Early Diagnosis of Oral Cancer and Lesions in Fanconi Anemia Patients: A Prospective and Longitudinal Study Using Saliva and Plasma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
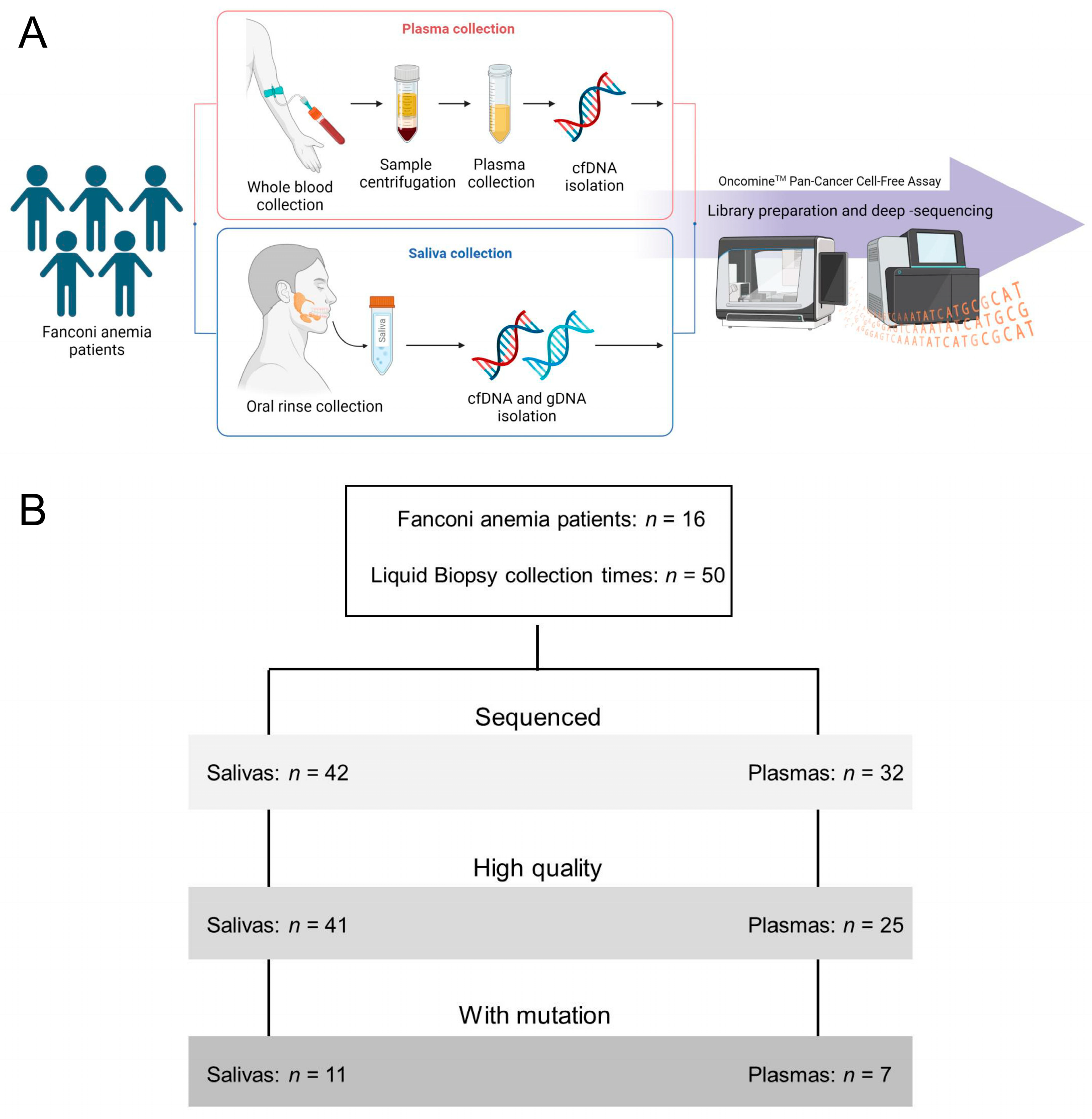
2.1. Study Design and Patient Cohort
2.2. Saliva and Plasma Collection and DNA Purification
2.3. Library Preparation and Deep-Sequencing
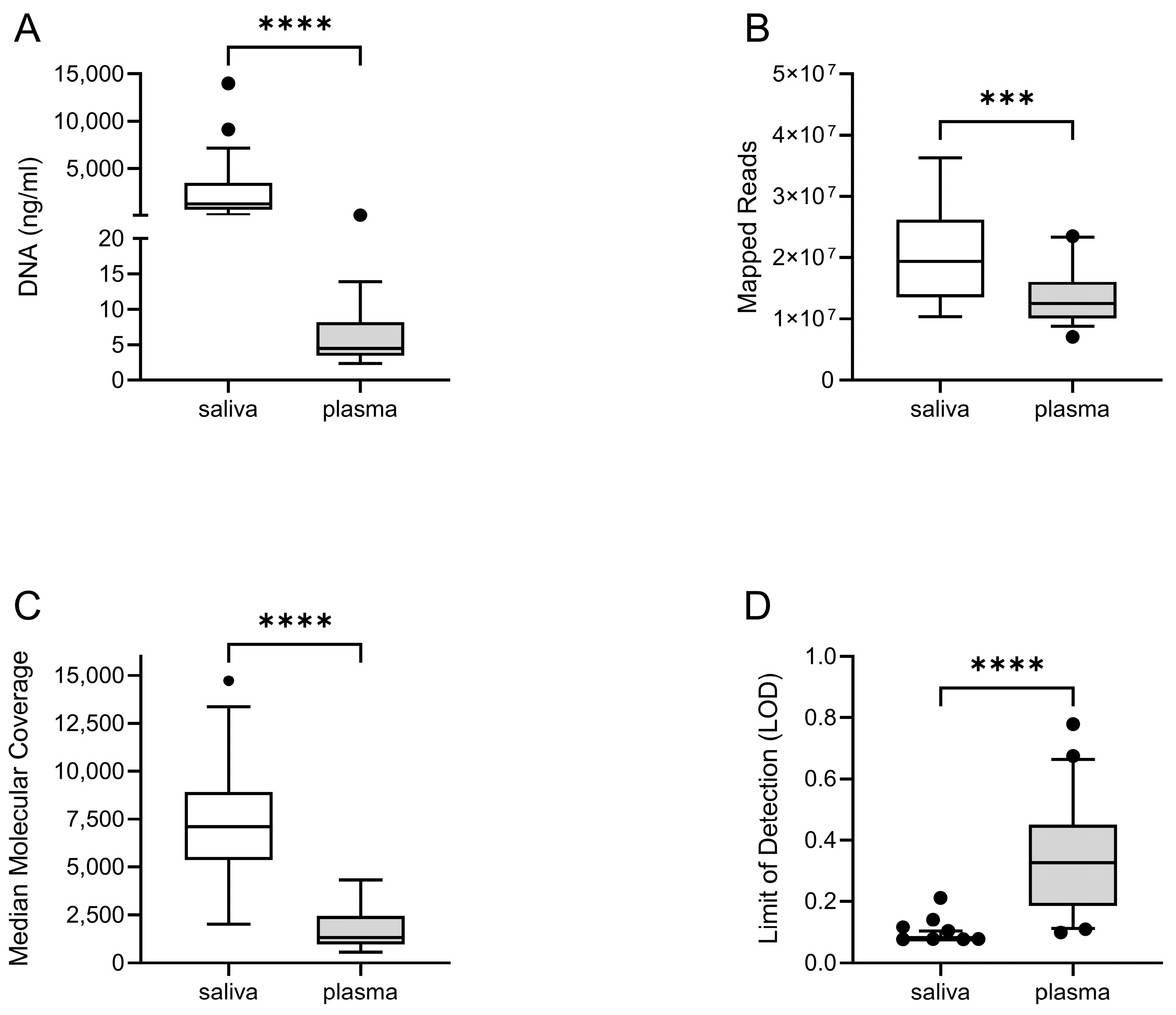
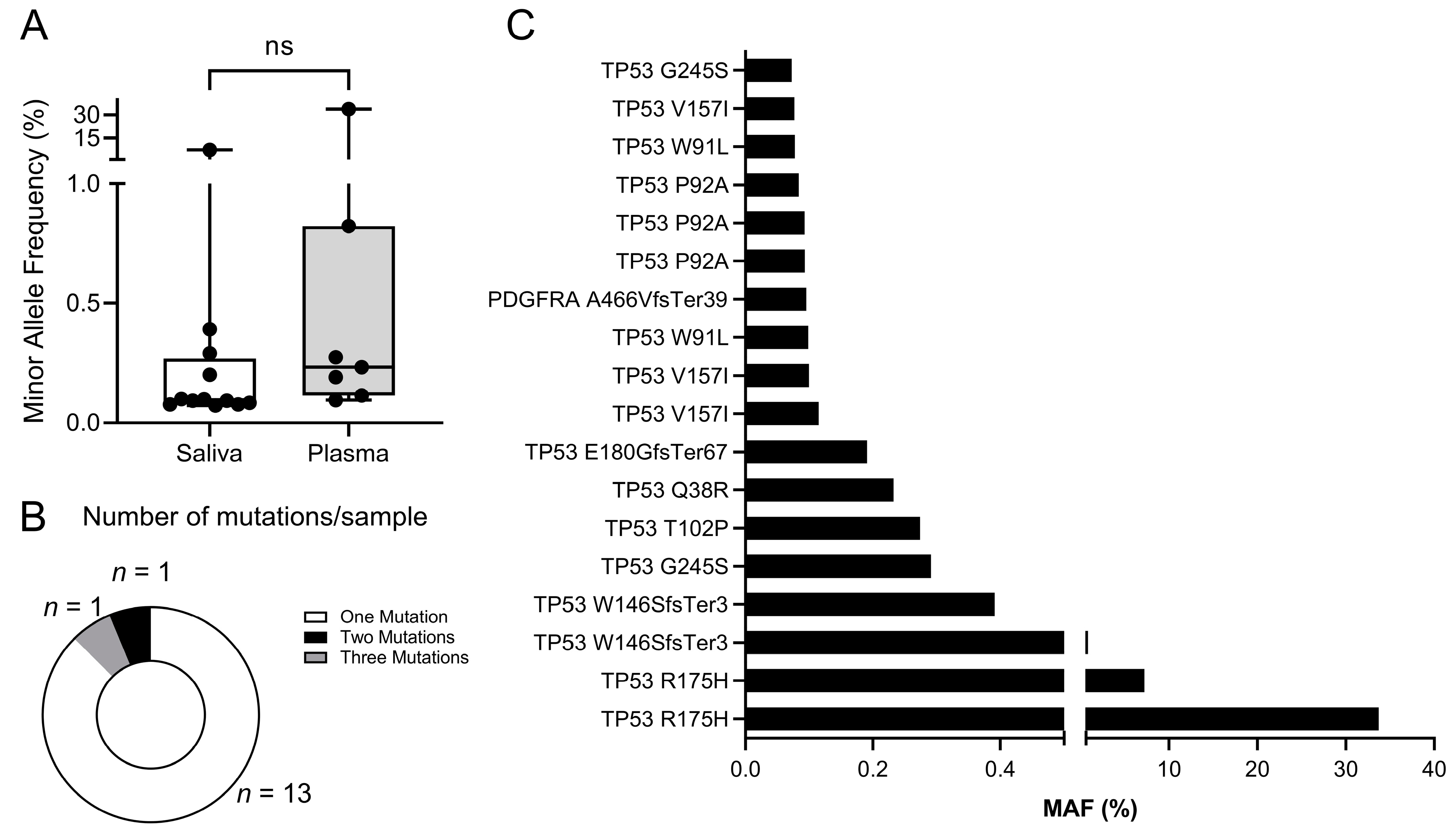
2.4. Sequencing Analysis
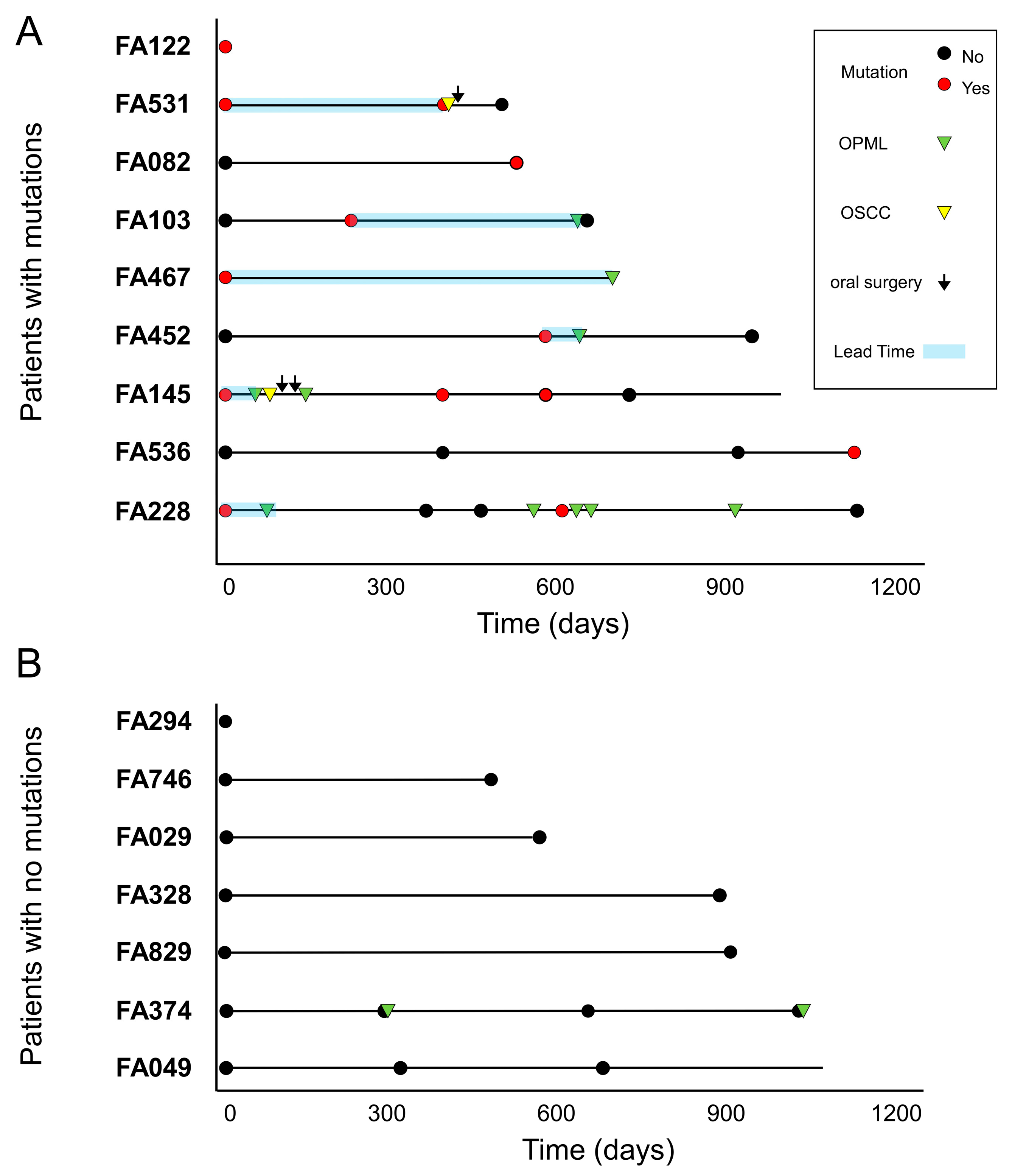
2.5. Survival Curves
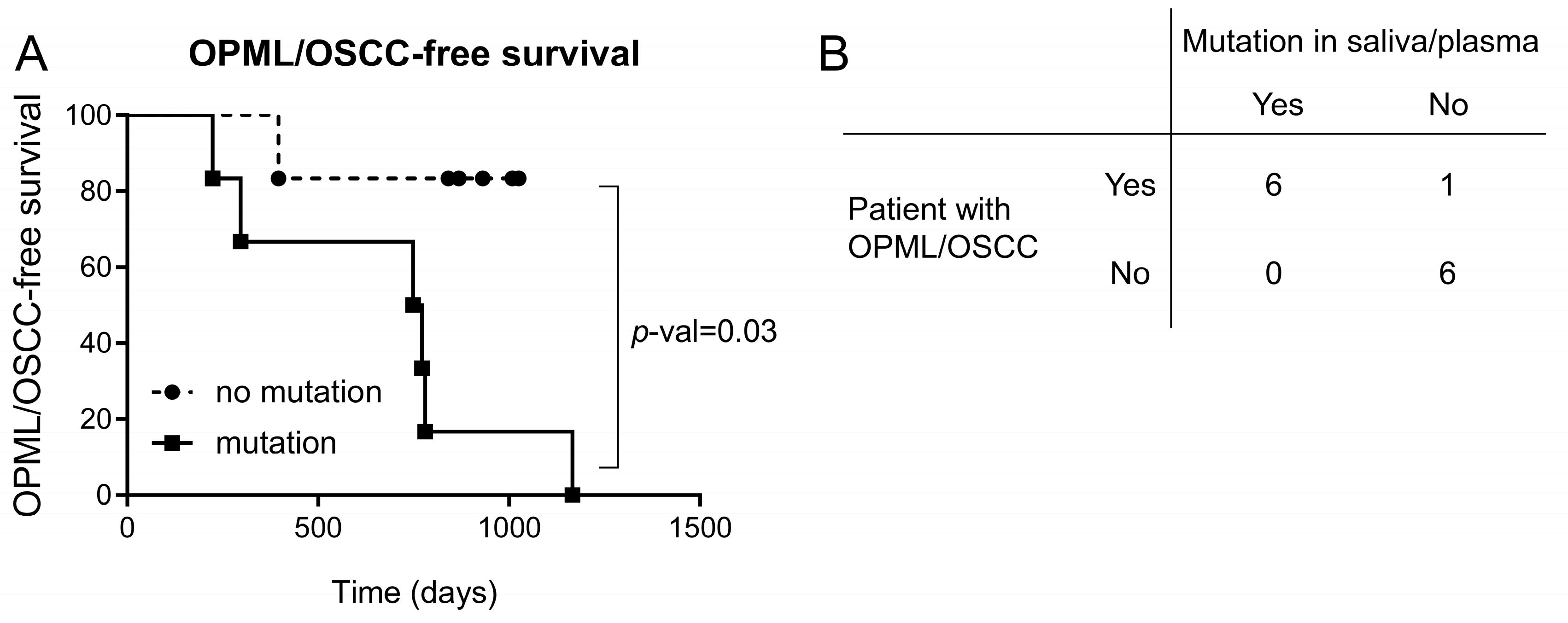
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.C.; Sarode, G.S.; Tupkari, J.V. Oral potentially malignant disorders: Precising the definition. Oral Oncol. 2012, 48, 759–760. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Udaltsova, N.; Engels, E.A.; Katzel, J.A.; Yanik, E.L.; Katki, H.A.; Lingen, M.W.; Silverberg, M.J. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2020, 112, 1047–1054. [Google Scholar] [CrossRef]
- Zhang, L.; Poh, C.F.; Williams, M.; Laronde, D.M.; Berean, K.; Gardner, P.J.; Jiang, H.; Wu, L.; Lee, J.J.; Rosin, M.P. Loss of heterozygosity (LOH) profiles--validated risk predictors for progression to oral cancer. Cancer Prev. Res. 2012, 5, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cascarini, L.; McCaul, J.A.; Kerawala, C.J.; Coombes, D.; Godden, D.; Brennan, P.A. How should we manage oral leukoplakia? Br. J. Oral Maxillofac. Surg. 2013, 51, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Brouns, E.; Baart, J.; Karagozoglu, K.; Aartman, I.; Bloemena, E.; van der Waal, I. Malignant transformation of oral leukoplakia in a well-defined cohort of 144 patients. Oral Dis. 2014, 20, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Tabor, M.P.; Brakenhoff, R.H.; van Houten, V.M.; Kummer, J.A.; Snel, M.H.; Snijders, P.J.; Snow, G.B.; Leemans, C.R.; Braakhuis, B.J. Persistence of genetically altered fields in head and neck cancer patients: Biological and clinical implications. Clin. Cancer Res. 2001, 7, 1523–1532. [Google Scholar]
- Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.M.; Sardella, A.; Kerr, A.R.; Carrassi, A.; MacDonald, L.C.; Worthington, H.V.; Cochrane Oral Health Group. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev. 2016, 7, CD001829. [Google Scholar] [CrossRef]
- Mehta, P.A.; Ebens, C. Fanconi Anemia. In GeneReviews(R); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Niraj, J.; Farkkila, A.; D’Andrea, A.D. The Fanconi Anemia Pathway in Cancer. Annu. Rev. Cancer Biol. 2019, 3, 457–478. [Google Scholar] [CrossRef]
- Rio, P.; Navarro, S.; Wang, W.; Sanchez-Dominguez, R.; Pujol, R.M.; Segovia, J.C.; Bogliolo, M.; Merino, E.; Wu, N.; Salgado, R.; et al. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 2019, 25, 1396–1401. [Google Scholar] [CrossRef]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica 2018, 103, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Kang, H.; Yom, S.S.; Smogorzewska, A.; Johnson, D.E.; Grandis, J.R. Treatment of Fanconi Anemia-Associated Head and Neck Cancer: Opportunities to Improve Outcomes. Clin. Cancer Res. 2021, 27, 5168–5187. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Grein Cavalcanti, L.; Lyko, K.F.; Araujo, R.L.; Amenabar, J.M.; Bonfim, C.; Torres-Pereira, C.C. Oral leukoplakia in patients with Fanconi anaemia without hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2015, 62, 1024–1026. [Google Scholar] [CrossRef]
- Archibald, H.; Kalland, K.; Kuehne, A.; Ondrey, F.; Roby, B.; Jakubowski, L. Oral Premalignant and Malignant Lesions in Fanconi Anemia Patients. Laryngoscope 2022. [Google Scholar] [CrossRef]
- Kisiel, J.B.; Papadopoulos, N.; Liu, M.C.; Crosby, D.; Srivastava, S.; Hawk, E.T. Multicancer early detection test: Preclinical, translational, and clinical evidence-generation plan and provocative questions. Cancer 2022, 128, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.; Park, B.H. Circulating Tumor DNA: Measurement and Clinical Utility. Annu. Rev. Med. 2018, 69, 223–234. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, J.H.; Slieker, F.J.B.; de Bree, R.; van Es, R.J.J.; Van Cann, E.M.; Willems, S.M. Cell-free nucleic acids in body fluids as biomarkers for the prediction and early detection of recurrent head and neck cancer: A systematic review of the literature. Oral Oncol. 2017, 75, 8–15. [Google Scholar] [CrossRef]
- Shanmugam, A.; Hariharan, A.K.; Hasina, R.; Nair, J.R.; Katragadda, S.; Irusappan, S.; Ravichandran, A.; Veeramachaneni, V.; Bettadapura, R.; Bhati, M.; et al. Ultrasensitive detection of tumor-specific mutations in saliva of patients with oral cavity squamous cell carcinoma. Cancer 2021, 127, 1576–1589. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.R.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef]
- van Zeeburg, H.J.; Snijders, P.J.; Wu, T.; Gluckman, E.; Soulier, J.; Surralles, J.; Castella, M.; van der Wal, J.E.; Wennerberg, J.; Califano, J.; et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J. Natl. Cancer Inst. 2008, 100, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Errazquin, R.; Page, A.; Sunol, A.; Segrelles, C.; Carrasco, E.; Peral, J.; Garrido-Aranda, A.; Del Marro, S.; Ortiz, J.; Lorz, C.; et al. Development of a mouse model for spontaneous oral squamous cell carcinoma in Fanconi anemia. Oral Oncol. 2022, 134, 106184. [Google Scholar] [CrossRef] [PubMed]
- Szilard, L. On the Nature of the Aging Process. Proc. Natl. Acad. Sci. USA 1959, 45, 30–45. [Google Scholar] [CrossRef]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.J.; Abascal, F.; Hall, M.W.J.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 362, 911–917. [Google Scholar] [CrossRef]
- Rieckher, M.; Garinis, G.A.; Schumacher, B. Molecular pathology of rare progeroid diseases. Trends Mol. Med. 2021, 27, 907–922. [Google Scholar] [CrossRef]
- Robinson, P.S.; Coorens, T.H.H.; Palles, C.; Mitchell, E.; Abascal, F.; Olafsson, S.; Lee, B.C.H.; Lawson, A.R.J.; Lee-Six, H.; Moore, L.; et al. Increased somatic mutation burdens in normal human cells due to defective DNA polymerases. Nat. Genet. 2021, 53, 1434–1442. [Google Scholar] [CrossRef]
- Webster, A.L.H.; Sanders, M.A.; Patel, K.; Dietrich, R.; Noonan, R.J.; Lach, F.P.; White, R.R.; Goldfarb, A.; Hadi, K.; Edwards, M.M.; et al. Fanconi Anemia Pathway Deficiency Drives Copy Number Variation in Squamous Cell Carcinomas. bioRxiv 2021. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- de Boer, D.V.; Brink, A.; Buijze, M.; Stigter-van Walsum, M.; Hunter, K.D.; Ylstra, B.; Bloemena, E.; Leemans, C.R.; Brakenhoff, R.H. Establishment and Genetic Landscape of Precancer Cell Model Systems from the Head and Neck Mucosal Lining. Mol. Cancer Res. 2019, 17, 120–130. [Google Scholar] [CrossRef]
- Poell, J.B.; Wils, L.J.; Brink, A.; Dietrich, R.; Krieg, C.; Velleuer, E.; Evren, I.; Brouns, E.R.; de Visscher, J.G.; Bloemena, E.; et al. Oral cancer prediction by noninvasive genetic screening. Int. J. Cancer 2023, 152, 227–238. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Fanconi Anemia Patients (n = 16) n (%) |
|---|---|
| Age (years) | |
| Median | 27 |
| Range | 14–46 |
| Sex (n) | |
| Female | 9 (56) |
| Male | 7 (44) |
| Patients with HSCT | 11 (69) |
| Median years from HSCT to study launch date | 8.4 |
| GVHD | 4 (24) |
| Follow-up time (days) | |
| Median | 719 |
| Range | 454–1118 |
| Patients with lesions/tumors | |
| Oral potentially malignant lesion | 6 (35) |
| Oral Squamous Cell Carcinoma | 2 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errazquin, R.; Carrasco, E.; Del Marro, S.; Suñol, A.; Peral, J.; Ortiz, J.; Rubio, J.C.; Segrelles, C.; Dueñas, M.; Garrido-Aranda, A.; et al. Early Diagnosis of Oral Cancer and Lesions in Fanconi Anemia Patients: A Prospective and Longitudinal Study Using Saliva and Plasma. Cancers 2023, 15, 1871. https://doi.org/10.3390/cancers15061871
Errazquin R, Carrasco E, Del Marro S, Suñol A, Peral J, Ortiz J, Rubio JC, Segrelles C, Dueñas M, Garrido-Aranda A, et al. Early Diagnosis of Oral Cancer and Lesions in Fanconi Anemia Patients: A Prospective and Longitudinal Study Using Saliva and Plasma. Cancers. 2023; 15(6):1871. https://doi.org/10.3390/cancers15061871
Chicago/Turabian StyleErrazquin, Ricardo, Estela Carrasco, Sonia Del Marro, Anna Suñol, Jorge Peral, Jessica Ortiz, Juan Carlos Rubio, Carmen Segrelles, Marta Dueñas, Alicia Garrido-Aranda, and et al. 2023. "Early Diagnosis of Oral Cancer and Lesions in Fanconi Anemia Patients: A Prospective and Longitudinal Study Using Saliva and Plasma" Cancers 15, no. 6: 1871. https://doi.org/10.3390/cancers15061871
APA StyleErrazquin, R., Carrasco, E., Del Marro, S., Suñol, A., Peral, J., Ortiz, J., Rubio, J. C., Segrelles, C., Dueñas, M., Garrido-Aranda, A., Alvarez, M., Belendez, C., Balmaña, J., & Garcia-Escudero, R. (2023). Early Diagnosis of Oral Cancer and Lesions in Fanconi Anemia Patients: A Prospective and Longitudinal Study Using Saliva and Plasma. Cancers, 15(6), 1871. https://doi.org/10.3390/cancers15061871






