Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting
Abstract
Simple Summary
Abstract
1. Introduction
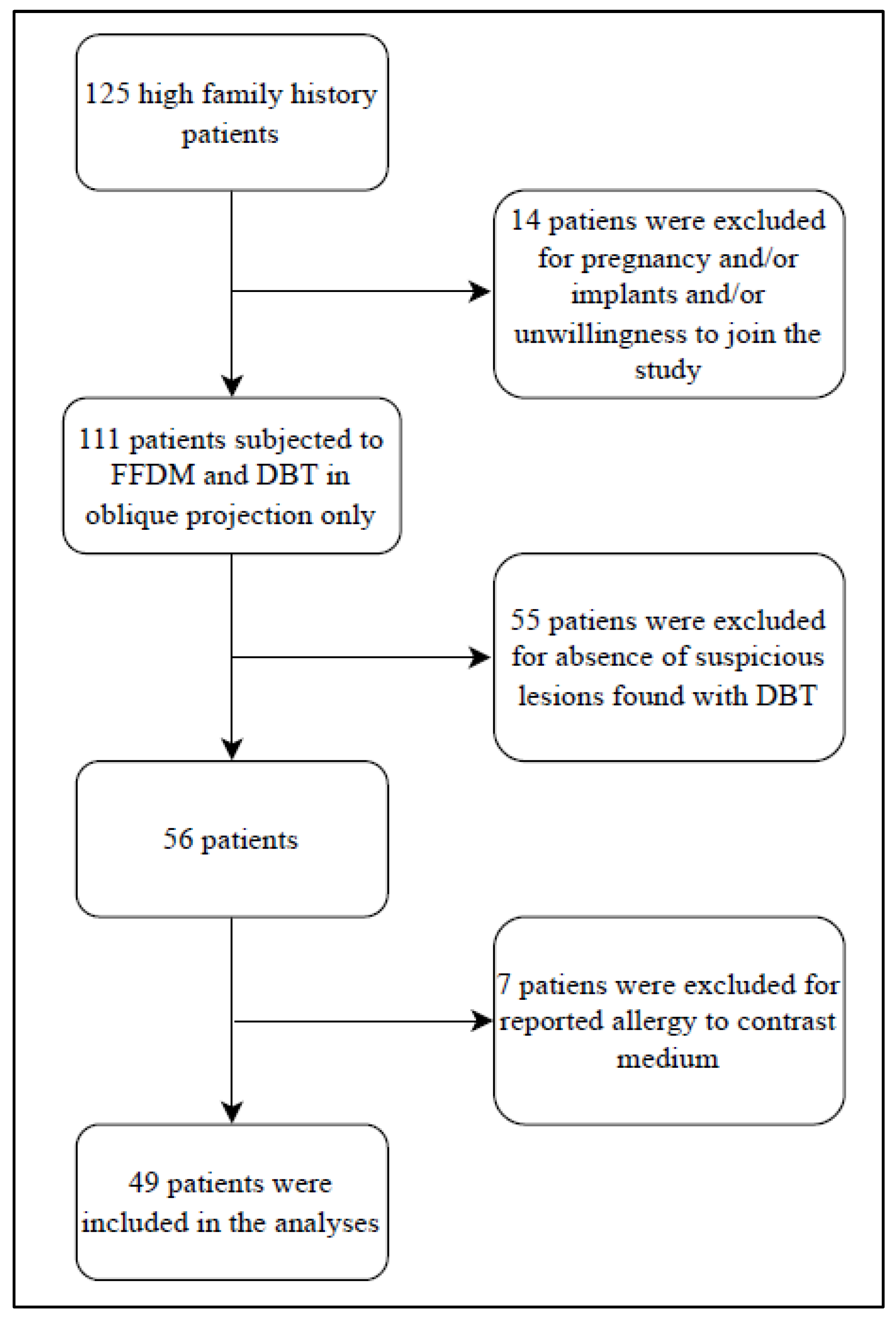
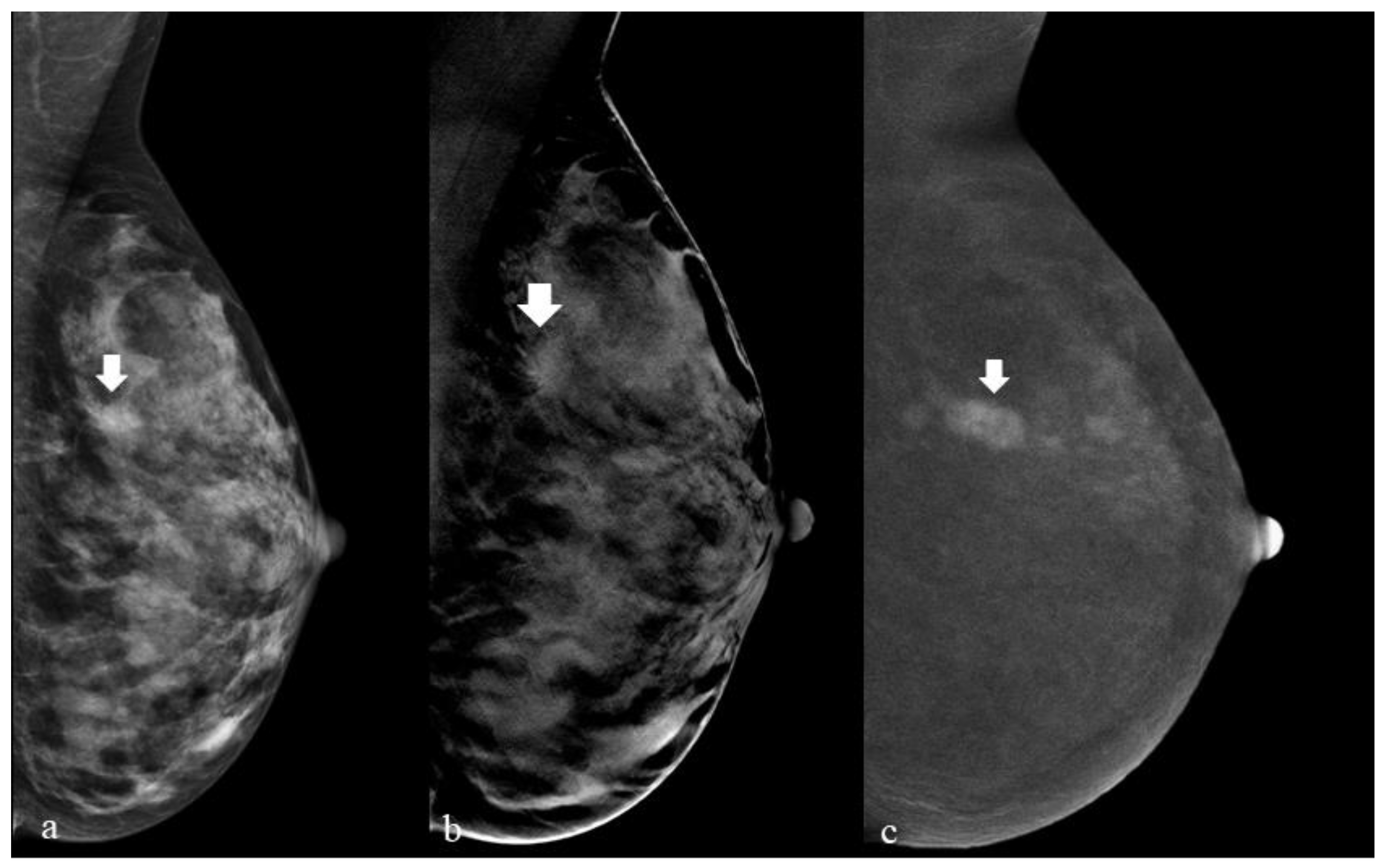
2. Materials and Methods
- B2: benign lesions.
- B3: high-risk lesions
- B5a: in situ lesions.
- B5b: invasive lesions.
2.1. Inclusion Criterion of the Study
- Asymptomatic patients undergoing mammography and tomosynthesis in which there is a dubious finding (BI-RADS > 3).
- Patients at high risk for the development of breast neoplasia (at least one first-degree relative with breast neoplasm).
- Patients with suspicious findings (BI-RADS > 3) undergoing CEM examination.
- Patients who have signed a specific, informed consent for the study.
- Patients with age > 18 years old.
2.2. Exclusion Criterion of the Study
- Patients with known allergy to iodinated contrast medium.
- Symptomatic patients with palpable breast lumps.
- Patients with breast implant(s).
- Patients with proven or supposed pregnancy.
2.3. Statistical Analysis
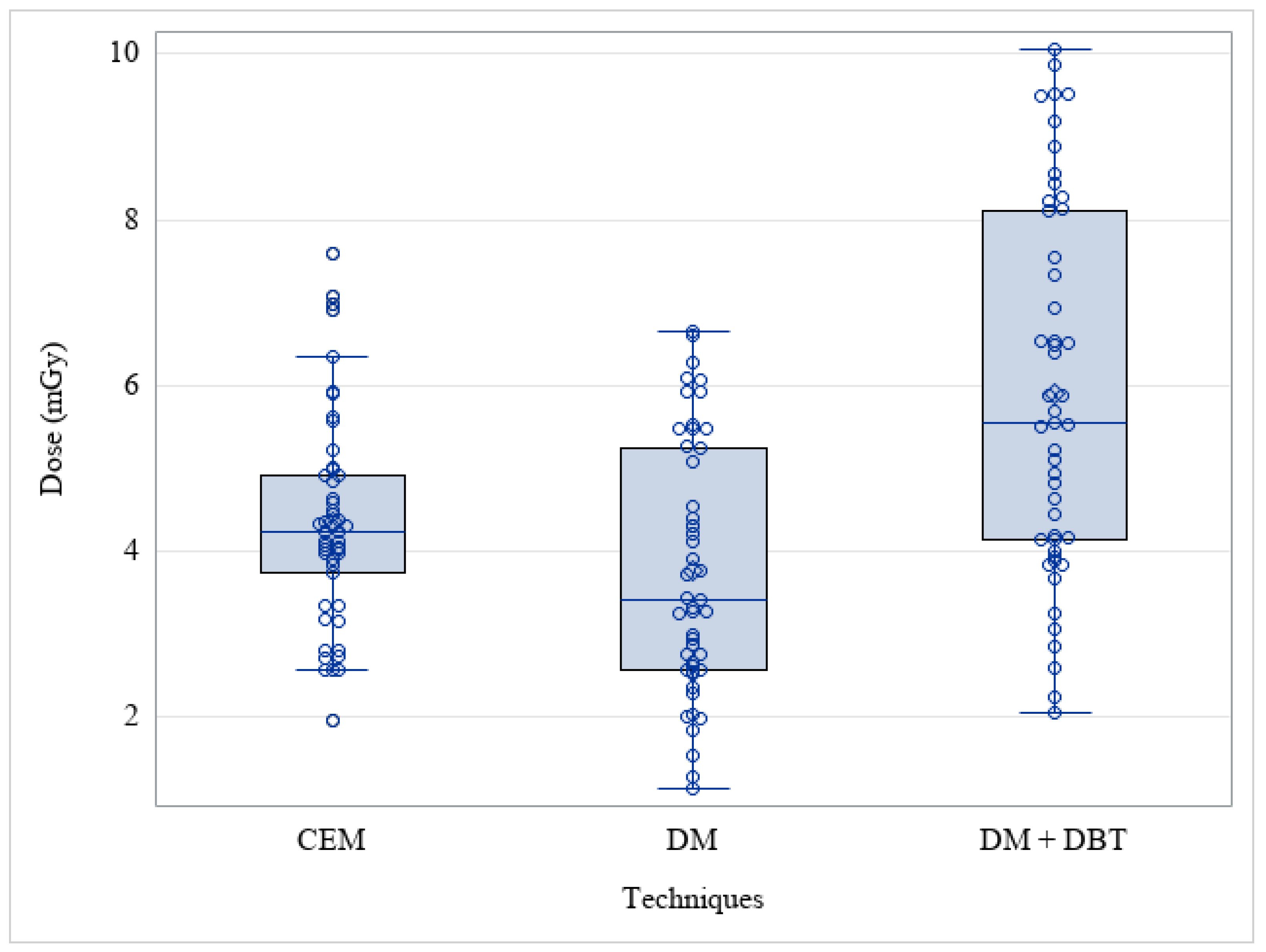
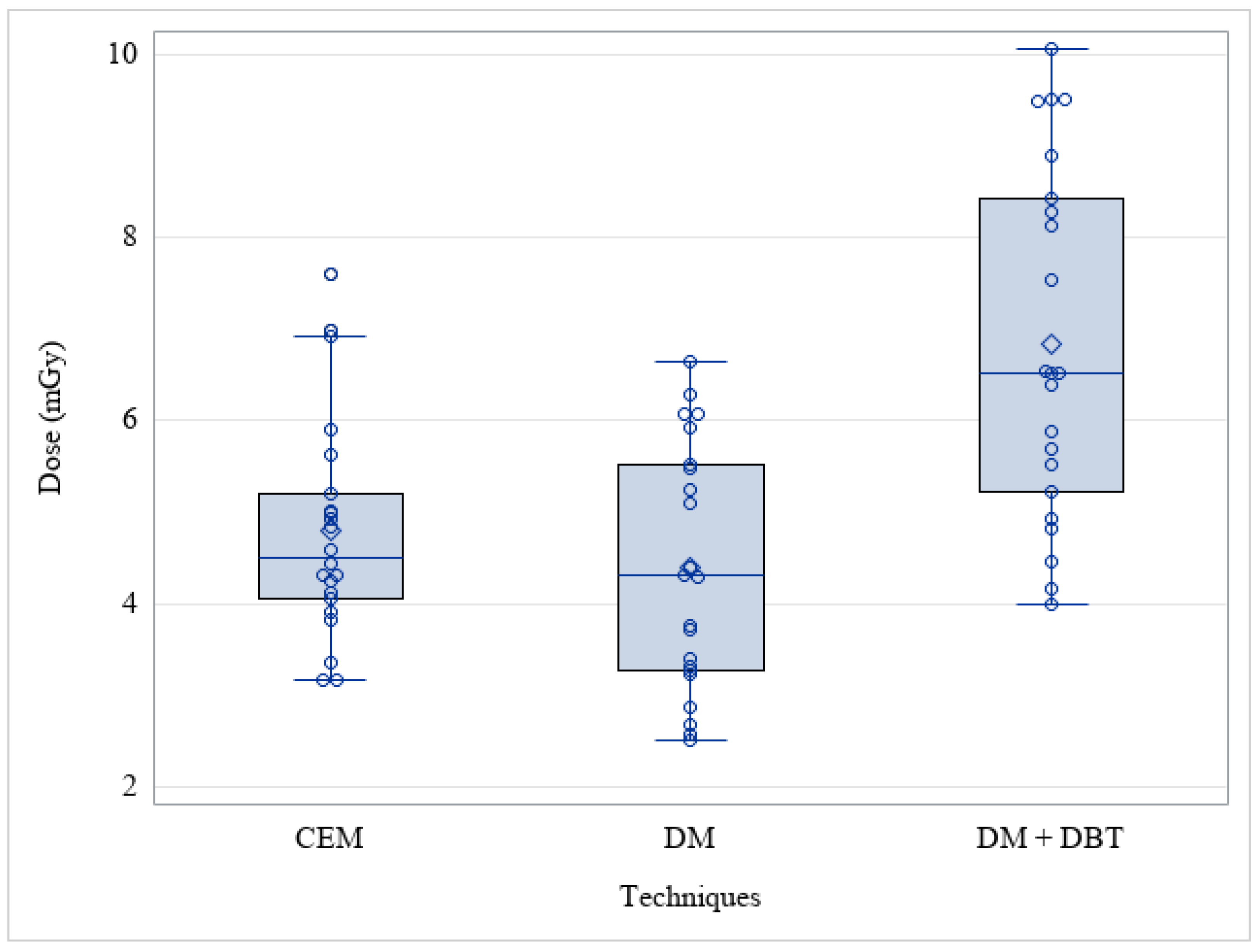
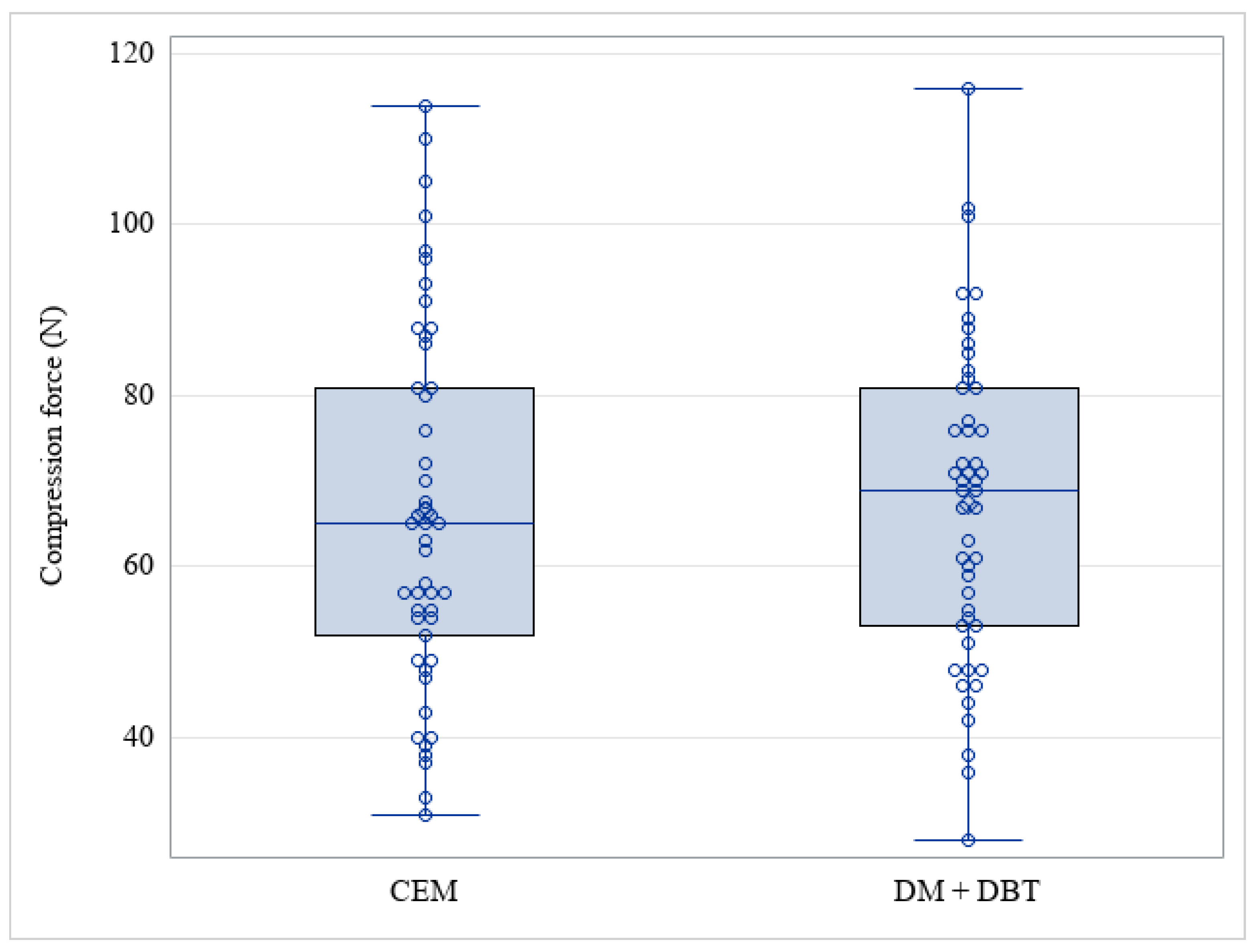
3. Results
- CEM mammograph: 10 (20%) Fuji Amulet Innovality; 34 (69%) GE Pristina; 5 (10%) Hologic 3Dimensions.
- DM + DBT mammograph: 26 (53%) GE Pristina; 23 (47%) Hologic 3Dimensions.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Breast Cancer 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 10 June 2022).
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—Summary document. Ann. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Bochud, F.O.; Valley, J.F.; Verdun, F.R.; Hessler, C.; Schnyder, P. Estimation of the noisy component of anatomical backgrounds. Med. Phys. 1999, 26, 1365–1370. [Google Scholar] [CrossRef]
- Caumo, F.; Montemezzi, S.; Romanucci, G.; Brunelli, S.; Bricolo, P.; Cugola, L.; Gennaro, G. Repeat Screening Outcomes with Digital Breast Tomosynthesis Plus Synthetic Mammography for Breast Cancer Detection: Results from the Prospective Verona Pilot Study. Radiology 2021, 298, 49–57. [Google Scholar] [CrossRef]
- Skaane, P. Breast cancer screening with digital breast tomosynthesis. Breast Cancer 2017, 24, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Pattacini, P.; Nitrosi, A.; Giorgi Rossi, P.; Iotti, V.; Ginocchi, V.; Ravaioli, S.; Vacondio, R.; Braglia, L.; Cavuto, S.; Campari, C.; et al. Digital Mammography versus Digital Mammography Plus Tomosynthesis for Breast Cancer Screening: The Reggio Emilia Tomosynthesis Randomized Trial. Radiology 2018, 288, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Li, T.; Houssami, N.; Noguchi, N.; Zeng, A.; Marinovich, M.L. Differential detection by breast density for digital breast tomosynthesis versus digital mammography population screening: A systematic review and meta-analysis. Br. J. Cancer 2022, 127, 116–125. [Google Scholar] [CrossRef]
- Libesman, S.; Zackrisson, S.; Hofvind, S.; Seidler, A.L.; Bernardi, D.; Lång, K.; Robledo, K.P.; Houssami, N. An individual participant data meta-analysis of breast cancer detection and recall rates for digital breast tomosynthesis versus digital mammography population screening. Clin. Breast Cancer 2022, 22, e647–e654. [Google Scholar] [CrossRef]
- Cozzi, A.; Magni, V.; Zanardo, M.; Schiaffino, S.; Sardanelli, F. Contrast-enhanced Mammography: A Systematic Review and Meta-Analysis of Diagnostic Performance. Radiology 2022, 302, 568–581. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Lalji, U.; Houwers, J.; Nijssen, E.C.; Nelemans, P.J.; van Roozendaal, L.; Smidt, M.L.; Heuts, E.; Wildberger, J.E. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur. Radiol. 2014, 24, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Schiaffino, S.; Fanizza, M.; Magni, V.; Menicagli, L.; Monaco, C.G.; Benedek, A.; Spinelli, D.; Di Leo, G.; Di Giulio, G.; et al. Contrast-enhanced mammography for the assessment of screening recalls: A two-centre study. Eur. Radiol. 2022, 32, 7388–7399. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Mihai, G.; Hassonjee, S.E.; Raj, S.D.; Palmer, M.R.; Brook, A.; Zhang, D. Comparative Dose of Contrast-Enhanced Spectral Mammography (CESM), Digital Mammography, and Digital Breast Tomosynthesis. AJR Am. J. Roentgenol. 2018, 211, 839–846. [Google Scholar] [CrossRef] [PubMed]
- James, J.R.; Pavlicek, W.; Hanson, J.A.; Boltz, T.F.; Patel, B.K. Breast Radiation Dose with CESM Compared with 2D FFDM and 3D Tomosynthesis Mammography. AJR Am. J. Roentgenol. 2017, 208, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, G.; Cozzi, A.; Schiaffino, S.; Sardanelli, F.; Caumo, F. Radiation Dose of Contrast-Enhanced Mammography: A Two-Center Prospective Comparison. Cancers 2022, 14, 1774. [Google Scholar] [CrossRef] [PubMed]
- Bicchierai, G.; Busoni, S.; Tortoli, P.; Bettarini, S.; Naro, F.D.; De Benedetto, D.; Savi, E.; Bellini, C.; Miele, V.; Nori, J. Single Center Evaluation of Comparative Breast Radiation dose of Contrast Enhanced Digital Mammography (CEDM), Digital Mammography (DM) and Digital Breast Tomosynthesis (DBT). Acad. Radiol. 2022, 29, 1342–1349. [Google Scholar] [CrossRef]
- Lång, K.; Andersson, I.; Rosso, A.; Tingberg, A.; Timberg, P.; Zackrisson, S. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: Results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur. Radiol. 2016, 26, 184–190. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas: Breast Imaging Re-Porting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- International Atomic Energy Agency. Dosimetry in Diagnostic Radiology: An International Code of Practice; International Atomic Energy Agency: Vienna, Austria, 2007; Volume 457. [Google Scholar]
- Dance, D.R.; Skinner, C.L.; Young, K.C.; Beckett, J.R.; Kotre, C.J. Additional factors for the estimation of mean glandular breast dose using the UK mammography dosimetry protocol. Phys. Med. Biol. 2000, 45, 3225–3240. [Google Scholar] [CrossRef]
- Lee, C.H.; Phillips, J.; Sung, J.S.; Lewin, J.M.; Newell, M.S. Contrast Enhanced Mammography (CEM) (A Supplement to ACR BI-RADS® Mammography 2013) Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2022. [Google Scholar]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Pesapane, F.; Meneghetti, L.; Pizzamiglio, M.; Abbate, F.; Latronico, A.; Bagnardi, V.; Frassoni, S.; et al. Breast Imaging Reporting and Data System and Contrast Enhancement Mammography: Lesion Conspicuity Likelihood of Malignancy and Relationship with Breast Tumor Receptor Status. Acad. Radiol. 2023, in press. [CrossRef]
- Hoon Tan, P.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Smith, R.A.; Duffy, S.W.; Gabe, R.; Tabar, L.; Yen, A.M.; Chen, T.H. The randomized trials of breast cancer screening: What have we learned? Radiol. Clin. N. Am. 2004, 42, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L.; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer: A cohort study. Ann. Intern. Med. 2015, 162, 673–681. [Google Scholar] [CrossRef]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef]
- Magnoni, F.; Corso, G. Progress in breast cancer surgical management. Eur. J. Cancer Prev. 2022, 31, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Svahn, T.M.; Houssami, N.; Sechopoulos, I.; Mattsson, S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Abbas, E.; Keshavarzi, S.; Fazelzad, R.; Bukhanov, K.; Kulkarni, S.; Au, F.; Ghai, S.; Alabousi, A.; Freitas, V. Supplemental Breast Cancer Screening in Women with Dense Breasts and Negative Mammography: A Systematic Review and Meta-Analysis. Radiology 2023, 306, e221785. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Leutner, C.; Schild, H.H.; Schrading, S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017, 283, 361–370. [Google Scholar] [CrossRef]
- Li, F.Y.; Hollingsworth, A.; Lai, W.T.; Yang, T.L.; Chen, L.J.; Wang, W.T.; Wang, J.L.; Morse, A.N. Feasibility of Breast MRI as the Primary Imaging Modality in a Large Asian Cohort. Cureus 2021, 13, e15095. [Google Scholar] [CrossRef]
- Sardanelli, F.; Cozzi, A.; Trimboli, R.M.; Schiaffino, S. Gadolinium Retention and Breast MRI Screening: More Harm Than Good? AJR Am. J. Roentgenol. 2020, 214, 324–327. [Google Scholar] [CrossRef]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef]
- Zuckerman, S.P.; Conant, E.F.; Keller, B.M.; Maidment, A.D.; Barufaldi, B.; Weinstein, S.P.; Synnestvedt, M.; McDonald, E.S. Implementation of Synthesized Two-dimensional Mammography in a Population-based Digital Breast Tomosynthesis Screening Program. Radiology 2016, 281, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Kleinknecht, J.H.; Ciurea, A.I.; Ciortea, C.A. Pros and cons for breast cancer screening with tomosynthesis—A review of the literature. Med. Pharm. Rep. 2020, 93, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Chikarmane, S. Synthetic Mammography: Review of Benefits and Drawbacks in Clinical Use. J. Breast Imaging 2022, 4, 124–134. [Google Scholar] [CrossRef]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Tyler, N.; Mackenzie, A. Technical Evaluation of SenoBright HD Contrast-Enhanced Mammography Functions of Senographe GE Pristina System Technical Report 2004. December 2020. Available online: https://medphys.royalsurrey.nhs.uk/nccpm/files/other/Tech_Eval_CESMGE_Pristina_NCCPMformatFinalV2.pdf (accessed on 7 April 2023).
- Kelly, M.; Rai, M.; Mackenzie, A. Technical Evaluation of Contrast Enhanced Mammography Functions Using Hologic I-View Software. Technical Report 2003. November 2020. Available online: https://medphys.royalsurrey.nhs.uk/nccpm/files/other/Tech_Eval_CESM_Hologic3Dimensions_Final.pdf (accessed on 7 April 2023).
| System | Anode/Filter DM 1 | Anode/Filter DBT 2 | Anode/Filter CEM 3 LE 4 | Anode/Filter CEM HE 5 |
|---|---|---|---|---|
| General Electric Pristina | Mo/Mo 6 Rh 7/Ag | Mo/Mo Rh/Ag | Mo/Mo Rh/Ag | Mo/Cu Rh/Cu |
| Hologic 3Dimensions | W 8/Rh W/Ag 11 | W/Al 9 | W/Rh W/Ag | W/Cu 10 |
| Fuji Amulet Innovality | W/Rh | W/Al | W/Rh | W/Cu |
| Variable | Level | Overall (N = 49) |
|---|---|---|
| Age at CEM 1 (y 2), median (min–max) | 52 (40–80) | |
| Type of lesion—DM 3 + DBT 4, N (%) | Microcalcifications | 31 (63) |
| Mass | 14 (29) | |
| Mass with microcalcifications | 1 (2) | |
| Architectural distortion | 3 (6) | |
| Density (ACR 5), N (%) | B | 12 (24) |
| C | 32 (65) | |
| D | 5 (10) | |
| BI-RADS–DBT, N (%) | 3 | 7 (14) |
| 4a | 25 (51) | |
| 4b | 8 (16) | |
| 4c | 9 (18) |
| Histological Results of the Biopsy | Overall (N = 49) | |
|---|---|---|
| B2 | Adenosis | 4 |
| Breast fibroadenoma | 4 | |
| Ductal hyperplasia without atypia | 1 | |
| Fibrocystic disease | 18 | |
| Fibrosis | 1 | |
| Flogosis | 2 | |
| B3 | Atypical ductal hyperplasia (Din1b) | 2 |
| Atypical lobular hyperplasia (LIN1) | 1 | |
| Flat epithelial atypia (FEA) | 1 | |
| Intraductal papilloma | 4 | |
| Radial scar | 1 | |
| B5a | High-grade ductal carcinoma in situ (DIN3) | 3 |
| Intermediate-grade ductal carcinoma in situ (DIN2) | 2 | |
| Low-grade ductal carcinoma in situ (DIN1c) | 2 | |
| B5b | Invasive ductal carcinoma | 2 |
| Invasive lobular carcinoma | 1 | |
| Histological Results | Visibility | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM 1 | DM + DBT 2 | Recombined (CEM) 3 | Low Energy (CEM) | Recombined + Low Energy | ||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| B2 (N = 30) | 7 | 23 | 0 | 30 | 25 | 5 | 8 | 22 | 6 | 24 |
| B3 (N = 9) | 2 | 7 | 0 | 9 | 6 | 3 | 2 | 7 | 2 | 7 |
| B5a (N = 7) | 1 | 6 | 0 | 7 | 2 | 5 | 1 | 6 | 0 | 7 |
| B5b (N = 3) | 1 | 2 | 0 | 3 | 0 | 3 | 2 | 1 | 0 | 3 |
| Total | 11 | 38 | 0 | 49 | 33 | 16 | 13 | 36 | 8 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, L.; Bozzini, A.C.; Pesapane, F.; Rotili, A.; Marinucci, I.; Signorelli, G.; Frassoni, S.; Bagnardi, V.; Origgi, D.; De Marco, P.; et al. Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting. Cancers 2023, 15, 2413. https://doi.org/10.3390/cancers15092413
Nicosia L, Bozzini AC, Pesapane F, Rotili A, Marinucci I, Signorelli G, Frassoni S, Bagnardi V, Origgi D, De Marco P, et al. Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting. Cancers. 2023; 15(9):2413. https://doi.org/10.3390/cancers15092413
Chicago/Turabian StyleNicosia, Luca, Anna Carla Bozzini, Filippo Pesapane, Anna Rotili, Irene Marinucci, Giulia Signorelli, Samuele Frassoni, Vincenzo Bagnardi, Daniela Origgi, Paolo De Marco, and et al. 2023. "Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting" Cancers 15, no. 9: 2413. https://doi.org/10.3390/cancers15092413
APA StyleNicosia, L., Bozzini, A. C., Pesapane, F., Rotili, A., Marinucci, I., Signorelli, G., Frassoni, S., Bagnardi, V., Origgi, D., De Marco, P., Abiuso, I., Sangalli, C., Balestreri, N., Corso, G., & Cassano, E. (2023). Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting. Cancers, 15(9), 2413. https://doi.org/10.3390/cancers15092413









