Prognostic Value of Combined Hematological/Biochemical Indexes and Tumor Clinicopathologic Features in Colorectal Cancer Patients—A Pilot Single Center Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Patient Characteristics
2.3. Preoperative Laboratory Measurements and Other Prognostic Scores
2.4. Tumor Characteristics
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Overall Patient Survival (OS)
3.3. Disease-Free Patient Survival (DFS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef]
- Naxerova, K.; Reiter, J.G.; Brachtel, E.; Lennerz, J.K.; van de Wetering, M.; Rowan, A.; Cai, T.; Clevers, H.; Swanton, C.; Nowak, M.A.; et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017, 357, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Percario, R.; Panaccio, P.; di Mola, F.F.; Grottola, T.; Di Sebastiano, P. The Complex Network between Inflammation and Colorectal Cancer: A Systematic Review of the Literature. Cancers 2021, 13, 6237. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yao, S.; Lu, P.; Ma, Y.; Xu, H.; Yin, Z.; Hu, J.; Liu, Y.; Wei, S. The Prognostic Value of New Index (LANR) Composed of Pre-operative Lymphocytes, Albumin, and Neutrophils in Patients with Resectable Colorectal Cancer. Front. Oncol. 2021, 11, 610264. [Google Scholar] [CrossRef]
- Hayama, T.; Ozawa, T.; Asako, K.; Kondo, R.; Ono, K.; Okada, Y.; Tsukamoto, M.; Fukushima, Y.; Shimada, R.; Nozawa, K.; et al. Impact of Colon Cancer Location on the Prognostic Significance of Nutritional Indexes and Inflammatory Markers. In Vivo 2021, 35, 1261–1269. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Iwasaki, Y.; Shibuya, N.; Kubota, K. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival After Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 900–907. [Google Scholar] [CrossRef]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’Reilly, D.S.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Man, W.; Lin, H.; Liu, Z.; Jin, L.; Wang, J.; Zhang, J.; Bai, Z.; Yao, H.; Zhang, Z.; Deng, W. Usefulness of Inflammation-Based Prognostic Scores for Predicting the Risk of Complications After Radical Resection of Colorectal Carcinoma. Cancer Manag. Res. 2020, 12, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Oikawa, M.; Kakita, T.; Okada, T.; Abe, T.; Yazawa, T.; Tsuchiya, H.; Akazawa, N.; Sato, M.; Ohira, T.; et al. Preoperative change of modified Glasgow prognostic score after stenting predicts the long-term outcomes of obstructive colorectal cancer. Surg. Today 2020, 50, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Republic of Serbia. Available online: https://www.zdravlje.gov.rs/view_file.php?file_id=648&cache=sr (accessed on 7 December 2022).
- Pfister, D.G.; Benson, A.B., 3rd; Somerfield, M.R. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N. Engl. J. Med. 2004, 350, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H.; GEKID Cancer Survival Working Group. Sex differences in colorectal cancer survival: Population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS ONE 2013, 8, e68077. [Google Scholar] [CrossRef]
- Clendenen, T.V.; Koenig, K.L.; Shore, R.E.; Levitz, M.; Arslan, A.A.; Zeleniuch-Jacquotte, A. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Cancer Epidemiol. Biomark. Prev. 2009, 18, 275–281. [Google Scholar]
- Barzi, A.; Lenz, A.M.; Labonte, M.J.; Lenz, H.J. Molecular pathways: Estrogen pathway in colorectal cancer. Clinical cancer research. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5842–5848. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Jiang, L.; Fei, H.; Yang, A.; Zhu, J.; Sun, J.; Liu, X.; Xu, W.; Yang, J.; Zhang, S. Estrogen inhibits the growth of colon cancer in mice through reversing extracellular vesicle-mediated immunosuppressive tumor microenvironment. Cancer Lett. 2021, 520, 332–343. [Google Scholar] [CrossRef]
- Salehi Far, S.; Soltani, M.; Zardast, M.; Zhu, J.; Sun, J.; Liu, X.; Xu, W.; Yang, J.; Zhang, S. Investigating the Factors Associated with the Level of Expression of Estrogen and Progesterone Receptors in Patients Suffering from Colorectal Cancer. J. Cancer Epidemiol. 2021, 2021, 4478155. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Duraes, L.C.; Stocchi, L.; Steele, S.R.; Kalady, M.F.; Church, J.M.; Gorgun, E.; Liska, D.; Kessler, H.; Lavryk, O.A.; Delaney, C.P. The Relationship between Clavien-Dindo Morbidity Classification and Oncologic Outcomes After Colorectal Cancer Resection. Ann. Surg. Oncol. 2018, 25, 188–196. [Google Scholar] [CrossRef]
- Aoyama, T.; Oba, K.; Honda, M.; Sadahiro, S.; Hamada, C.; Mayanagi, S.; Kanda, M.; Maeda, H.; Kashiwabara, K.; Sakamoto, J.; et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: Analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med. 2017, 6, 1573–1580. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Xia, L.J.; Li, W.; Zhai, J.C.; Yan, C.W.; Chen, J.B.; Yang, H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer 2020, 20, 208. [Google Scholar] [CrossRef]
- Mizuno, H.; Yuasa, N.; Takeuchi, E.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Miyata, K. Blood cell markers that can predict the long-term outcomes of patients with colorectal cancer. PLoS ONE 2019, 14, e0220579. [Google Scholar] [CrossRef]
- Ozawa, T.; Ishihara, S.; Nishikawa, T.; Tanaka, T.; Tanaka, J.; Kiyomatsu, T.; Hata, K.; Kawai, K.; Nozawa, H.; Kazama, S.; et al. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. Int. J. Color. Dis. 2015, 30, 1165–1171. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Son, W.; Shin, S.J.; Park, S.H.; Lee, S.K.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Clinical Impact of Combined Modified Glasgow Prognostic Score and C-Reactive Protein/Albumin Ratio in Patients with Colorectal Cancer. Diagnostics 2020, 10, 859. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Impact of the preoperative prognostic nutritional index on postoperative and survival outcomes in colorectal cancer patients who underwent primary tumor resection: A systematic review and meta-analysis. Int. J. Color. Dis. 2019, 34, 681–689. [Google Scholar] [CrossRef]
- Climent, M.; Ryan, É.J.; Stakelum, Á.; Khaw, Y.L.; Creavin, B.; Lloyd, A.; Alhassan, D.; Mohan, H.M.; Kennelly, R.; Sheahan, K.; et al. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int. J. Color. Dis. 2019, 34, 1069–1078. [Google Scholar] [CrossRef]
- Nagashima, Y.; Funahashi, K.; Kagami, S.; Ushigome, M.; Kaneko, T.; Miura, Y.; Yoshida, K.; Koda, T.; Kurihara, A. Which preoperative immunonutritional index best predicts postoperative mortality after palliative surgery for malignant bowel obstruction in patients with late-stage cancer? A single-center study in Japan comparing the modified Glasgow prognostic score (mGPS), the prognostic nutritional index (PNI), and the controlling nutritional status (CONUT). Surg. Today 2023, 53, 22–30. [Google Scholar]
- Tamai, K.; Okamura, S.; Makino, S.; Yamamura, N.; Fukuchi, N.; Ebisui, C.; Inoue, A.; Yano, M. C-reactive protein/albumin ratio predicts survival after curative surgery in elderly patients with colorectal cancer. Updates Surg. 2022, 74, 153–162. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; Gomez, D.; Prasad, K.R. C-reactive protein in liver cancer surgery. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2008, 34, 727–729. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, W.; Hou, H.; Ning, S.; Li, J.; Huang, B.; Liu, K.; Zhang, L. Prognostic Value of C-Reactive Protein, Glasgow Prognostic Score, and C-Reactive Protein-to-Albumin Ratio in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 637650. [Google Scholar] [CrossRef]
- Nakazaki, H. Preoperative and postoperative cytokines in patients with cancer. Cancer 1992, 70, 709–713. [Google Scholar] [CrossRef]
- McMillan, D.C.; Watson, W.S.; O’Gorman, P.; Preston, T.; Scott, H.R.; McArdle, C.S. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr. Cancer 2001, 39, 210–213. [Google Scholar] [CrossRef]
- Finn, O.J. A Believer’s Overview of Cancer Immunosurveillance and Immunotherapy. J. Immunol. 2018, 200, 385–391. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.; Li, C.; Wu, Y.; Song, W.; Guo, T.; Chen, S.; Cai, S.; Huang, D.; Xu, Y. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann. Transl. Med. 2019, 7, 769. [Google Scholar] [CrossRef]
- Lord, A.C.; D’Souza, N.; Pucher, P.H.; Moran, B.J.; Abulafi, A.M.; Wotherspoon, A.; Rasheed, S.; Brown, G. Significance of extranodal tumour deposits in colorectal cancer: A systematic review and meta-analysis. Eur. J. Cancer 2017, 82, 92–102. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Knijn, N.; Hugen, N.; Marshall, H.C.; Sugihara, K.; Tot, T.; Ueno, H.; Quirke, P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1119–1127. [Google Scholar] [CrossRef]
- Jakovljevic, K.; Malisic, E.; Cavic, M.; Krivokuca, A.; Dobricic, J.; Jankovic, R. KRAS and BRAF mutations in Serbian patients with colorectal cancer. J. BUON Off. J. Balk. Union Oncol. 2012, 17, 575–580. [Google Scholar]

| Characteristics of Patients | n (%) |
|---|---|
| Sex | |
| Male | 63 (56.8) |
| Age (years) † | 67 (32−88) |
| <40 years | 2 (1.8) |
| 41–50 years | 3 (2.7) |
| 51–60 years | 11 (9.9) |
| >60 years | 95 (85.57) |
| ASA Score ‡ | |
| 1 | 18 (16.2) |
| 2 | 50 (45.0) |
| 3 | 43 (38.7) |
| 4 | 0 (0) |
| 5 | 0 (0) |
| Ten most common comorbidities | |
| Arterial hypertension (HTA) | 81 (73.0) |
| Sideropenic anemia | 30 (27.0) |
| Diabetes mellitus | 29 (26.1) |
| Ischemic heart disease | 21 (18.9) |
| Cardiac arrhythmias | 15 (13.5) |
| Benign prostatic hyperplasia (BPH) | 12 (10.8) |
| Chronic obstructive pulmonary disease | 8 (7.2) |
| Hypothyroidism | 7 (6.3) |
| Stroke | 5 (4.5) |
| Renal failure | 3 (1.8) |
| Hematological/biochemical values and Indexes § | |
| Leukocytes (109/L) | 7 (5.70−8.4) |
| Erythrocytes (109/L) | 4.51 (4.17−4.88) |
| Platelets (109/L) | 293 (243−377) |
| Neutrophils (109/L) | 4.72 (3.78−5.85) |
| Lymphocytes (109/L) | 1.47 (1.14−1.94) |
| Monocytes (109/L) | 0.35 (0.28−0.44) |
| Hemoglobin (g/dL) | 12.10 (10.40−13.60) |
| Hematocrit (%) | 37.70 (33.60−41.80) |
| RBC (RDW-CV) (%) | 14.6 (13.3−17.4) |
| Serum albumin (g/L) | 39 (35.00−42.00) |
| CRP (C-reactive protein) (mg/L) | 4.80 (2.00−17.10) |
| CEA (ng/mL) | 3.37 (2.02−9.11) |
| CA 19-9 (U/mL) | 11.44 (6.28−28.46) |
| Cortisol 8 h (nmol/L) | 425 (349.10−526.70) |
| Testosterone (nmol/L) | 3.12 (0.32−10.64) |
| Estradiol (pmol/L) | 21.18 (13.57−50.56) |
| Estadiol of males | 30.465 (18.35–60.015) |
| Estadiol of females | 18.35 (5–18.35) |
| Estadiol of females <60 years | 30.75 (18.35–201.005) |
| Estadiol of females >60 years | 18.35 (5–18.35) |
| NLR (Neutrophile to Lymphocyte Ratio) | 3.09 (2.21−4.54) |
| MLR (Monocyte to Lymphocyte Ratio) | 0.23 (0.18−0.32) |
| PLR (Platelets to Lymphocyte Ratio) | 190.63 (141.60−276.14) |
| RLR (RBC to Lymphocyte Ratio) | 10.23 (7.62−14.1) |
| MPR (MPV to Platelets Ratio) | 0.03 (0.02−0.04) |
| CAR (CRP to Serum Albumin Ratio) | 0.12 (0.05−0.45) |
| PNI (Prognostic Nutritive Index) | 46.56 (42.30−50.85) |
| LANR (Lymphocyte, Serum Albumin, Neutrophile Ratio) | 12.43 (7.78−17.76) |
| mGPS (modified Glasgow Prognostic Score) ¶: | |
| 0 | 66 (59.5) |
| 1 | 31 (27.9) |
| 2 | 14 (12.6) |
| Parameters | Death | p | Relapse of Disease | p | ||
|---|---|---|---|---|---|---|
| No N (%) | Yes N (%) | No n (%) | Yes n (%) | |||
| Sex | 0.300 * | 0.892 * | ||||
| Male | 44 (60.3) | 19 (50) | 41 (57.7) | 16 (59.3) | ||
| Female | 29 (39.7) | 19 (50) | 30 (42.3) | 11 (40.7) | ||
| Age (years) † | 66 (62−73) | 68 (62−80) | 0.117 ** | 67 (63−75) | 63 (60−68) | 0.045 ** |
| ASA Score | 0.248 * | 0.032 * | ||||
| 1 | 11 (15.1) | 7 (18.4) | 9 (12.7) | 8 (29.6) | ||
| 2 | 37 (50.7) | 13 (34.2) | 31 (43.7) | 14 (51.9) | ||
| 3 | 25 (34.2) | 18 (47.4) | 31 (43.7) | 5 (18.5) | ||
| 4 | / | / | / | / | ||
| 5 | / | / | / | / | ||
| Diabetes mellitus | 21 (28.8) | 8 (21.1) | 0.380 * | 21 (29.6) | 5 (18.5) | 0.268 * |
| HTA | 53 (72.6) | 28 (73.7) | 0.903 * | 53 (74.6) | 17 (63.0) | 0.253 * |
| Leukocytes † | 6.9 (5.80−8.10) | 7.65 (5.6−8.8) | 0.218 ** | 7 (5.88−8.1) | 7.7 (5.4−8.7) | 0.811 ** |
| Erythrocytes † | 4.55 (4.29−4.89) | 4.3 (3.88−4.79) | 0.059 ** | 4.48 (4.17−4.86) | 4.57 (4.14−5.14) | 0.375 ** |
| Platelets † | 282 (228−373) | 317 (258−389) | 0.090 ** | 282 (228−375) | 285 (230−392) | 0.431 ** |
| Neutrophils † | 4.59 (3.78−5.66) | 5.18 (3.84−7.12) | 0.196 ** | 4.68 (3.86−5.77) | 5.05 (3.08−6.58) | 0.880 ** |
| Lymphocytes † | 1.54 (1.25−1.94) | 1.33 (1.05−1.84) | 0.254 ** | 1.51 (1.25−1.94) | 1.56 (1−2) | 0.990 ** |
| Monocytes † | 0.34 (0.29−0.42) | 0.35 (0.26−0.48) | 0.751 ** | 0.36 (0.29−0.44) | 0.32 (0.25−0.39) | 0.183 ** |
| NLR † | 2.89 (2.22−4.44) | 3.73 (2.21−4.8) | 0.160 ** | 2.9 (2.25−4.52) | 2.9 (1.83−4.51) | 0.833 ** |
| MLR † | 0.22 (0.17−0.29) | 0.25 (0.19−0.35) | 0.234 ** | 0.22 (0.18−0.32) | 0.22 (0.17−0.27) | 0.278 ** |
| PLR † | 188.3 (138.14−235.62) | 211.64 (153.26−324.6) | 0.056 ** | 187.5 (140.3−245.03) | 193.88 (138.97−322.45) | 0.559 ** |
| MPR † | 0.03 (0.03−0.04) | 0.03 (0.025−0.04) | 0.229 ** | 0.03 (0.03−0.43) | 0.03 (0.02−0.043) | 0.933 ** |
| RLR † | 9.375 (7.64−12.14) | 12.3 (7.56−16.82) | 0.093 ** | 9.71 (7.64−13.73) | 9.3 (7.51−14.1) | 0.759 ** |
| CRP † | 3.5 (1.7−9.8) | 10.3 (4−34.9) | 0.001 ** | 4 (2−12) | 6.1 (1.7−15.2) | 0.605 ** |
| Serum Albumin † | 40 (36−42) | 37 (33−41) | 0.005 ** | 40 (36−42) | 41 (35−42) | 1.000 ** |
| Hemoglobin † | 12.4 (10.8−13.7) | 11 (9.4−12.9) | 0.037 ** | 12 (10−13.6) | 12.3 (10.4−13.7) | 0.535 ** |
| Hematocrit † | 38.6 (35.2−41.9) | 35.2 (31.9−41.7) | 0.027 ** | 37.7 (33.6−41.7) | 38.7 (32.6−42.9) | 0.580 ** |
| RBC (RDW-CV) † | 14.2 (13.2−16.7 | 14.9 (14−19.8) | 0.041 ** | 14.7 (13.3−17.4) | 14.4 (13.2−16.7) | 0.404 ** |
| CAR † | 0.08 (0.04−0.24) | 0.257 (0.11−0.85) | 0.001 ** | 0.09 (0.05−0.32) | 0.16 (0.04−0.38) | 0.611 ** |
| PNI † | 47.3 (43.8−51.25) | 54.1 (39.6−48.6) | 0.013 ** | 46.7 (43.3−51.15) | 47 (43.1−50.85) | 0.849 ** |
| LANR † | 13.27 (8.86−18.9) | 9.85 (7.09−16.28) | 0.043 ** | 13.27 (8.69−16.89) | 12.43 (7.72−19) | 0.852 ** |
| CEA † | 2.99 (1.88−6.48) | 5.2 (2.41−10.05) | 0.030 ** | 2.76 (1.88−6.31) | 3.59 (1.94−12.74) | 0.361 ** |
| CA 19−9 † | 10.41 (6.4−27.59) | 14.33 (6.22−37.16) | 0.230 ** | 10.92 (6.28−20.84) | 12.01 (6.22−41.8) | 0.324 ** |
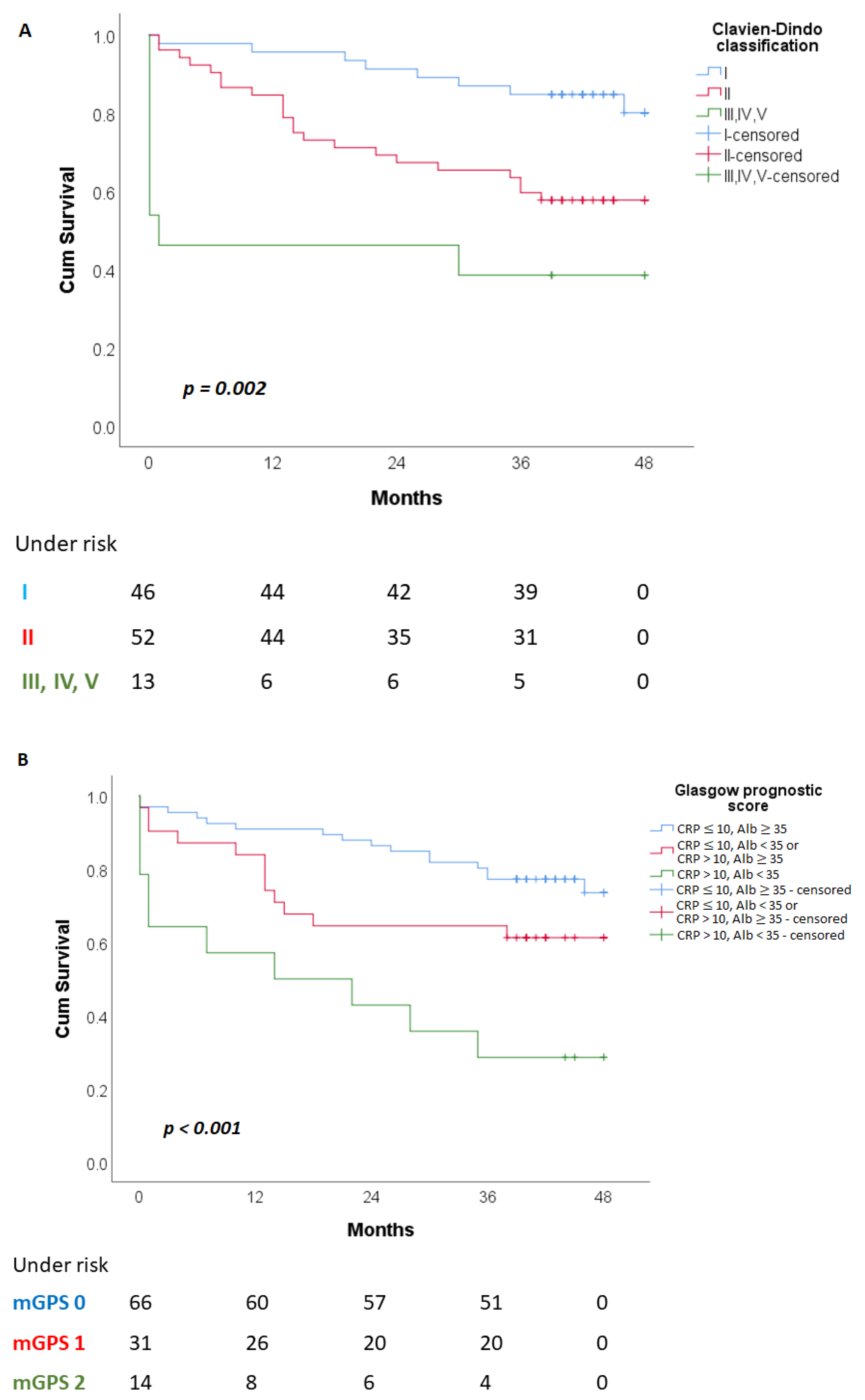
| mGPS | 0.003 * | 0.700 * | ||||
| 0 | 50 (68.5) | 16 (42.1) | 46 (64.8) | 15 (55.6) | ||
| 1 | 19 (26) | 12 (31.6) | 19 (26.8) | 9 (33.3) | ||
| 2 | 4 (5.5) | 10 (26.3) | 6 (8.5) | 3 (11.1) | ||
| Tumor Location | 0.748 * | 0.929 * | ||||
| Right Colon | 25 (34.7) | 14 (37.8) | 24 (34.3) | 9 (33.3) | ||
| Left Colon and Rectum | 47 (65.3) | 23 (62.2) | 46 (65.7) | 18 (66.7) | ||
| Max Diameter of Tumor † | 40 (30−55) | 40 (30−55) | 40 (30−52) | 40 (30−60) | 0.582 ** | |
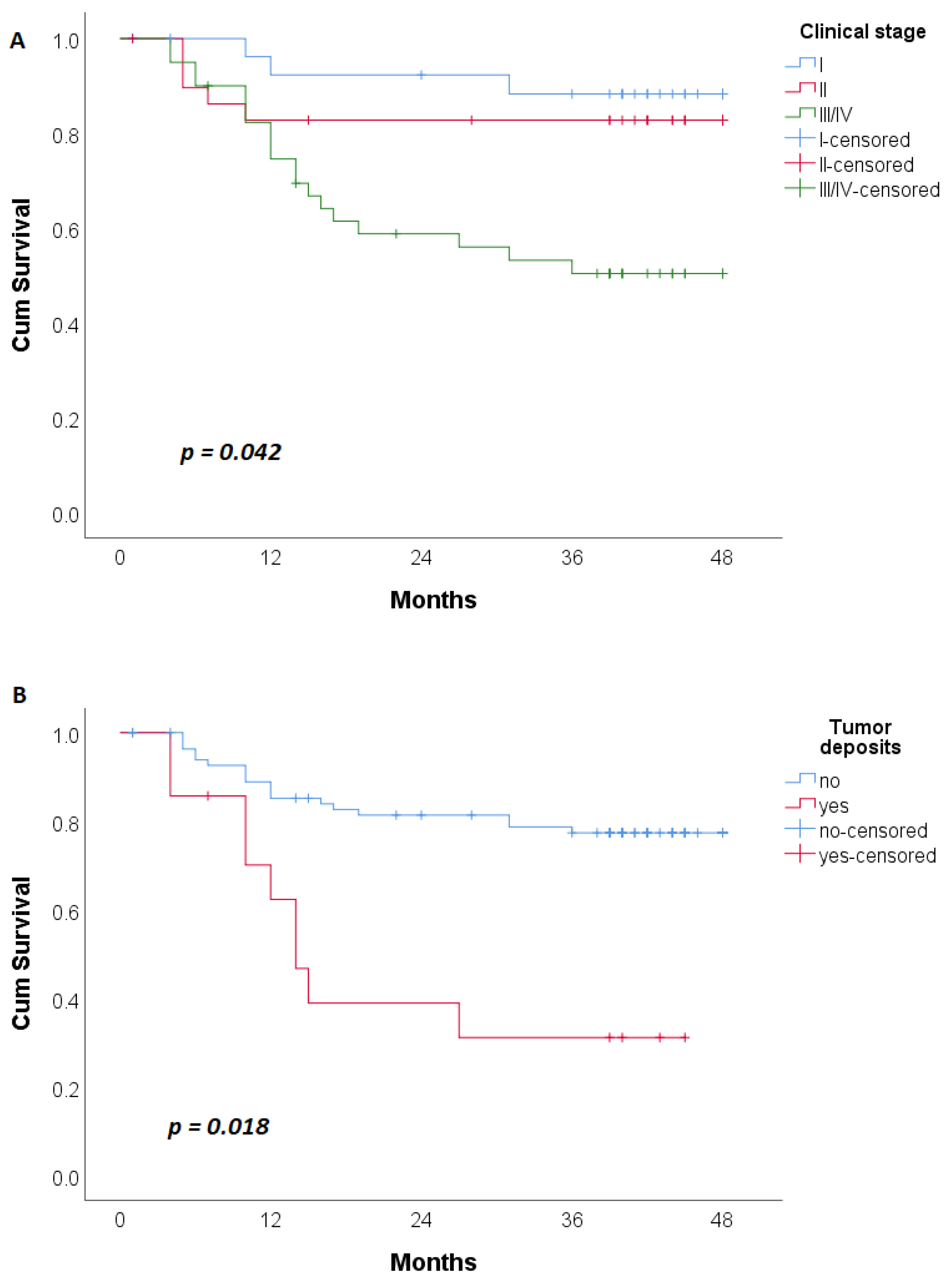
| TNM Stage | 0.009 * | <0.001 * | ||||
| I/II | 46 (63) | 14 (36.8) | 50 (70.4) | 8 (29.6) | ||
| III/IV | 27 (37) | 24 (63.2) | 21 (29.6) | 19 (70.4) | ||
| Number of Lymph Nodes † | 17 (12−21) | 14 (12−17) | 0.120 * | 15 (12−21) | 18 (13−21) | 0.444 ** |
| Positive Lymph Nodes † | 0 (0−1) | 2 (0−5) | 0.003 ** | 0 (0−1) | 1 (0−5) | 0.008 ** |
| Lymphonodal Ratio (LNR) | 0 (0−0.048) | 0.113 (0−0.353) | 0.001 ** | 0 (0−0.043) | 0 (0−0.231) | 0.008 ** |
| Tumor Configuration | 0.262 * | 0.731 * | ||||
| Exophytic | 29 (39.7) | 11 (28.9) | 29 (40.8) | 10 (37.0) | ||
| Endophytic | 44 (60.3) | 27 (71.1) | 42 (59.2) | 17 (63.0) | ||
| Tumor Gradus | 0.148 * | 0.193 * | ||||
| G1 | 10 (13.7) | 7 (18.4) | 10 (14.1) | 5 (18.5) | ||
| G2 | 60 (82.2) | 26 (68.4) | 59 (83.1) | 19 (70.4) | ||
| G3 | 3 (4.1) | 5 (13.2) | 2 (2.8) | 3 (11.1) | ||
| TIL ‡ | 0.380 * | 0.520 * | ||||
| Without/Easy to Moderate | 52 (71.2) | 30 (78.9) | 52 (73.2) | 18 (66.7) | ||
| Expressed | 21 (28.8) | 8 (21.1) | 19 (26.8) | 9 (33.3) | ||
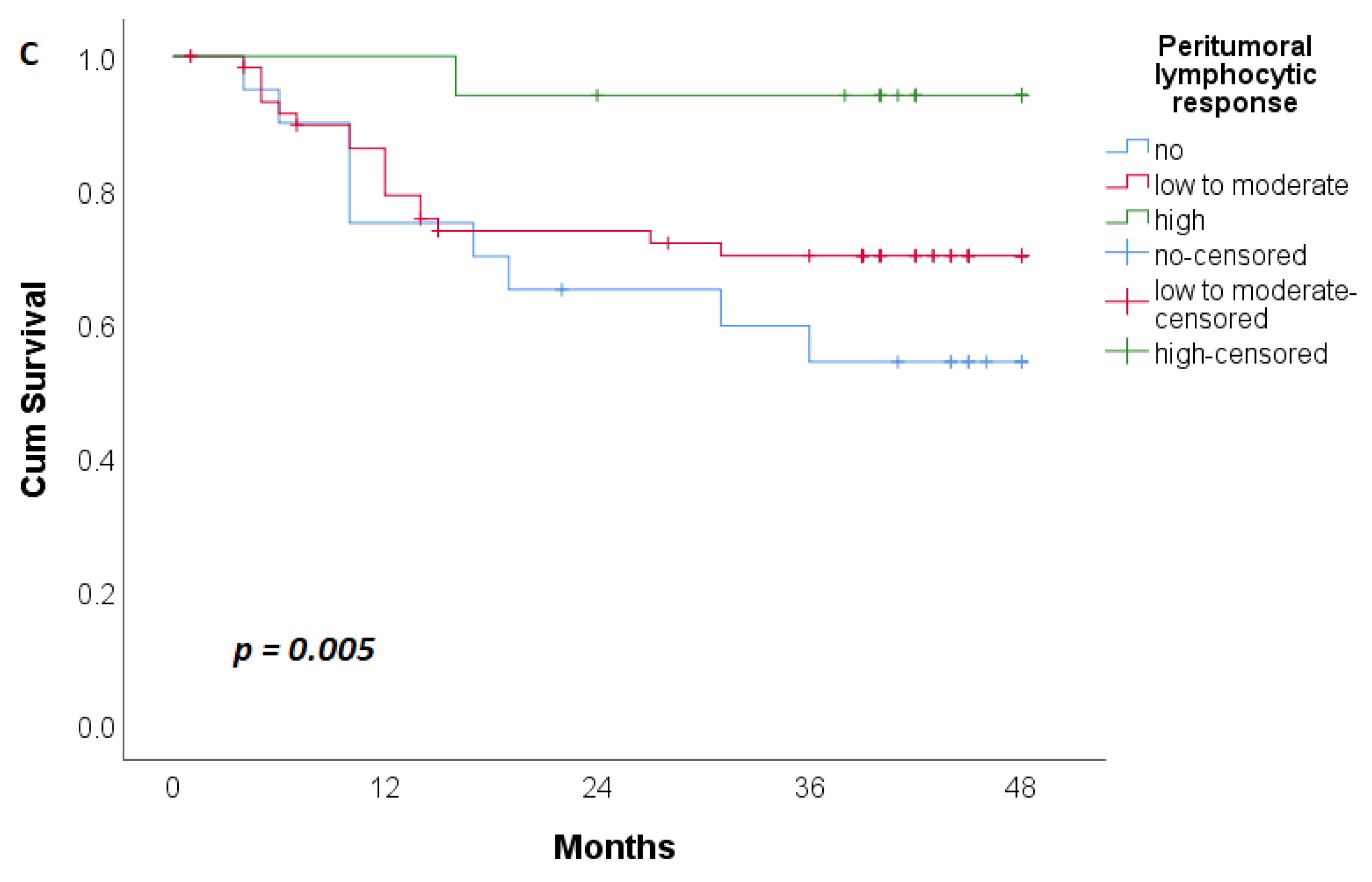
| PTL Response § | 0.019 * | 0.029 * | ||||
| Without | 14 (19.2) | 12 (31.6) | 11 (15.5) | 9 (33.3) | ||
| Easy to Moderate | 43 (58.9) | 25 (65.8) | 44 (62.0) | 17 (63.0) | ||
| Expressed | 16 (21.9) | 1 (2.6) | 16 (22.5) | 1 (3.7) | ||
| Mucosal Component of The Tumor | 0.401 * | 0.292 * | ||||
| Yes | 23 (31.5) | 15 (39.5) | 21 (29.6) | 11 (40.7) | ||
| No | 50 (68.5) | 23 (60.5) | 50 (70.4) | 16 (59.3) | ||
| Lymphovascular Invasion | 0.205 * | 0.038 * | ||||
| Yes | 45 (61.6) | 28 (73.7) | 39 (54.9) | 21 (77.8) | ||
| No | 28 (38.4) | 10 (26.3) | 32 (45.1) | 6 (22.2) | ||
| Venous Invasion | 0.015 * | 0.020 * | ||||
| Yes | 0 (0) | 3 (7.9) | 0 (0) | 2 (7.4) | ||
| No | 73 (100) | 35 (92.1) | 71 (100) | 25 (92.6) | ||
| Perineural Invasion | 0.001 * | 0.018 * | ||||
| Yes | 10 (13.7) | 16 (42.1) | 9 (12.7) | 9 (33.3) | ||
| No | 63 (86.3) | 22 (57.9) | 62 (87.3) | 18 (66.7) | ||
| Tumor Deposits | 0.001 * | 0.001a * | ||||
| Yes | 7 (9.6) | 13 (34.2) | 5 (7.0) | 9 (33.3) | ||
| No | 66 (90.4) | 25 (65.8) | 66 (93.0) | 18 (66.7) | ||
| Tumor Budding | 0.037 * | 0.838 * | ||||
| Yes | 55 (75.3) | 34 (91.9) | 56 (78.9) | 21 (80.8) | ||
| No | 18 (24.7) | 3 (8.1) | 15 (21.1) | 5 (19.2) | ||
| Tumor Growth | 0.113 * | 0.752 * | ||||
| Expansive | 32 (44.4) | 11 (28.9) | 31 (44.3) | 11 (40.7) | ||
| Infiltrative | 40 (55.6) | 27 (71.1) | 39 (55.7) | 16 (59.3) | ||
| Approach | 0.092 * | 0.926 * | ||||
| Open | 61 (83.6) | 36 (94.7) | 61 (85.9) | 23 (85.2) | ||
| Laparoscopic | 12 (16.4) | 2 (5.3) | 10 (14.1) | 4 (14.8) | ||
| C-D Clasiffication ¶ | 0.003 * | 0.598 * | ||||
| I | 38 (52.1) | 8 (21.1) | 32 (45.1) | 11 (40.7) | ||
| II | 30 (41.1) | 22 (57.9) | 33 (46.5) | 15 (55.6) | ||
| III, IV, V | 5 (6.8) | 8 (21.1) | 6 (8.5) | 1 (3.7) | ||
| Adjuvant CT # | 0.644 * | <0.001 * | ||||
| Yes | 20 (27.4) | 12 (31.6) | 13 (18.3) | 15 (55.6) | ||
| No | 53 (72.6) | 26 (68.4) | 58 (81.7) | 12 (44.4) | ||
| Neoadjuvant CRT ## | 0.619 * | 0.744 * | ||||
| Yes | 4 (5.5) | 3 (7.9) | 4 (5.6) | 2 (7.4) | ||
| No | 69 (94.5) | 35 (92.1) | 67 (94.4) | 25 (92.6) | ||
| Parameters | Univariate | p Value | Multivariate | p Value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| TNM Stage (III/IV) † | 2.430 | 1.254−4.711 | 0.009 | / | / | / |
| LNR (Higher) | 16.706 | 4.890−57.074 | <0.001 | 6.862 | 1.635−28.808 | 0.009 |
| PTL Reponse ‡ (Emphasized) | 0.531 | 0.317−0.890 | 0.016 | / | / | / |
| Perineural Invasion (Presence) | 2.988 | 1.563−5.709 | 0.001 | / | / | / |
| Tumor Deposits (Presence) | 3.254 | 1.652−6.409 | 0.001 | 3.089 | 1.447−6.593 | 0.004 |
| Tumor Budding (Presence) | 3.233 | 0.992−10.540 | 0.052 | / | / | / |
| C-D Classification Gradus III, IV, V | 2.528 | 1.574−4.061 | <0.001 | 2.609 | 1.437−4.737 | 0.002 |
| Hemoglobin (g/dL) | 0.844 | 0.723−0.985 | 0.031 | / | / | / |
| Hematocrit (%) | 0.938 | 0.885−0.994 | 0.030 | / | / | / |
| CRP (mg/L) | 1.009 | 1.003−1.016 | 0.006 | / | / | / |
| Serum Albumin (g/L) | 0.897 | 0.843−0.955 | 0.001 | / | / | / |
| CEA (ng/mL) | 1.010 | 1.000−1.019 | 0.041 | / | / | / |
| PLR | 1.002 | 1.000−1.005 | 0.045 | / | / | / |
| LANR | 0.946 | 0.898−0.996 | 0.035 | / | / | / |
| CAR | 1.335 | 1.102−1.617 | 0.003 | / | / | / |
| PNI | 0.924 | 0.877−0.974 | 0.003 | / | / | / |
| mGPS 2 | 2.145 | 1.431−3.215 | <0.001 | 2.188 | 1.413−3.387 | <0.001 |
| Parameters | Univariate | p Value | Multivariate | p Value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age | 0.961 | 0.926−0.998 | 0.041 | / | / | / |
| TNM Stage (III/IV) † | 2.486 | 1.390−4.445 | 0.002 | 1.888 | 1.024−3.481 | 0.042 |
| LNR (Higher) | 5.588 | 0.835−37.388 | 0.076 | / | / | / |
| PTL Response (Emphasized) | 0.465 | 0.252−0.858 | 0.014 | 0.391 | 0.196−0.780 | 0.005 |
| Lymphovascular Invasion (Presence) | 2.322 | 0.936−5.756 | 0.069 | / | / | / |
| Perineural Invasion (Presence) | 2.374 | 1.064−5.299 | 0.035 | / | / | / |
| Tumor Deposits (Presence) | 4.194 | 1.869−9.411 | 0.001 | 3.049 | 1.206−7.706 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuk, V.; Karamarkovic, A.; Juloski, J.; Arbutina, D.; Radulovic, R.; Milic, L.; Kovacevic, B.; De Luka, S.; Grahovac, J. Prognostic Value of Combined Hematological/Biochemical Indexes and Tumor Clinicopathologic Features in Colorectal Cancer Patients—A Pilot Single Center Study. Cancers 2023, 15, 1761. https://doi.org/10.3390/cancers15061761
Cuk V, Karamarkovic A, Juloski J, Arbutina D, Radulovic R, Milic L, Kovacevic B, De Luka S, Grahovac J. Prognostic Value of Combined Hematological/Biochemical Indexes and Tumor Clinicopathologic Features in Colorectal Cancer Patients—A Pilot Single Center Study. Cancers. 2023; 15(6):1761. https://doi.org/10.3390/cancers15061761
Chicago/Turabian StyleCuk, Vladica, Aleksandar Karamarkovic, Jovan Juloski, Dragana Arbutina, Radosav Radulovic, Ljiljana Milic, Bojan Kovacevic, Silvio De Luka, and Jelena Grahovac. 2023. "Prognostic Value of Combined Hematological/Biochemical Indexes and Tumor Clinicopathologic Features in Colorectal Cancer Patients—A Pilot Single Center Study" Cancers 15, no. 6: 1761. https://doi.org/10.3390/cancers15061761
APA StyleCuk, V., Karamarkovic, A., Juloski, J., Arbutina, D., Radulovic, R., Milic, L., Kovacevic, B., De Luka, S., & Grahovac, J. (2023). Prognostic Value of Combined Hematological/Biochemical Indexes and Tumor Clinicopathologic Features in Colorectal Cancer Patients—A Pilot Single Center Study. Cancers, 15(6), 1761. https://doi.org/10.3390/cancers15061761





