ≥50 | DFS | 0.99 (0.78–1.28) | ||||||||||||||
| 40–49 | OS | 0.97 (0.72–1.32) | ||||||||||||||
| ≥50 | OS | 1.14 (0.85–1.52) | ||||||||||||||
| Patridge et al. [19] | 2016 | 2000–2007 | United States | 2886 | ≤40 | 51–60 | BCSS | 1.3 (0.90–1.70) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Radosa et al. [44] | 2017 | 1998–2011 | United States | 1930 | <40 | ≥40 | LRFS | 0.91 (0.41–1.75) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| DRFS | 1.38 (0.99–1.95) | |||||||||||||||
| Ryu et al. [45] | 2017 | 2003–2010 | Korea | 5875 | 20–29 | 40–49 | OS | 1.14 (0.76–1.71) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 30–39 | 40–49 | OS | 1.20 (1.01–1.42) | |||||||||||||
| Saifi et al. [46] | 2022 | 2010–2015 | United States | 12,761 | <40 | 40–60 | BCSS | 1.15 (0.84–1.56) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| >60 | BCSS | 0.77 (0.59–1.09) | ||||||||||||||
| 40–60 | OS | 0.91 (0.71–1.27) | ||||||||||||||
| >60 | OS | 0.48 (0.35–0.62) | ||||||||||||||
| Tang et al. [47] | 2015 | 2003–2012 | China | 672 | <40 | 40–50 | OS | 0.9 (0.22–3.77) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| DFS | 1.38 (0.76–2.52) | |||||||||||||||
| Tzikas et al. [48] | 2020 | 2007–2015 | Sweden | 524 | <40 | >74 | RFS | 1.45 (0.36–5.88) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| DDFS | 1.72 (0.43–6.67) | |||||||||||||||
| BCSS | 1.35 (0.31–5.88) | |||||||||||||||
| Verdial et al. [49] | 2022 | 2013–2018 | United States | 394 | ≤40 | 41–60 | pCR | 2.01 (1.21–3.34) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| ≥61 | pCR | 2.63(1.4–5.09) | ||||||||||||||
| Villarreal-Garza et al. [50] | 2016 | 2007–2013 | Mexico | 287 | ≤40 | >40 | pCR | 1.9 (1.1–3.4) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| OS | 0.91 (0.45–2.00) | |||||||||||||||
| DFS | 1.11 (0.59–2.00) | |||||||||||||||
| von Waldenfels et al. [51] | 2018 | 1998–2010 | Germany | 1638 | <40 | >65 | DDFS | 1.01 (0.68–1.51) | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| LRRFS | 1.76 (0.94–3.29) | |||||||||||||||
| DFS | 1.22 (0.83–1.78) | |||||||||||||||
| OS | 1.05 (0.68–1.61) | |||||||||||||||
| pCR | 2.21 (1.27–3.84) | |||||||||||||||
| Wei et al. [52] | 2013 | 2002–2004 | China | 309 | <35 | ≥35 | DFS | 1.80 (1.08–3.02) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| OS | 1.87 (1.05–3.31) | |||||||||||||||
| Yang et al. [53] | 2022 | 2008–2018 | China | 1158 | <35 | 35–50 | LRFS | 1.49 (0.75–2.49) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| >50 | LRFS | 1.05 (0.54–2.08) | ||||||||||||||
| 35–50 | DFS | 1.20 (0.77–1.89) | ||||||||||||||
| >50 | DFS | 0.74 (0.47–1.14) | ||||||||||||||
| 35–50 | OS | 0.85 (0.46–1.59) | ||||||||||||||
| >50 | OS | 0.51 (0.28–0.93) | ||||||||||||||
| Yao et al. [54] | 2018 | 2010–2014 | China | 22,802 | <40 | 40–60 | BCSS | 1.02 (0.89–1.15) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| >60 | BCSS | 0.81 (0.71–0.93) | ||||||||||||||
| 40–60 | OS | 0.96 (0.85–1.09) | ||||||||||||||
| >60 | OS | 0.67 (0.59–0.75) | ||||||||||||||
| Yoon et al. [55] | 2017 | 1989–2008 | Korea | 1792 | <35 | ≥35 | DFS | 1.06 (0.72–1.56) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| LRRFS | 1.42 (0.76–2.65) | |||||||||||||||
| LRFS | 2.25 (0.94–5.38) | |||||||||||||||
| DMFS | 1.05 (0.65–1.68) | |||||||||||||||
| BCSS | 1.00 (0.65–1.53) | |||||||||||||||
| Yoon et al. [56] | 2019 | 1989–2008 | Korea | 845 | <35 | ≥35 | Contralateral breast recurrence | 2.00 (1.20–3.33) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Zenzola et al. [57] | 2018 | 1999–2014 | Spain | 201 | ≤40 | >40 | DFS | 2.80 (0.51–15.31) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Zhang et al. [58] | 2012 | 2003–2004 | China | 356 | <35 | ≥35 | DFS | 2.11 (1.08–4.13) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basmadjian, R.B.; Chow, K.; Kim, D.; Kenney, M.; Lukmanji, A.; O’Sullivan, D.E.; Xu, Y.; Quan, M.L.; Cheung, W.Y.; Lupichuk, S.; et al. The Association between Early-Onset Diagnosis and Clinical Outcomes in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1923. https://doi.org/10.3390/cancers15071923
Basmadjian RB, Chow K, Kim D, Kenney M, Lukmanji A, O’Sullivan DE, Xu Y, Quan ML, Cheung WY, Lupichuk S, et al. The Association between Early-Onset Diagnosis and Clinical Outcomes in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(7):1923. https://doi.org/10.3390/cancers15071923
Chicago/Turabian StyleBasmadjian, Robert B., Kristian Chow, Dayoung Kim, Matthew Kenney, Aysha Lukmanji, Dylan E. O’Sullivan, Yuan Xu, May Lynn Quan, Winson Y. Cheung, Sasha Lupichuk, and et al. 2023. "The Association between Early-Onset Diagnosis and Clinical Outcomes in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis" Cancers 15, no. 7: 1923. https://doi.org/10.3390/cancers15071923
APA StyleBasmadjian, R. B., Chow, K., Kim, D., Kenney, M., Lukmanji, A., O’Sullivan, D. E., Xu, Y., Quan, M. L., Cheung, W. Y., Lupichuk, S., & Brenner, D. R. (2023). The Association between Early-Onset Diagnosis and Clinical Outcomes in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Cancers, 15(7), 1923. https://doi.org/10.3390/cancers15071923






