Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
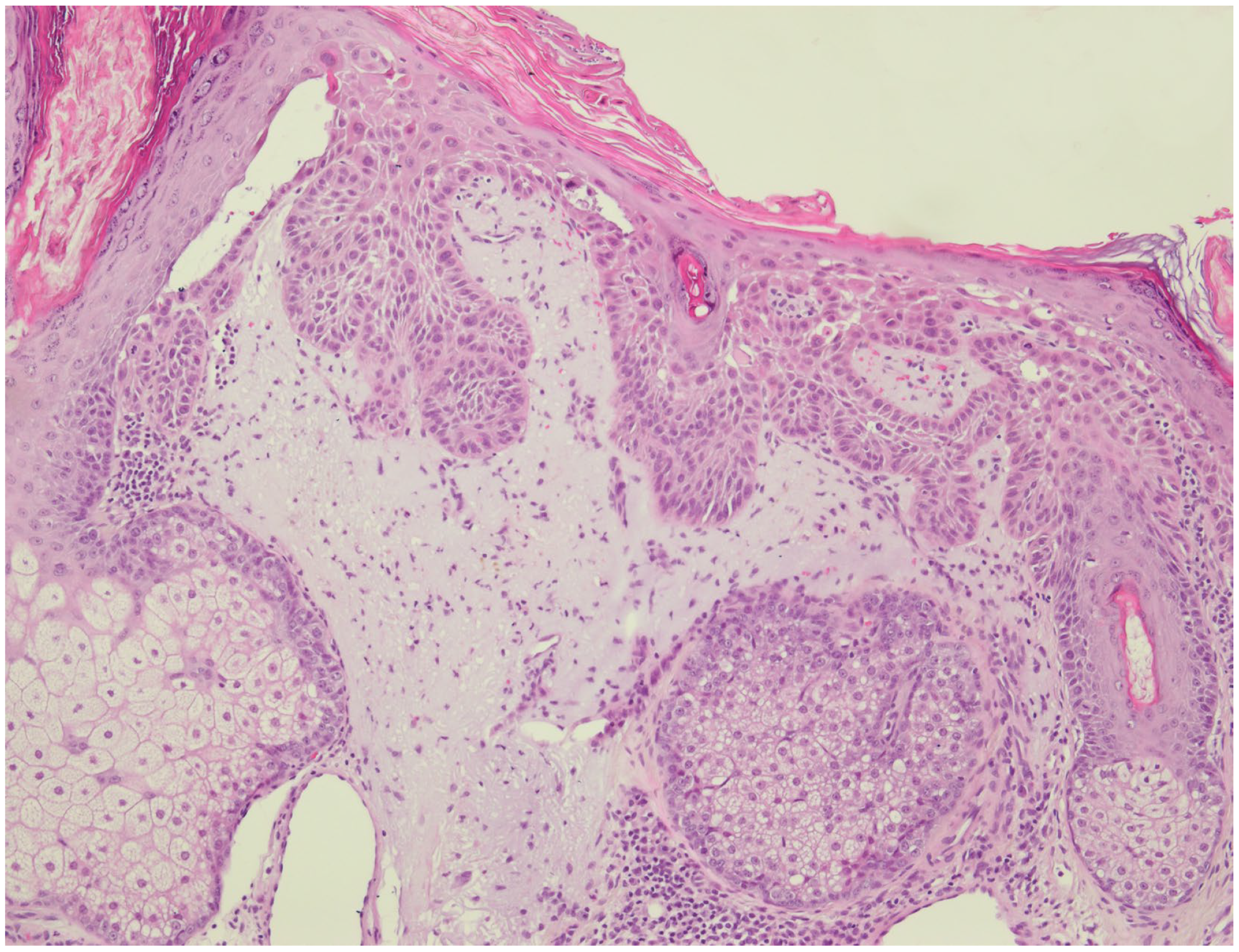
2.2. Microscopic Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ackerman, A.B. Solar keratosis is squamous cell carcinoma. Arch. Dermatol. 2003, 139, 1216–1217. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, M.R., Jr.; Ackerman, A.B. The nature of solar keratosis: A critical review in historical perspective. J. Am. Acad. Dermatol. 2000, 43, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Salasche, S.J. Epidemiology of actinic keratoses and squamous cell carcinoma. J. Am. Acad. Dermatol. 2000, 42, 4–7. [Google Scholar] [CrossRef]
- Memon, A.A.; Tomenson, J.A.; Bothwell, J.; Friedmann, P.S. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br. J. Dermatol. 2000, 142, 1154–1159. [Google Scholar] [CrossRef]
- Stockfleth, E.; Ulrich, C.; Meyer, T.; Christophers, E. Epithelial malignancies in organ transplant patients: Clinical presentation and new methods of treatment. Recent Results Cancer Res. 2002, 160, 251–258. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Jensen, P.; Hansen, S.; Møller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef]
- Hartevelt, M.M.; Bavinck, J.N.; Kootte, A.M.; Vermeer, B.J.; Vandenbroucke, J.P. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation 1990, 49, 506–509. [Google Scholar] [CrossRef]
- Stockfleth, E.; Kerl, H. Guideline Subcommittee of the European Dermatology Forum. Guidelines for the management of actinic keratoses. Eur. J. Dermatol. 2006, 16, 599–606. [Google Scholar]
- Boyle, J.; MacKie, R.M.; Briggs, J.D.; Junor, B.J.; Aitchison, T.C. Cancer, warts, and sunshine in renal transplant patients. A case-control study. Lancet 1984, 1, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Figueras, M.T.; Carrato, C.; Sáenz, X.; Puig, L.; Musulen, E.; Ferrándiz, C.; Ariza, A. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 991–997. [Google Scholar] [CrossRef]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic keratosis is an early in situ squamous cell carcinoma: A proposal for reclassification. Br. J. Dermatol. 2007, 156, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Gambichler, T.; Gupta, G.; Stücker, M.; Stockfleth, E.; Szeimies, R.M.; Dirschka, T. Actinic keratoses show variable histological basal growth patterns—A proposed classification adjustment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Gambichler, T.; Kost, C.; Gupta, G.; Stücker, M.; Stockfleth, E.; Dirschka, T. Cutaneous squamous cell carcinomas are associated with basal proliferating actinic keratoses. Br. J. Dermatol. 2019, 180, 916–921. [Google Scholar] [CrossRef]
- Schmitz, L.; Brehmer, A.; Falkenberg, C.; Gambichler, T.; Heppt, M.V.; Steeb, T.; Gupta, G.; Malvehy, J.; Dirschka, T. Treatment-resistant actinic keratoses are characterized by distinct clinical and histological features. Ital. J. Dermatol. Venerol. 2021, 156, 213–219. [Google Scholar] [CrossRef]
- Heppt, M.V.; Leiter, U.; Steeb, T.; Amaral, T.; Bauer, A.; Becker, J.C.; Breitbart, E.; Breuninger, H.; Diepgen, T.; Dirschka, T.; et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma-short version, part 1: Diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J. Dtsch. Dermatol. Ges. 2020, 18, 275–294. [Google Scholar] [CrossRef]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Merks, I.; Nelemans, P.J.; Kelleners-Smeets, N.W.J.; Mosterd, K.; Essers, B.A.B. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br. J. Dermatol. 2020, 183, 738–744. [Google Scholar] [CrossRef]
- Ulrich, C.; Schmook, T.; Nindl, I.; Meyer, T.; Sterry, W.; Stockfleth, E. Cutaneous precancers in organ transplant recipients: An old enemy in a new surrounding. Br. J. Dermatol. 2003, 149, 40–42. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, H.S.; Yoon, H.S.; Cho, S. Claudin-1 expression decreases with increasing pathological grade in actinic keratosis and may be a marker of high-risk actinic keratosis. Clin. Exp. Dermatol. 2019, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Gopal, S.K.; Simpson, R.J. Contribution of cells undergoing epithelial-mesenchymal transition to the tumour microenvironment. J. Proteom. 2013, 78, 545–557. [Google Scholar] [CrossRef]
- Guarino, M. Epithelial-mesenchymal transition and tumour invasion. Int. J. Biochem. Cell Biol. 2007, 39, 2153–2160. [Google Scholar] [CrossRef]
- Saenz-Sardà, X.; Carrato, C.; Pérez-Roca, L.; Puig, L.; Ferrándiz, C.; Ariza, A.; Fernández-Figueras, M.T. Epithelial-to-mesenchymal transition contributes to invasion in squamous cell carcinomas originated from actinic keratosis through the differentiated pathway, whereas proliferation plays a more significant role in the classical pathway. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Mühleisen, B.; Petrov, I.; Gächter, T.; Kurrer, M.; Schärer, L.; Dummer, R.; French, L.E.; Hofbauer, G.F. Progression of cutaneous squamous cell carcinoma in immunosuppressed patients is associated with reduced CD123+ and FOXP3+ cells in the perineoplastic inflammatory infiltrate. Histopathology 2009, 55, 67–76. [Google Scholar] [CrossRef]
- Harwood, C.A.; Proby, C.M.; McGregor, J.M.; Sheaff, M.T.; Leigh, I.M.; Cerio, R. Clinicopathologic features of skin cancer in organ transplant recipients: A retrospective case-control series. J. Am. Acad. Dermatol. 2006, 54, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Nissinen, L.; Farshchian, M.; Riihilä, P.; Kähäri, V.M. New perspectives on role of tumor microenvironment in progression of cutaneous squamous cell carcinoma. Cell Tissue Res. 2016, 365, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Kahl, P.; Majores, M.; Bierhoff, E.; Stockfleth, E.; Dirschka, T. Actinic keratosis: Correlation between clinical and histological classification systems. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S.; Stasko, T.; Cameron, G.S.; Russell, M.; King, L.E., Jr. Histologic features of actinic keratoses in solid organ transplant recipients and healthy controls. J. Am. Acad. Dermatol. 2001, 45, 217–221. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n | (%) | |
|---|---|---|---|
| Sex | Male | 34 | (79.1) |
| Female | 9 | (20.9) | |
| Age, years | 69.3 | (8.1) * | |
| Solid organ transplant | Kidney | 35 | (81.4) |
| Heart | 7 | (16.3) | |
| Kidney + heart | 1 | (2.3) | |
| Number of transplants received per patient | 1 | 37 | (86.0) |
| 2 | 4 | (9.3) | |
| 3 | 2 | (4.7) | |
| Duration of immunosuppression, years | 11 | (7–20.5) # | |
| <5 years | 9 | (20.9) | |
| 5–10 years | 9 | (20.9) | |
| >10 years | 25 | (58.1) | |
| Immunosuppressant medication | Single/dual therapy | 27 | (62.8) |
| Triple therapy | 16 | (37.2) | |
| History of invasive skin cancer | Any type of skin cancer | 39 | (88.6) |
| Non-melanoma skin cancer | 39 | (88.6) | |
| Histological Characteristics | Overall (N = 222) | sOTRs (N = 111) | ICG (N = 111) | p-Value (sOTRs vs. Controls) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| AK Histological Severity | 0.0078 * | |||
| AK I | 89 (40) | 54 (48.6) | 35 (31.5) | 0.0094 * |
| AK II | 84 (37.8) | 38 (34.2) | 46 (41.4) | 0.2693 |
| AK III | 49 (22.1) | 19 (17.1) | 30 (27) | 0.0757 |
| AK Basal Growth Grading | <0.0001 * | |||
| PRO 0 | 10 (4.5) | 1 (0.9) | 9 (8.1) | 0.0098 * |
| PRO I | 32 (14.4) | 5 (4.5) | 27 (24.3) | <0.0001 * |
| PRO II | 54 (24.3) | 16 (14.4) | 38 (34.2) | 0.0006 * |
| PRO III | 126 (56.8) | 89 (80.2) | 37 (33.3) | <0.0001 * |
| Acantholysis | 102 (45.9) | 66 (59.5) | 36 (32.4) | <0.0001 * |
| Elastosis | 0.1107 | |||
| Unknown | 8 (3.6) | 7 (6.3) | 1 (0.9) | 0.0311 * |
| None | 17 (7.7) | 17 (15.3) | 0 (0) | 0.0004 * |
| Mild | 28 (12.6) | 12 (10.8) | 16 (14.4) | 0.8507 |
| Moderate | 42 (18.9) | 21 (18.9) | 21 (18.9) | 1.0000 |
| Severe | 70 (31.5) | 30 (27) | 40 (36) | 0.1495 |
| Very severe | 57 (25.7) | 24 (21.6) | 33 (29.7) | 0.1677 |
| Follicular Involvement | 170 (76.6) | 93 (83.8) | 77 (69.4) | <0.0001 * |
| Hyperkeratosis | <0.0001 * | |||
| None | 15 (6.8) | 3 (2.7) | 12 (10.8) | 0.0163 * |
| Mild | 60 (27) | 20 (18) | 40 (36) | 0.0026 |
| Moderate | 84 (37.8) | 47 (42.3) | 37 (33.3) | 0.1674 |
| Severe | 39 (17.6) | 26 (23.4) | 13 (11.7) | 0.0222 * |
| Very severe | 24 (10.8) | 15 (13.5) | 9 (8.1) | 0.1957 |
| Infiltrate | 0.4373 | |||
| None | 8 (3.6) | 2 (1.8) | 6 (5.4) | 0.1507 |
| Mild | 73 (32.9) | 44 (39.6) | 29 (26.1) | 0.0325 * |
| Moderate | 98 (44.1) | 44 (39.6) | 54 (48.6) | 0.1775 |
| Severe | 36 (16.2) | 14 (12.6) | 22 (19.8) | 0.1461 |
| Very severe | 7 (3.2) | 7 (6.3) | 0 (0) | 0.0073 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkenberg, C.; Dirschka, T.; Gilbert, G.; Stockfleth, E.; Homey, B.; Schmitz, L. Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients. Cancers 2023, 15, 1765. https://doi.org/10.3390/cancers15061765
Falkenberg C, Dirschka T, Gilbert G, Stockfleth E, Homey B, Schmitz L. Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients. Cancers. 2023; 15(6):1765. https://doi.org/10.3390/cancers15061765
Chicago/Turabian StyleFalkenberg, Conrad, Thomas Dirschka, Georgia Gilbert, Eggert Stockfleth, Bernhard Homey, and Lutz Schmitz. 2023. "Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients" Cancers 15, no. 6: 1765. https://doi.org/10.3390/cancers15061765
APA StyleFalkenberg, C., Dirschka, T., Gilbert, G., Stockfleth, E., Homey, B., & Schmitz, L. (2023). Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients. Cancers, 15(6), 1765. https://doi.org/10.3390/cancers15061765








