Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Management
2.2. Lymphatic Mapping, Sentinel Lymph Node Biopsy, and Axillary Treatment
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients and Axillary Surgery
3.2. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef]
- Veronesi, U.; Viale, G.; Paganelli, G.; Zurrida, S.; Luini, A.; Galimberti, V.; Veronesi, P.; Intra, M.; Maisonneuve, P.; Zucca, F.; et al. Sentinel Lymph Node Biopsy in Breast Cancer: Ten-Year Results: Of a Randomized Controlled Study. Ann. Surg. 2010, 251, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary Dissection vs No Axillary Dissection in Women with Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2017, 318, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Gentile, D.; Gatzemeier, W.; Sagona, A.; Barbieri, E.; Testori, A.; Errico, V.; Bottini, A.; Marrazzo, E.; Dani, C.; et al. Preservation of Axillary Lymph Nodes Compared with Complete Dissection in T1–2 Breast Cancer Patients Presenting One or Two Metastatic Sentinel Lymph Nodes: The SINODAR-ONE Multicenter Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 5732–5744. [Google Scholar] [CrossRef]
- Montagna, G.; Mamtani, A.; Knezevic, A.; Brogi, E.; Barrio, A.V.; Morrow, M. Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2020, 27, 4515–4522. [Google Scholar] [CrossRef]
- Fisher, C.S.; Margenthaler, J.A.; Hunt, K.K.; Schwartz, T. The Landmark Series: Axillary Management in Breast Cancer. Ann. Surg. Oncol. 2020, 27, 724–729. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA-J. Am. Med. Assoc. 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicentre Cohort Study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J. Clin. Oncol. 2015, 33, 258–263. [Google Scholar] [CrossRef]
- Cohen, L.F.; Breslin, T.M.; Kuerer, H.M.; Ross, M.I.; Hunt, K.K.; Sahin, A.A. Identification and Evaluation of Axillary Sentinel Lymph Nodes in Patients with Breast Carcinoma Treated with Neoadjuvant Chemotherapy. Am. J. Surg. Pathol. 2000, 24, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.K.M.; Goh, B.K.P.; Fook-Chong, S.; Khin, L.W.; Wong, W.K.; Yong, W.S. The Feasibility and Accuracy of Sentinel Lymph Node Biopsy in Clinically Node-Negative Patients after Neoadjuvant Chemotherapy for Breast Cancer—A Systematic Review and Meta-Analysis. J. Surg. Oncol. 2011, 104, 97–103. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Brown, A.; Anderson, S.; Smith, R.; Julian, T.; Miller, B.; Bear, H.D.; Caldwell, C.B.; Walker, A.P.; Mikkelson, W.M.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2005, 23, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Foy, M.; Cox, D.D.; Kuerer, H.M.; Hunt, K.K.; Cormier, J.N. Meta-Analysis of Sentinel Lymph Node Biopsy after Preoperative Chemotherapy in Patients with Breast Cancer. Br. J. Surg. 2006, 93, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Terribile, D.; Franco, A.; Martullo, A.; Orlandi, A.; Magno, S.; Di Leone, A.; Moschella, F.; Natale, M.; D’archi, S.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment. J. Pers. Med. 2021, 11, 172. [Google Scholar] [CrossRef]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Neoadjuvant Chemotherapy Use in Breast Cancer Is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann. Surg. Oncol. 2018, 25, 2241–2248. [Google Scholar] [CrossRef]
- Takada, M.; Toi, M. Neoadjuvant Treatment for HER2-Positive Breast Cancer. Chinese Clin. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-Positive Breast Cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Newman, E.A.; Sabel, M.S.; Nees, A.V.; Schott, A.; Diehl, K.M.; Cimmino, V.M.; Chang, A.E.; Kleer, C.; Hayes, D.F.; Newman, L.A. Sentinel Lymph Node Biopsy Performed after Neoadjuvant Chemotherapy Is Accurate in Patients with Documented Node-Positive Breast Cancer at Presentation. Ann. Surg. Oncol. 2007, 14, 2946–2952. [Google Scholar] [CrossRef]
- Van Nijnatten, T.J.A.; Schipper, R.J.; Lobbes, M.B.I.; Nelemans, P.J.; Beets-Tan, R.G.H.; Smidt, M.L. The Diagnostic Performance of Sentinel Lymph Node Biopsy in Pathologically Confirmed Node Positive Breast Cancer Patients after Neoadjuvant Systemic Therapy: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2015, 41, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.M.; Van Nijnatten, T.J.A.; Van Der Pol, C.C.; Luiten, E.J.T.; Koppert, L.B.; Smidt, M.L. Diagnostic Accuracy of Different Surgical Procedures for Axillary Staging after Neoadjuvant Systemic Therapy in Node-Positive Breast Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. 2019, 269, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; Desnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patientswith Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.T.; et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-Positive Breast Cancer Patients. Ann. Surg. 2022, 276, E553–E562. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, P.K.; Tayeh, S.; Michell, M.J.; Mokbel, K. The Evolving Role of Marked Lymph Node Biopsy (Mlnb) and Targeted Axillary Dissection (Tad) after Neoadjuvant Chemotherapy (Nact) for Node-positive Breast Cancer: Systematic Review and Pooled Analysis. Cancers 2021, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Kahler-Ribeiro-Fontana, S.; Pagan, E.; Magnoni, F.; Vicini, E.; Morigi, C.; Corso, G.; Intra, M.; Canegallo, F.; Ratini, S.; Leonardi, M.C.; et al. Long-Term Standard Sentinel Node Biopsy after Neoadjuvant Treatment in Breast Cancer: A Single Institution Ten-Year Follow-Up. Eur. J. Surg. Oncol. 2020, 47, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody, H.S.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients with Node-Positive Breast Cancer Treated with Sentinel Node Biopsy Alone after Neoadjuvant Chemotherapy—A Rare Event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar] [CrossRef]
- Martelli, G.; Barretta, F.; Miceli, R.; Folli, S.; Maugeri, I.; Listorti, C.; Scaperrotta, G.; Baili, P.; Pruneri, G.; Capri, G.; et al. Sentinel Node Biopsy Alone or With Axillary Dissection in Breast Cancer Patients After Primary Chemotherapy: Long-Term Results of a Prospective Interventional Study. Ann. Surg. 2022, 276, E544–E552. [Google Scholar] [CrossRef] [PubMed]
- Piltin, M.A.; Hoskin, T.L.; Day, C.N.; Davis, J.; Boughey, J.C. Oncologic Outcomes of Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Ann. Surg. Oncol. 2020, 27, 4795–4801. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Tinterri, C.; Canavese, G.; Bruzzi, P.; Dozin, B. NEONOD 2: Rationale and Design of a Multicenter Non-Inferiority Trial to Assess the Effect of Axillary Surgery Omission on the Outcome of Breast Cancer Patients Presenting Only Micrometastasis in the Sentinel Lymph Node after Neoadjuvant Chemotherapy. Contemp. Clin. Trials Commun. 2020, 17, 100496. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Aebi, S.; et al. Customizing Local and Systemic Therapies for Women with Early Breast Cancer: The St. Gallen International Consensus Guidelines for Treatment of Early Breast Cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Diego, E.J.; McAuliffe, P.F.; Soran, A.; McGuire, K.P.; Johnson, R.R.; Bonaventura, M.; Ahrendt, G.M. Axillary Staging after Neoadjuvant Chemotherapy for Breast Cancer: A Pilot Study Combining Sentinel Lymph Node Biopsy with Radioactive Seed Localization of Pre-Treatment Positive Axillary Lymph Nodes. Ann. Surg. Oncol. 2016, 23, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Reimer, T.; Gerber, B.; Stubert, J.; Stengel, B.; Stachs, A. Wire Localization of Clip-Marked Axillary Lymph Nodes in Breast Cancer Patients Treated with Primary Systemic Therapy. Eur. J. Surg. Oncol. 2018, 44, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hieken, T.J.; Glazebrook, K.N.; Boughey, J.C. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann. Surg. Oncol. 2017, 24, 3011–3016. [Google Scholar] [CrossRef]
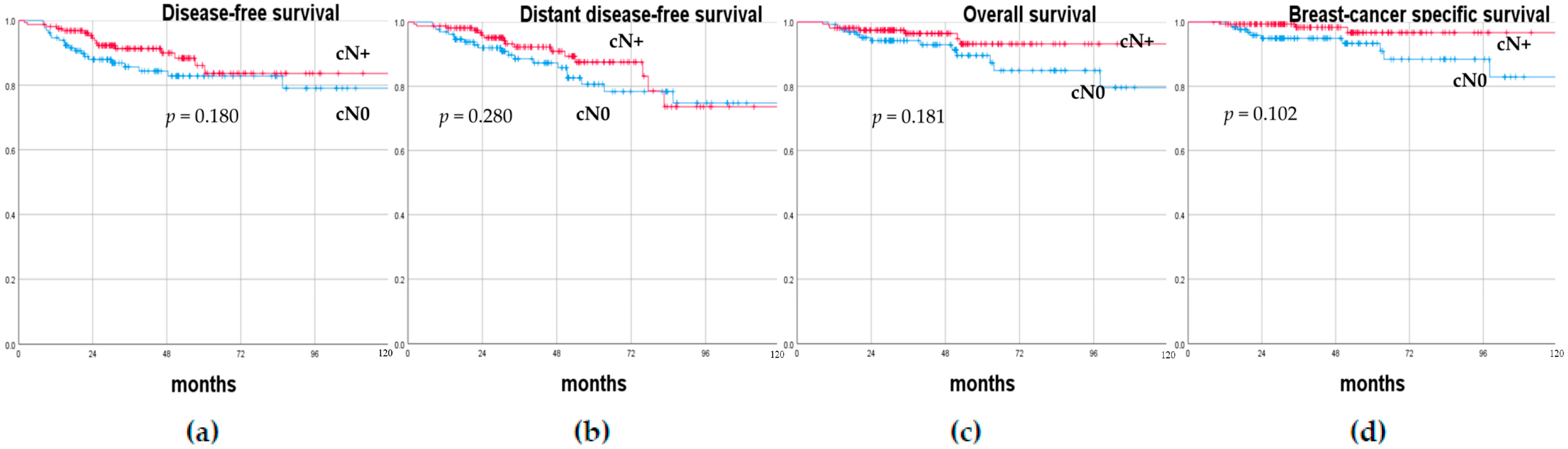
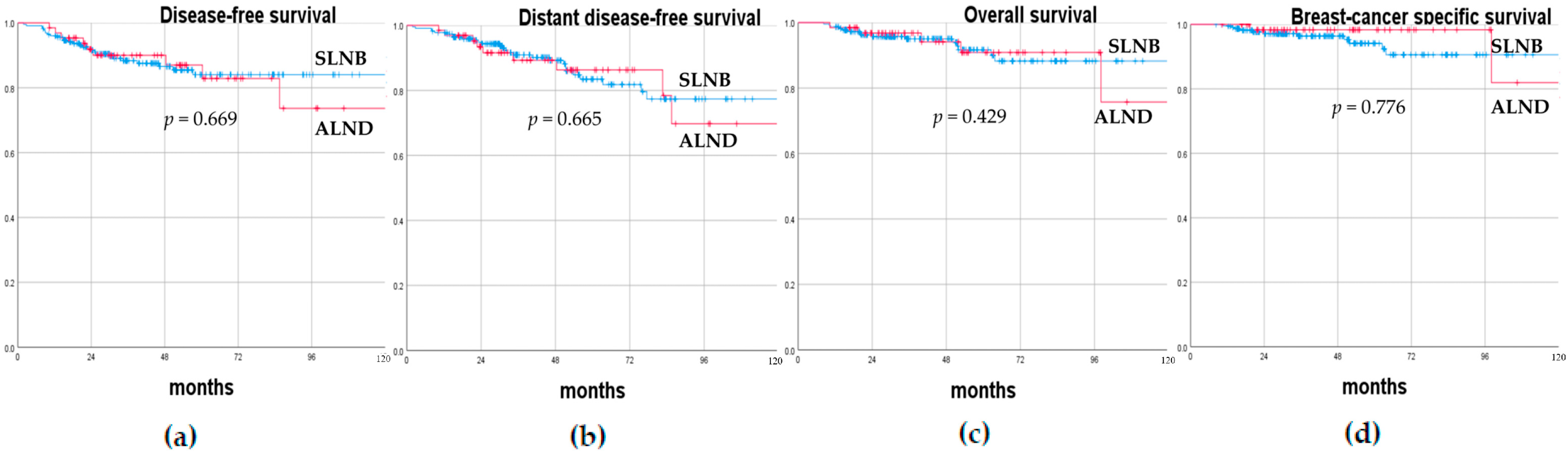
| cN0 (No. 131) Tot. (%)/ Median (Range) | cN+ (No. 160) Tot. (%)/ Median (Range) | Univariate Analysis p-Value | |
| Patient | |||
| Age (years) | 48 (28–79) | 50 (29–87) | 0.144 |
| Post-menopausal | 63 (48.1%) | 81 (50.6%) | 0.668 |
| Tumor | |||
| Dimension pre-NAC (mm) | 30 (12–90) | 30 (7–100) | 0.288 |
| Clinical T pre-NAC | |||
| 0 (0%) | 1 (0.6%) | 0.627 |
| 23 (17.6%) | 24 (15.0%) | - |
| 89 (67.9%) | 110 (68.8%) | - |
| 15 (11.5%) | 19 (11.9%) | - |
| 4 (3.0%) | 6 (3.7%) | - |
| 0 (0%) | 2 (1.3%) | 0.200 |
| Multifocality/multicentricity | 30 (22.9%) | 55 (34.4%) | 0.469 |
| Anthracycline only | 10 (7.6%) | 7 (4.4%) | 0.094 |
| Anthracycline and taxanes | 67 (51.2%) | 75 (46.9%) | - |
| Anthracycline, taxanes, and Trastuzumab | 53 (40.5%) | 75 (46.9%) | - |
| Anthracycline, taxanes, and Pertuzumab | 1 (0.7%) | 2 (1.3%) | - |
| CDK inhibitor | 0 (0%) | 1 (0.6%) | - |
| Subtype | |||
| 32 (24.4%) | 43 (26.9%) | 0.715 |
| 56 (42.8%) | 78 (48.8%) | - |
| 43 (32.8%) | 39 (24.3%) | - |
| Histotype | |||
| 127 (97.0%) | 151 (94.4%) | 0.366 |
| 2 (1.5%) | 7 (4.4%) | - |
| 2 (1.5%) | 1 (0.6%) | - |
| 0 (0%) | 1 (0.6%) | - |
| Vascular invasion | 22 (16.8%) | 25 (15.6%) | 0.788 |
| Ki67 | 15 (2–90) | 10 (1–85) | 0.091 |
| Dimension post-NAC (mm) | 8 (0–70) | 7 (0–60) | 0.086 |
| Pathological T post-NAC | |||
| 22 (16.8%) | 38 (23.8%) | 0.051 |
| 19 (14.5%) | 20 (12.5%) | - |
| 2 (1.5%) | 8 (5.0%) | - |
| 8 (6.1%) | 8 (5.0%) | - |
| 22 (16.8%) | 26 (16.3%) | - |
| 27 (20.6%) | 39 (24.3%) | - |
| 28 (21.5%) | 20 (12.5%) | - |
| 2 (1.5%) | 1 (0.6%) | - |
| 1 (0.7%) | 0 (0%) | - |
| Surgery | |||
| 81 (61.8%) | 100 (62.5%) | 0.907 |
| 50 (38.2%) | 60 (37.5%) | - |
| 18 (13.7%) | 47 (29.4%) | 0.001 a |
| Post-operative treatment | |||
| 20 (15.3%) | 18 (11.3%) | 0.313 |
| 97 (74.1%) | 133 (83.1%) | 0.059 |
| 66 (50.4%) | 86 (53.8%) | 0.569 |
| 34 (26.0%) | 42 (26.3%) | 0.434 |
| cN0 (No. 131) Tot. (%)/ Median (Range) | cN+ (No. 160) Tot. (%)/ Median (Range) | Univariate Analysis p-Value | |
| Intra-operative SLN status | |||
| Number of SLNs | 1 (1–5) | 1 (1–6) | 0.004 a |
| Number of patients with positive SLNs | 19 (14.5%) | 58 (36.3%) | <0.001 a |
| Pathological N post-NAC | |||
| 112 (85.5%) | 102 (63.8%) | <0.001 a |
| 0 (0%) | 1 (0.6%) | - |
| 5 (3.8%) | 12 (7.5%) | - |
| 11 (8.4%) | 29 (18.1%) | - |
| 1 (0.8%) | 13 (8.1%) | - |
| 2 (1.5%) | 3 (1.9%) | - |
| Non-SLN status at pathological evaluation | |||
| Number of evaluated non-SLNs | 12 (3–28) | 13 (5–27) | 0.903 |
| Number of positive non-SLNs | 0 (0–13) | 1 (0–26) | 0.516 |
| Number of patients with 1 positive non-SLN | 3 (2.3%) | 7 (4.4%) | 0.140 |
| Number of patients with 2 positive non-SLNs | 1 (0.8%) | 6 (3.7%) | - |
| Number of patients with 3 positive non-SLNs | 0 (0%) | 3 (1.9%) | - |
| Number of patients with >3 positive non-SLNs | 3 (2.3%) | 12 (7.5%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinterri, C.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Caraceni, G.; Ambrogi, G.; Jacobs, F.; Biondi, E.; Scardina, L.; Gentile, D. Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy. Cancers 2023, 15, 1719. https://doi.org/10.3390/cancers15061719
Tinterri C, Sagona A, Barbieri E, Di Maria Grimaldi S, Caraceni G, Ambrogi G, Jacobs F, Biondi E, Scardina L, Gentile D. Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy. Cancers. 2023; 15(6):1719. https://doi.org/10.3390/cancers15061719
Chicago/Turabian StyleTinterri, Corrado, Andrea Sagona, Erika Barbieri, Simone Di Maria Grimaldi, Giulia Caraceni, Giacomo Ambrogi, Flavia Jacobs, Ersilia Biondi, Lorenzo Scardina, and Damiano Gentile. 2023. "Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy" Cancers 15, no. 6: 1719. https://doi.org/10.3390/cancers15061719
APA StyleTinterri, C., Sagona, A., Barbieri, E., Di Maria Grimaldi, S., Caraceni, G., Ambrogi, G., Jacobs, F., Biondi, E., Scardina, L., & Gentile, D. (2023). Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy. Cancers, 15(6), 1719. https://doi.org/10.3390/cancers15061719








