Investigation of 11p15.5 Methylation Defects Associated with Beckwith-Wiedemann Spectrum and Embryonic Tumor Risk in Lateralized Overgrowth Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic Test Algorithm
2.3. MS-MLPA
2.4. Statistical Methods
3. Results
3.1. Clinical Characteristic
3.2. Molecular Results
3.3. The Incidence of Embryonal Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalish, J.M.; Biesecker, L.G.; Brioude, F.; Deardorff, M.A.; Di Cesare-Merlone, A.; Druley, T.; Ferrero, G.B.; Lapunzina, P.; Larizza, L.; Maas, S.; et al. Nomenclature and definition in asymmetric regional body overgrowth. Am. J. Med. Genet. A 2017, 173, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Griff, J.R.; Duffy, K.A.; Kalish, J.M. Characterization and childhood tumor risk assessment of genetic and epigenetic syndromes associated with lateralized overgrowth. Front. Pediatr. 2020, 8, 613260. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Russo, S.; Larizza, L.; Riccio, A.; Ferrero, G.B. (Epi) genotype-phenotype correlations in Beckwith-Wiedemann syndrome: A paradigm for genomic medicine. Clin. Genet. 2016, 89, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrerro, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Cielo, C.M.; Cohen, J.L.; Gonzalez-Gandolfi, C.X.; Griff, J.R.; Hathaway, E.R.; Kupa, J.; Taylor, J.A.; Wang, K.H.; Ganguly, A.; et al. Characterization of the Beckwith-Wiedemann spectrum: Diagnosis and management. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 693–708. [Google Scholar] [CrossRef]
- Ibrahim, A.; Kirby, G.; Hardy, C.; Dias, R.P.; Tee, L.; Lim, D.; Berg, J.; MacDonald, F.; Nightingale, P.; Maher, E.R. Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin. Epigenet. 2014, 6, 11. [Google Scholar] [CrossRef]
- Shuman, C.; Beckwith, J.B.; Weksberg, R. Beckwith-Wiedemann Syndrome. In GeneReviews (®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Eds.; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- Mussa, A.; Carli, D.; Cardaropoli, S.; Ferrero, G.B.; Resta, N. Lateralized and Segmental Overgrowth in Children. Cancers 2021, 13, 6166. [Google Scholar] [CrossRef]
- Mussa, A.; Russo, S.; De Crescenzo, A.; Freschi, A.; Calzari, L.; Maitz, S.; Macchiaiolo, M.; Molinatto, C.; Baldassarre, G.; Mariani, M.; et al. (Epi)genotype-phenotype correlations in Beckwith- Wiedemann syndrome. Eur. J. Hum. Genet. 2016, 24, 183–190. [Google Scholar] [CrossRef]
- Brioude, F.; Lacoste, A.; Netchine, I.; Vazquez, M.P.; Auber, F.; Audry, G.; Gauthier-Villars, M.; Brugieres, L.; Gicquel, C.; Le Bouc, Y.; et al. Beckwith-Wiedemann syndrome: Growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Hor. Res. Paediatr. 2013, 80, 457–465. [Google Scholar] [CrossRef]
- Baker, S.W.; Duffy, K.A.; Richards-Yutz, J.; Deardorff, M.A.; Kalish, J.M.; Ganguly, A. Improved molecular detection of mosaicism in Beckwith Wiedemann Syndrome. J. Med. Genet. 2021, 68, 178–184. [Google Scholar] [CrossRef]
- Duffy, K.A.; Getz, K.D.; Hathaway, E.R.; Byrne, M.E.; MacFarland, S.P.; Kalish, J.M. Characteristics associated with tumor development in individuals diagnosed with Beckwith– Wiedemann spectrum: Novel tumor-(epi)genotype-phenotype associations in the BWSp Population. Genes 2021, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.M.; Vansenne, F.; Kadouch, D.J.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.; Maher, E.R.; Hennekam, R.C. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am. J. Med. Genet. A 2016, 170, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Molinatto, C.; Baldassarre, G.; Riberi, E.; Russo, S.; Larizza, L.; Riccio, A.; Ferrero, G.B. Cancer Risk in Beckwith-Wiedemann Syndrome: A systematic review and meta-analysis outlining a novel (epi) genotype specific histotype targeted screening protocol. J. Pediatr. 2016, 176, 142–149. [Google Scholar] [CrossRef]
- MacFarland, S.P.; Duffy, K.A.; Bhatti, T.R.; Bagatell, R.; Balamuth, N.J.; Brodeur, G.M.; Ganguly, A.; Mattei, P.A.; Surrey, L.F.; Balis, F.M.; et al. Diagnosis of Beckwith-Wiedemann syndrome in children presenting with Wilms tumor. Pediatr. Blood Cancer 2018, 65, e27296. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Calzari, L.; Mussa, A.; Mainini, E.; Cassina, M.; Di Candia, S.; Clementi, M.; Guzzetti, S.; Tabano, S.; Miozzo, M.; et al. A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver-Russell and Beckwith-Wiedemann syndromes. Clin. Epigenet. 2016, 8, 23. [Google Scholar] [CrossRef]
- Choufani, S.; Shuman, C.; Weksberg, R. Beckwith–Wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2010, 154C, 343–354. [Google Scholar] [CrossRef]
- Radley, J.A.; Connolly, M.; Sabir, A.; Kanani, F.; Carley, H.; Jones, R.L.; Hyder, Z.; Gompertz, L.; Reardon, W.; Richardson, R.; et al. Isolated- and Beckwith-Wiedemann syndrome related- lateralised overgrowth (hemihypertrophy): Clinical and molecular correlations in 94 individuals. Clin. Genet. 2021, 100, 292–297. [Google Scholar] [CrossRef]
- Bliek, J.; Maas, S.; Alders, M.; Merks, J.H.M.; Mannens, M. Epigenotype, phenotype, and tumors in patients with isolated hemihyperplasia. J. Pediatr. 2008, 153, 95–100. [Google Scholar] [CrossRef]
- Tüysüz, B.; Günes, N.; Geyik, F.; Yesil, G.; Celkan, T.; Vural, M. Investigation of (epi)genotype causes and follow-up manifestations in the patients with classical and atypical phenotype of Beckwith-Wiedemann spectrum. Am. J. Med. Genet. A 2021, 185, 1721–1731. [Google Scholar] [CrossRef]
- Kalish, J.M.; Boodhansingh, K.E.; Bhatti, T.R.; Ganguly, A.; Conlin, L.K.; Becker, S.A.; Givler, S.; Mighion, L.; Palladino, A.A.; Adzick, N.S.; et al. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J. Med. Genet. 2016, 53, 53–61. [Google Scholar] [CrossRef]
- Carli, D.; De Pellegrin, M.; Franceschi, L.; Zinali, F.; Paonessa, M.; Spolaore, S.; Cardaropoli, S.; Cravino, M.; Marcucci, L.; Andreacchio, A.; et al. Evolution over time of leg length discrepancy in patients with syndromic and isolated lateralized overgrowth. J. Pediatr. 2021, 234, 123–127. [Google Scholar] [CrossRef]
- Romaris, M.J.; Caino, S.; Adamo, P.; Fano, V. Isolated lateralized overgrowth: Clinical, radiological, and auxological characteristics of a single-site cohort of 76 cases. Arch. Argent. Pediatr. 2022, 120, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Parada-Avendaño, I.; Salvador, H.; García, R.G.; Martorell-Sampol, L.; Fontecha, C.G.; Torner-Rubies, F.; Perez-Lopez, L.M. Lateralized overgrowth as a guiding sign of abdominal neoplasms for pediatric orthopedic surgeons. Jt. Dis. Relat. Surg. 2023, 34, 3–8. [Google Scholar] [CrossRef]
- Mussa, A.; Di Candia, S.; Russo, S.; Catania, S.; De Pellegrin, M.; Di Luzio, L.; Ferrari, M.; Tortora, C.; Meazzini, M.C.; Brusati, R.; et al. Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur. J. Med. Genet. 2016, 59, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Deardorff, M.A. Tumor screening in Beckwith-Wiedemann syndrome-To screen or not to screen? Am. J. Med. Genet. A 2016, 170, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Eggermann, T.; Maher, E.R.; Kratz, C.P.; Prawitt, D. Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice. Cancers 2022, 14, 3083. [Google Scholar] [CrossRef]
- Coktu, S.; Spix, C.; Kaiser, M.; Beygo, J.; Kleinle, S.; Bachmann, N.; Kohlschmidt, N.; Prawitt, D.; Beckmann, A.; Klaes, R.; et al. Cancer incidence and spectrum among children with genetically confirmed Beckwith-Wiedemann spectrum in Germany: A retrospective cohort study. Br. J. Cancer. 2020, 123, 619–623. [Google Scholar] [CrossRef]
- Mussa, A.; Duffy, K.A.; Carli, D.; Griff, J.R.; Fagiano, R.; Kupa, J.; Brodeur, G.M.; Ferrero, G.B.; Kalish, J.M. The effectiveness of Wilms tumor screening in Beckwith Wiedemann Spectrum. J. Cancer. Res. Clin. Oncol. 2019, 145, 3115–3123. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Seaver, L.H.; Jones, K.L.; Procopio, F.; Crooks, W.; Feingold, M. Isolated hemihyperplasia (hemihypertrophy): Report of a prospective multicenter study of the incidence of neoplasia and review. Am. J. Med. Genet. 1998, 79, 274–278. [Google Scholar] [CrossRef]
- Dempsey-Robertson, M.; Wilkes, D.; Stall, A.; Bush, P. Incidence of abdominal tumors in syndromic and idiopathic hemihypertrophy/isolated hemihyperplasia. J. Pediatr. Orthop. 2012, 32, 322–326. [Google Scholar] [CrossRef]
- Shuman, C.; Smith, A.C.; Steele, L.; Ray, P.N.; Clericuzio, C.; Zackai, E.; Parisi, M.A.; Meadows, A.T.; Kelly, T.; Tichauer, D.; et al. Constitutional UPD for chromosome 11p15 in individuals with isolated hemihypertrophy is associated with high tumour risk and occurs following assisted reproductive technologies. Am. J. Med. Genet. A 2006, 140A, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Maas, S.M.; Kadouch, D.J.M.; van der Lip, K.; Bliek, J.; van der Horst, C.M.A.M.; Mannens, M.M.M.M. Methylation analysis in tongue tissue of BWS patients identifies the (EPI) genetic cause in three patients with normal methylation levels in Blood. Eur. J. Med. Genet. 2014, 57, 293–297. [Google Scholar] [CrossRef] [PubMed]

| Localization | Severe (%) | Mild (%) | Total (%) |
|---|---|---|---|
| Right side (upper/lower limb) | 15 (17.6%) | 20 (23.6%) | 35 (40.3%) |
| Left side (upper/lower limb) | 4 (4.7%) | 10 (11.8%) | 14 (16.1%) |
| Contralateral (upper/lower limb) | 2 (2.4%) | 1 (1.1%) | 3 (3.4%) |
| Isolated right lower limb | 12 (13.8%) | 11 (12.5%) | 23 (26.4%) |
| Isolated left lower limb | 3 (3.5%) | 6 (7.05%) | 9 (10.3%) |
| Isolated left upper limb | 0 | 2 (2.3%) | 2 (2.3%) |
| Isolated upper right limb | 0 | 1 (1.2%) | 1 (1.2%) |
| Overall | 36 (41.4%) | 51 (58.6%) | 87 (100%) |
| Clinical Phenotype | Epigenetic Change | Negative Testing | Total | |||
|---|---|---|---|---|---|---|
| ICR1- GOM | ICR2- LOM | 11p UPD | Total | |||
| Atypical and Classical | 2 (6.2%) | 15 (46.9%) | 9 (28.1%) | 26 (81.25%) | 6 (18.7%) | 32 (100%) |
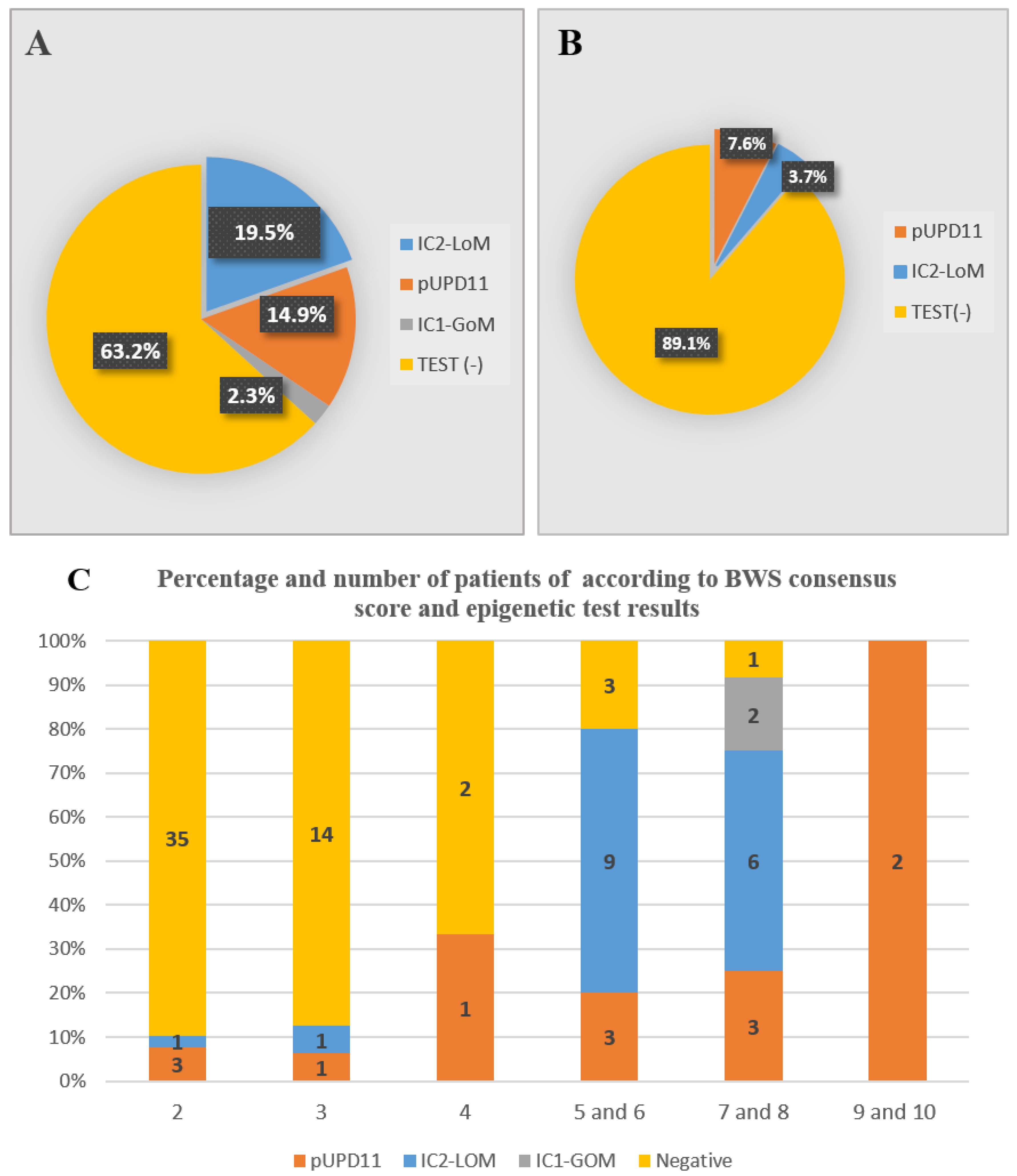
| ILO | - | 2 (3.7%) | 4 (7.6%) | 6 (10.9%) | 49 (89.1%) | 55 (100%) |
| Total | 2 (2.3%) | 17 (19.5%) | 13 (14.9%) | 32 (36.8%) | 55 (63.2%) | 87 (100%) |
| Severity of LO | Epigenetic Change | Negative Testing | Total | p Value * | |||
|---|---|---|---|---|---|---|---|
| IC1 GOM | IC2 LOM | 11p UPD | Total | ||||
| ILO group | p = 0.033 < 0.05 | ||||||
| Severe | 0 | 2 | 3 | 5 | 17 | 22 | |
| Mild | 0 | 0 | 1 | 1 | 32 | 33 | |
| Total | 0 | 2 | 4 | 6 | 49 | 55 | |
| Atypical/classical group Severe | 0 | 5 | 4 | 9 | 4 | 13 | p = 0.194 > 0.05 |
| Mild | 2 | 10 | 5 | 17 | 2 | 19 | |
| Total | 2 | 15 | 9 | 26 | 6 | 32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tüysüz, B.; Bozlak, S.; Uludağ Alkaya, D.; Ocak, S.; Kasap, B.; Sunamak Çifçi, E.; Seker, A.; Bayhan, I.A.; Apak, H. Investigation of 11p15.5 Methylation Defects Associated with Beckwith-Wiedemann Spectrum and Embryonic Tumor Risk in Lateralized Overgrowth Patients. Cancers 2023, 15, 1872. https://doi.org/10.3390/cancers15061872
Tüysüz B, Bozlak S, Uludağ Alkaya D, Ocak S, Kasap B, Sunamak Çifçi E, Seker A, Bayhan IA, Apak H. Investigation of 11p15.5 Methylation Defects Associated with Beckwith-Wiedemann Spectrum and Embryonic Tumor Risk in Lateralized Overgrowth Patients. Cancers. 2023; 15(6):1872. https://doi.org/10.3390/cancers15061872
Chicago/Turabian StyleTüysüz, Beyhan, Serdar Bozlak, Dilek Uludağ Alkaya, Süheyla Ocak, Büşra Kasap, Evrim Sunamak Çifçi, Ali Seker, Ilhan Avni Bayhan, and Hilmi Apak. 2023. "Investigation of 11p15.5 Methylation Defects Associated with Beckwith-Wiedemann Spectrum and Embryonic Tumor Risk in Lateralized Overgrowth Patients" Cancers 15, no. 6: 1872. https://doi.org/10.3390/cancers15061872
APA StyleTüysüz, B., Bozlak, S., Uludağ Alkaya, D., Ocak, S., Kasap, B., Sunamak Çifçi, E., Seker, A., Bayhan, I. A., & Apak, H. (2023). Investigation of 11p15.5 Methylation Defects Associated with Beckwith-Wiedemann Spectrum and Embryonic Tumor Risk in Lateralized Overgrowth Patients. Cancers, 15(6), 1872. https://doi.org/10.3390/cancers15061872





