CDK4/6 Inhibitors—Overcoming Endocrine Resistance Is the Standard in Patients with Hormone Receptor-Positive Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
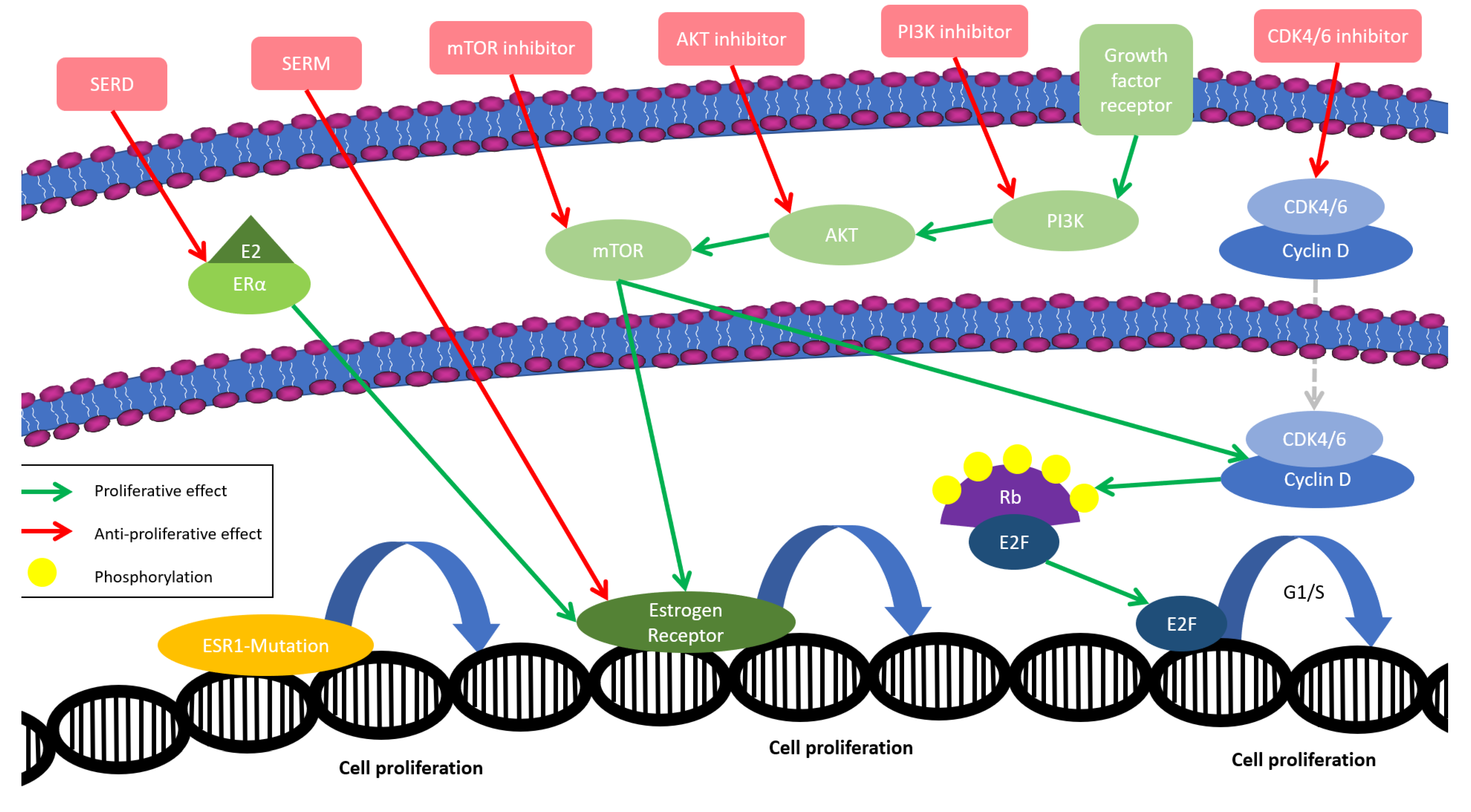
2. The Early Development of CDK4/6 Inhibitors in Patients with Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer
2.1. Impact on Progression-Free Survival
2.2. Improvement in Overall Survival
2.3. CDK4/6i vs. Chemotherapy
2.4. Resistance Mechanisms and Mutations
3. Advancements in the Endocrine Treatment of Hormone Receptor-Positive, HER2-Negative Early-Stage Breast Cancer Patients
3.1. Palbociclib Failing to Improve Invasive-Disease-Free Survival
3.2. Abemaciclib as the First New Drug in Two Decades to Complement Curative ET in Node-Positive Patients
3.3. Ribociclib with the Potential of Covering the Unmet Need in Stage II Disease
3.4. CDK4/6i as Neoadjuvant Therapy
4. Impact on Patients’ Adherence and Quality of Life
5. Modern Therapy Approaches and New Opportunities
5.1. The Role of HER2
5.2. Novel Combination Partners
5.3. Further CDK Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beatson, G. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Lancet 1896, 148, 162–165. [Google Scholar] [CrossRef]
- Cole, M.P.; Jones, C.T.; Todd, I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer 1971, 25, 270–275. [Google Scholar] [CrossRef]
- Nabholtz, J.M.; Buzdar, A.; Pollak, M.; Harwin, W.; Burton, G.; Mangalik, A.; Steinberg, M.; Webster, A.; von Euler, M. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: Results of a North American multicenter randomized trial. Arimidex Study Group. J. Clin. Oncol. 2000, 18, 3758–3767. [Google Scholar] [CrossRef]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Perez-Carrion, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Janicke, F.; et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001, 19, 2596–2606. [Google Scholar] [CrossRef]
- Osborne, C.K.; Pippen, J.; Jones, S.E.; Parker, L.M.; Ellis, M.; Come, S.; Gertler, S.Z.; May, J.T.; Burton, G.; Dimery, I.; et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J. Clin. Oncol. 2002, 20, 3386–3395. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative, G.; Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.; Aihara, T.; Bliss, J.; Boccardo, F.; Coates, A.; Coombes, R.C.; et al. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative, G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar]
- Pagani, O.; Francis, P.A.; Fleming, G.F.; Walley, B.A.; Viale, G.; Colleoni, M.; Lang, I.; Gomez, H.L.; Tondini, C.; Pinotti, G.; et al. Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results from TEXT and SOFT. J. Clin. Oncol. 2020, 38, 1293–1303. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Bachelot, T.; Cottu, P.; Chabaud, S.; Dalenc, F.; Allouache, D.; Delaloge, S.; Jacquin, J.P.; Grenier, J.; Venat Bouvet, L.; Jegannathen, A.; et al. Everolimus Added to Adjuvant Endocrine Therapy in Patients with High-Risk Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. J. Clin. Oncol. 2022, 40, 3699–3708. [Google Scholar] [CrossRef]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Ditsch, N.; Wocke, A.; Untch, M.; Jackisch, C.; Albert, U.S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.U.; Budach, W.; Dall, P.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2022. Breast Care 2022, 17, 403–420. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef]
- Mills, J.N.; Rutkovsky, A.C.; Giordano, A. Mechanisms of resistance in estrogen receptor positive breast cancer: Overcoming resistance to tamoxifen/aromatase inhibitors. Curr. Opin. Pharmacol. 2018, 41, 59–65. [Google Scholar] [CrossRef]
- Watt, A.C.; Goel, S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022, 24, 17. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.Y.; Zhang, P.; Tong, Z.; Sun, T.; Li, W.; Ouyang, Q.; Hu, X.; Cheng, Y.; Yan, M.; et al. LBA16 Dalpiciclib plus letrozole or anastrozole as first-line treatment for HR+/HER2- advanced breast cancer (DAWNA-2): A phase III trial. Ann. Oncol. 2022, 33, S1384–S1385. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, P.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: A randomized, phase 3 trial. Nat. Med. 2021, 27, 1904–1909. [Google Scholar] [CrossRef]
- Nabieva, N.; Fasching, P.A. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers 2021, 13, 5643. [Google Scholar] [CrossRef]
- Sledge, G.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Okera, M.; Masuda, N.; Kaufman, P.A.; Koh, H.; et al. Final Overall Survival Analysis of MONARCH-2: A Phase 3 trial of Abemaciclib Plus Fulvestrant in Patients with Hormone Receptor-positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Manso Sanchez, L.M.; Paluch-Shimon, S.; et al. LBA15 MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann. Oncol. 2022, 33, S1384. [Google Scholar] [CrossRef]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Gregor, M.C.M.; Bananis, E.; et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL + LET) versus placebo plus letrozole (PBO + LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40, LBA1003. [Google Scholar]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martin, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Abstract PD2-04: Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Cancer Res. 2021, 81, PD2-04. [Google Scholar]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and Pharmacologic Differences of CDK4/6 Inhibitors in Breast Cancer. Front. Oncol. 2021, 11, 693104. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Ettl, J.; Luftner, D.; Beckmann, M.W.; Belleville, E.; Fasching, P.A.; Fehm, T.N.; Geberth, M.; Haberle, L.; Hadji, P.; et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer—Data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast 2020, 54, 88–95. [Google Scholar]
- Engler, T.; Fasching, P.A.; Luftner, D.; Hartkopf, A.D.; Muller, V.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Haberle, L.; Ettl, J.; et al. Implementation of CDK4/6 Inhibitors and its Influence on the Treatment Landscape of Advanced Breast Cancer Patients—Data from the Real-World Registry PRAEGNANT. Geburtshilfe Frauenheilkd. 2022, 82, 1055–1067. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Munoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Ciruelos, E.M.; Munoz, M.; Bermejo, B.; Margeli, M.; Csoszi, T.; Anton, A.; et al. Overall survival with palbociclib plus endocrine therapy versus capecitabine in postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer in the PEARL study. Eur. J. Cancer 2022, 168, 12–24. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Bin Mohd Mahidin, E.I.; Azim, H.; Eralp, Y.; Yap, Y.-S.; Im, S.-A.; Rihani, J.; Bowles, J.; Alfaro, T.D.; Wu, J.; et al. Primary Results From the Randomized Phase II RIGHT Choice Trial of Premenopausal Patients with Aggressive HR+/HER2− Advanced Breast Cancer Treated with Ribociclib + Endocrine Therapy vs Physician’s Choice Combination Chemotherapy. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; Raptis, G.; Baer, L.N.; Oh, S.Y.; et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J. Clin. Oncol. 2022, 40, LBA1004. [Google Scholar]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 15 December 2022).
- Bidard, F.C.; Hardy-Bessard, A.C.; Dalenc, F.; Bachelot, T.; Pierga, J.Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.S.; Ferrero, J.M.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.-S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for HR+, HER2−, node-positive, high-risk early breast cancer: Results from a pre-planned monarchE overall survival interim analysis, including 4-year efficacy outcomes. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef]
- Loibl, S.; Marme, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.B.; Bear, H.; McCarthy, N.; Mele Olive, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovszky, G.; Burstein, H.J.; Bellet-Ezquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Royce, M.; Osgood, C.; Mulkey, F.; Bloomquist, E.; Pierce, W.F.; Roy, A.; Kalavar, S.; Ghosh, S.; Philip, R.; Rizvi, F.; et al. FDA Approval Summary: Abemaciclib with Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. 2022, 40, 1155–1162. [Google Scholar] [CrossRef]
- Martin, M.; Hegg, R.; Kim, S.B.; Schenker, M.; Grecea, D.; Garcia-Saenz, J.A.; Papazisis, K.; Ouyang, Q.; Lacko, A.; Oksuzoglu, B.; et al. Treatment with Adjuvant Abemaciclib Plus Endocrine Therapy in Patients with High-risk Early Breast Cancer Who Received Neoadjuvant Chemotherapy: A Prespecified Analysis of the monarchE Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1190–1194. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative, G. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar]
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef]
- Cottu, P.; D’Hondt, V.; Dureau, S.; Lerebours, F.; Desmoulins, I.; Heudel, P.E.; Duhoux, F.P.; Levy, C.; Mouret-Reynier, M.A.; Dalenc, F.; et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 2018, 29, 2334–2340. [Google Scholar] [CrossRef]
- Delaloge, S.; Dureau, S.; D’Hondt, V.; Desmoulins, I.; Heudel, P.E.; Duhoux, F.P.; Levy, C.; Lerebours, F.; Mouret-Reynier, M.A.; Dalenc, F.; et al. Survival outcomes after neoadjuvant letrozole and palbociclib versus third generation chemotherapy for patients with high-risk oestrogen receptor-positive HER2-negative breast cancer. Eur. J. Cancer 2022, 166, 300–308. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Press, M.F.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.S.; Barriga, S.; et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR(+)/HER2(−) Breast Cancer. Clin. Cancer Res. 2020, 26, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Munoz, M.; Pare, L.; Gonzalez Farre, B.; Fernandez, P.L.; Galvan, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nabieva, N.; Kellner, S.; Fehm, T.; Haberle, L.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; et al. Influence of patient and tumor characteristics on early therapy persistence with letrozole in postmenopausal women with early breast cancer: Results of the prospective Evaluate-TM study with 3941 patients. Ann. Oncol. 2018, 29, 186–192. [Google Scholar] [CrossRef]
- Nabieva, N.; Fehm, T.; Haberle, L.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; Warm, M.; et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: Results of the prospective EvAluate-TM study. Eur. J. Cancer 2018, 96, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wallwiener, M.; Nabieva, N.; Feisst, M.; Fehm, T.; de Waal, J.; Rezai, M.; Baier, B.; Baake, G.; Kolberg, H.C.; Guggenberger, M.; et al. Influence of patient and tumor characteristics on therapy persistence with letrozole in postmenopausal women with advanced breast cancer: Results of the prospective observational EvAluate-TM study. BMC Cancer 2019, 19, 611. [Google Scholar] [CrossRef] [PubMed]
- Chirgwin, J.H.; Giobbie-Hurder, A.; Coates, A.S.; Price, K.N.; Ejlertsen, B.; Debled, M.; Gelber, R.D.; Goldhirsch, A.; Smith, I.; Rabaglio, M.; et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1-98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J. Clin. Oncol. 2016, 34, 2452–2459. [Google Scholar] [CrossRef]
- Muller, V.; Nabieva, N.; Haberle, L.; Taran, F.A.; Hartkopf, A.D.; Volz, B.; Overkamp, F.; Brandl, A.L.; Kolberg, H.C.; Hadji, P.; et al. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast 2018, 37, 154–160. [Google Scholar] [CrossRef]
- Kaufman, P.A.; Toi, M.; Neven, P.; Sohn, J.; Grischke, E.M.; Andre, V.; Stoffregen, C.; Shekarriz, S.; Price, G.L.; Carter, G.C.; et al. Health-Related Quality of Life in MONARCH 2: Abemaciclib plus Fulvestrant in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer after Endocrine Therapy. Oncologist 2020, 25, e243–e251. [Google Scholar] [CrossRef]
- Goetz, M.P.; Martin, M.; Tokunaga, E.; Park, I.H.; Huober, J.; Toi, M.; Stoffregen, C.; Shekarriz, S.; Andre, V.; Gainford, M.C.; et al. Health-Related Quality of Life in MONARCH 3: Abemaciclib plus an Aromatase Inhibitor as Initial Therapy in HR+, HER2− Advanced Breast Cancer. Oncologist 2020, 25, e1346–e1354. [Google Scholar] [CrossRef]
- Harbeck, N.; Franke, F.; Villanueva-Vazquez, R.; Lu, Y.S.; Tripathy, D.; Chow, L.; Babu, G.K.; Im, Y.H.; Chandiwana, D.; Gaur, A.; et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: Results from a phase III randomized clinical trial (MONALEESA-7). Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Beck, J.T.; Chan, A.; De Laurentiis, M.; Esteva, F.J.; Jerusalem, G.; Neven, P.; Pivot, X.; Bianchi, G.V.; Martin, M.; et al. Ribociclib plus fulvestrant for advanced breast cancer: Health-related quality-of-life analyses from the MONALEESA-3 study. Breast 2020, 54, 148–154. [Google Scholar] [CrossRef]
- Verma, S.; O’Shaughnessy, J.; Burris, H.A.; Campone, M.; Alba, E.; Chandiwana, D.; Dalal, A.A.; Sutradhar, S.; Monaco, M.; Janni, W. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2. Breast Cancer Res. Treat. 2018, 170, 535–545. [Google Scholar] [CrossRef]
- Rugo, H.S.; Dieras, V.; Gelmon, K.A.; Finn, R.S.; Slamon, D.J.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.R.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef]
- Harbeck, N.; Iyer, S.; Turner, N.; Cristofanilli, M.; Ro, J.; Andre, F.; Loi, S.; Verma, S.; Iwata, H.; Bhattacharyya, H.; et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: Patient-reported outcomes from the PALOMA-3 trial. Ann. Oncol. 2016, 27, 1047–1054. [Google Scholar] [CrossRef]
- Hart, L.L.; Bardia, A.; Beck, J.T.; Chan, A.; Neven, P.; Hamilton, E.P.; Sohn, J.; Sonke, G.S.; Bachelot, T.; Spring, L.; et al. Impact of ribociclib (RIB) dose modifications (mod) on overall survival (OS) in patients (pts) with HR+/HER2- advanced breast cancer (ABC) in MONALEESA(ML)-2. J. Clin. Oncol. 2022, 40, 1017. [Google Scholar] [CrossRef]
- Burris, H.A.; Chan, A.; Bardia, A.; Thaddeus Beck, J.; Sohn, J.; Neven, P.; Tripathy, D.; Im, S.A.; Chia, S.; Esteva, F.J.; et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br. J. Cancer 2021, 125, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Fesl, C.; Hlauschek, D.; Garcia-Estevez, L.; Burstein, H.J.; Zdenkowski, N.; Wette, V.; Miller, K.D.; Balic, M.; Mayer, I.A.; et al. Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib With Adjuvant Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03). J. Clin. Oncol. 2022, 40, 449–458. [Google Scholar]
- Rugo, H.S.; O’Shaughnessy, J.; Boyle, F.; Toi, M.; Broom, R.; Blancas, I.; Gumus, M.; Yamashita, T.; Im, Y.H.; Rastogi, P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 2022, 33, 616–627. [Google Scholar] [CrossRef]
- Maculaitis, M.C.; Liu, X.; Will, O.; Hanson, M.; McRoy, L.; Berk, A.; Crastnopol, M. Oncologist and Patient Preferences for Attributes of CDK4/6 Inhibitor Regimens for the Treatment of Advanced/Metastatic HR Positive/HER2 Negative Breast Cancer: Discrete Choice Experiment and Best-Worst Scaling. Patient Prefer Adherence 2020, 14, 2201–2214. [Google Scholar] [CrossRef]
- Sinclair, W.D.; Cui, X. The Effects of HER2 on CDK4/6 Activity in Breast Cancer. Clin. Breast Cancer 2022, 22, e278–e285. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.; Villagrasa, P.; Pascual, T.; Oliveira, M.; Pernas, S.; Pare, L.; Escriva-de-Romani, S.; Manso, L.; Adamo, B.; Martinez, E.; et al. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res. 2020, 26, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Pernas, S.; Tan-Wasielewski, Z.; Barry, W.T.; Bardia, A.; Rees, R.; Andrews, C.; Tahara, R.K.; Trippa, L.; Mayer, E.L.; et al. Ribociclib Plus Trastuzumab in Advanced HER2-Positive Breast Cancer: Results of a Phase 1b/2 Trial. Clin. Breast Cancer 2019, 19, 399–404. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.F.; Troso-Sandoval, T.A.; Ricci, F.; Im, S.A.; Kim, S.B.; Johnston, S.R.; Chan, A.; et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): A randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Clark, S.L.; Li, T.; Goel, S.; Tayob, N.; Viscosi, E.; Abraham, E.; Juric, D.; Isakoff, S.J.; Mayer, E.; et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. NPJ Breast Cancer 2021, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Batra, K.; Sahoo, S.; Froehlich, T.; Klemow, D.; Unni, N.; Ahn, C.; Rodriguez, M.; Hullings, M.; Frankel, A.E. A Phase I/Ib Trial of PD 0332991 (Palbociclib) and T-DM1 in HER2-Positive Advanced Breast Cancer after Trastuzumab and Taxane Therapy. Clin. Breast Cancer 2021, 21, 417–424. [Google Scholar] [CrossRef]
- Thill, M.; Luftner, D.; Kolberg-Liedtke, C.; Albert, U.S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.U.; Budach, W.; Dall, P.; Fallenberg, E.M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2022. Breast Care 2022, 17, 421–429. [Google Scholar] [CrossRef]
- Janni, W.; Fehm, T.; Müller, V.; Schochter, F.; De Gregorio, A.; Decker, T.; Hartkopf, A.; Just, M.; Sagasser, J.; Schmidt, M.; et al. Omission of chemotherapy in the treatment of HER2-positive and hormone-receptor positive metastatic breast cancer—Interim results from the randomized phase 3 DETECT V trial. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Canino, F.; Piacentini, F.; Omarini, C.; Toss, A.; Barbolini, M.; Vici, P.; Dominici, M.; Moscetti, L. Role of Intrinsic Subtype Analysis with PAM50 in Hormone Receptors Positive HER2 Negative Metastatic Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 7079. [Google Scholar] [CrossRef]
- Prat, A.; Cheang, M.C.; Galvan, P.; Nuciforo, P.; Pare, L.; Adamo, B.; Munoz, M.; Viladot, M.; Press, M.F.; Gagnon, R.; et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor-Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncol. 2016, 2, 1287–1294. [Google Scholar] [CrossRef]
- Jacobson, A. Ribociclib Improves Overall Survival in HR+/HER2− Metastatic Breast Cancer Across Common Genomic and Clinical Subtypes. Oncologist 2022, 27, S11–S12. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sáez, O.; Tolosa, P.; Sánchez De Torre, A.; Pascual, T.; Brasó-Maristany, F.; Rodriguez Hernandez, A.; Parrilla, L.; Roncero, A.M.; Ruano, Y.; Chic, N.; et al. 23P CDK4/6 inhibition and endocrine therapy (ET) in the HER2-enriched subtype (HER2-E) in hormone receptor-positive/HER2-negative (HR+/HER2−) advanced breast cancer (ABC): A retrospective analysis of real-world data. Ann. Oncol. 2021, 32, S30. [Google Scholar] [CrossRef]
- Mayer, E.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Jolly, T.; et al. PACE: Palbociclib After CDK and Endocrine Therapy A Randomized Phase II Study of Fulvestrant +/− Palbociclib after Progression on CDK4/6 inhibitor for HR+/HER2− Metastatic Breast Cancer. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Damodaran, S.; Plourde, P.V.; Moore, H.C.F.; Anderson, I.C.; Portman, D.J. Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J. Clin. Oncol. 2022, 40, 1022. [Google Scholar] [CrossRef]
- Tan, A.R.; Wright, G.S.; Thummala, A.R.; Danso, M.A.; Popovic, L.; Pluard, T.J.; Han, H.S.; Vojnovic, Z.; Vasev, N.; Ma, L.; et al. Trilaciclib Prior to Chemotherapy in Patients with Metastatic Triple-Negative Breast Cancer: Final Efficacy and Subgroup Analysis from a Randomized Phase II Study. Clin. Cancer Res. 2022, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.N.; Chuang, Y.S.; Lin, Y.C.; Su, Y.; Chao, T.C. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol. Rep. 2022, 47, 105. [Google Scholar] [CrossRef]

| Study Name | ET Partner | Sample Size | Rando-mization | Median PFS in Months (PFS = Primary Endpoint) | Median OS in Months (OS = Secondary Endpoint) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| with CDK4/6 Inhibitor | without CDK4/6 Inhibitor | HR | 95% CI | Statistically Significant as per Protocol | with CDK4/6 Inhibitor | without CDK4/6 Inhibitor | HR | 95% CI | Statistically Significant as per Protocol | |||||
| ET +/− Abemaciclib | MONARCH-2 [24,28] | Fulvestrant | 669 | 2:1 | 16.4 | 9.3 | 0.55 | 0.45–0.68 | yes | 45.8 | 37.3 | 0.78 | 0.64–0.96 | yes |
| MONARCH-3 [23,29] | AI | 493 | 2:1 | 28.2 | 14.8 | 0.54 | 0.42–0.70 | yes | 67.1 | 54.5 | 0.75 | 0.58–0.97 | Final analys is not reported yet 1 | |
| ET +/− Dalpiciclib | DAWNA-1 [26] | Fulvestrant | 361 | 2:1 | 13.6 2 | 7.7 2 | 0.45 2 | 0.32–0.64 2 | yes 2 | Final analys is not reported yet | ||||
| DAWNA-2 [25] | AI | 456 | 2:1 | 30.6 | 18.2 | 0.51 | 0.38–0.69 | yes | Final analys is not reported yet | |||||
| ET +/− Palbociclib | PALOMA-2 [18,30] | AI | 666 | 2:1 | 24.8 | 14.5 | 0.58 | 0.46–0.72 | yes | 53.9 | 51.2 | 0.96 | 0.78–1.18 | no |
| PALOMA-3 [19,31] | Fulvestrant | 521 | 2:1 | 9.5 | 4.6 | 0.46 | 0.36–0.59 | yes | 34.9 | 28.0 | 0.81 | 0.64–1.03 | no | |
| ET +/− Ribociclib | MONALEESA-2 [22,32] | AI | 668 | 1:1 | 25.3 | 16.0 | 0.57 | 0.46–0.70 | yes | 63.9 | 51.4 | 0.76 | 0.63–0.93 | yes |
| MONALEESA-3 [21,33] | Fulvestrant | 726 | 2:1 | 20.5 | 12.8 | 0.59 | 0.48–0.73 | yes | 53.7 | 41.5 | 0.73 | 0.59–0.90 | yes | |
| MONALEESA-7 [20,34] | OFS plus tamoxifen or AI | 672 | 1:1 | 23.8 | 13.0 | 0.55 | 0.44–0.69 | yes | 58.7 | 48.0 | 0.76 | 0.61–0.96 | yes | |
| Study Name | ET Partner | Sample Size | Rando-mization | Duration of CDK4/6 Inhibitor Therapy | DFS Rate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latest Analysis | with CDK4/6 Inhibitor | without CDK4/6 Inhibitor | HR | 95% CI | Statistically Significant as per Protocol | ||||||
| ET +/− Abemaciclib | monarchE [46] | AI or Tam +/− OFS | 5637 | 1:1 | 2 years | year 4 | 85.8% | 79.4% | 0.66 | 0.58–0.76 | yes |
| ET +/− Dalpiciclib | SHR6390-III-303 [44] | - 1 | 4350 | 1:1 | - 1 | not reported yet | |||||
| ET +/− Palbociclib | PALLAS [47] | AI or Tam +/− OFS | 5796 | 1:1 | 2 years | year 4 | 84.2% | 84.5% | 0.96 | 0.81–1.14 | no |
| Penelope-B [48] | AI or Tam +/− OFS | 1250 | 1:1 | 1 year | year 3 | 81.2% | 77.7% | 0.93 | 0.74–1.17 | no | |
| ET +/− Ribociclib | NATALEE | AI +/− OFS | 5101 | 1:1 | 3 years | not reported yet | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabieva, N.; Fasching, P.A. CDK4/6 Inhibitors—Overcoming Endocrine Resistance Is the Standard in Patients with Hormone Receptor-Positive Breast Cancer. Cancers 2023, 15, 1763. https://doi.org/10.3390/cancers15061763
Nabieva N, Fasching PA. CDK4/6 Inhibitors—Overcoming Endocrine Resistance Is the Standard in Patients with Hormone Receptor-Positive Breast Cancer. Cancers. 2023; 15(6):1763. https://doi.org/10.3390/cancers15061763
Chicago/Turabian StyleNabieva, Naiba, and Peter A. Fasching. 2023. "CDK4/6 Inhibitors—Overcoming Endocrine Resistance Is the Standard in Patients with Hormone Receptor-Positive Breast Cancer" Cancers 15, no. 6: 1763. https://doi.org/10.3390/cancers15061763
APA StyleNabieva, N., & Fasching, P. A. (2023). CDK4/6 Inhibitors—Overcoming Endocrine Resistance Is the Standard in Patients with Hormone Receptor-Positive Breast Cancer. Cancers, 15(6), 1763. https://doi.org/10.3390/cancers15061763






