Safety and Quality of Life in Women with Immediate Reconstruction with Polyurethane Implants after Neoadjuvant Chemotherapy: Outcomes from The Preq-20 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Method
2.1. Inclusion and Exclusion Criteria
- Study group: Patients diagnosed with infiltrating breast carcinoma who required systemic therapy with chemotherapy (with or without antibodies) prior to surgery. The indication for primary systemic therapy (PST) was agreed upon by the Breast Unit’s Tumour Committee. In the manuscript, the authors use NAC and PST as synonyms to define the patients in the study group who received chemotherapy (with or without antibodies) before surgery.
- Control group 1: Patients diagnosed with infiltrating breast carcinoma who underwent adjuvant systemic therapy with chemotherapy (with or without antibodies) after surgery.
- Control group 2: Patients diagnosed with an infiltrating carcinoma or in situ ductal carcinoma who did not require chemotherapy. In this group of women, the Tumour Committee assessed the indication for radiation therapy and adjuvant hormone therapy according to each patient’s staging and biological characteristics.
2.2. Preoperative Evaluation
2.3. Surgical Method
2.4. Postoperative Follow-Up
2.5. Neoadjuvant and Adjuvant Therapies
2.6. Evaluation of Satisfaction and Quality of Life
2.7. Statistical Method
3. Results
3.1. Postoperative Complications
3.2. Bilateral Mastectomy
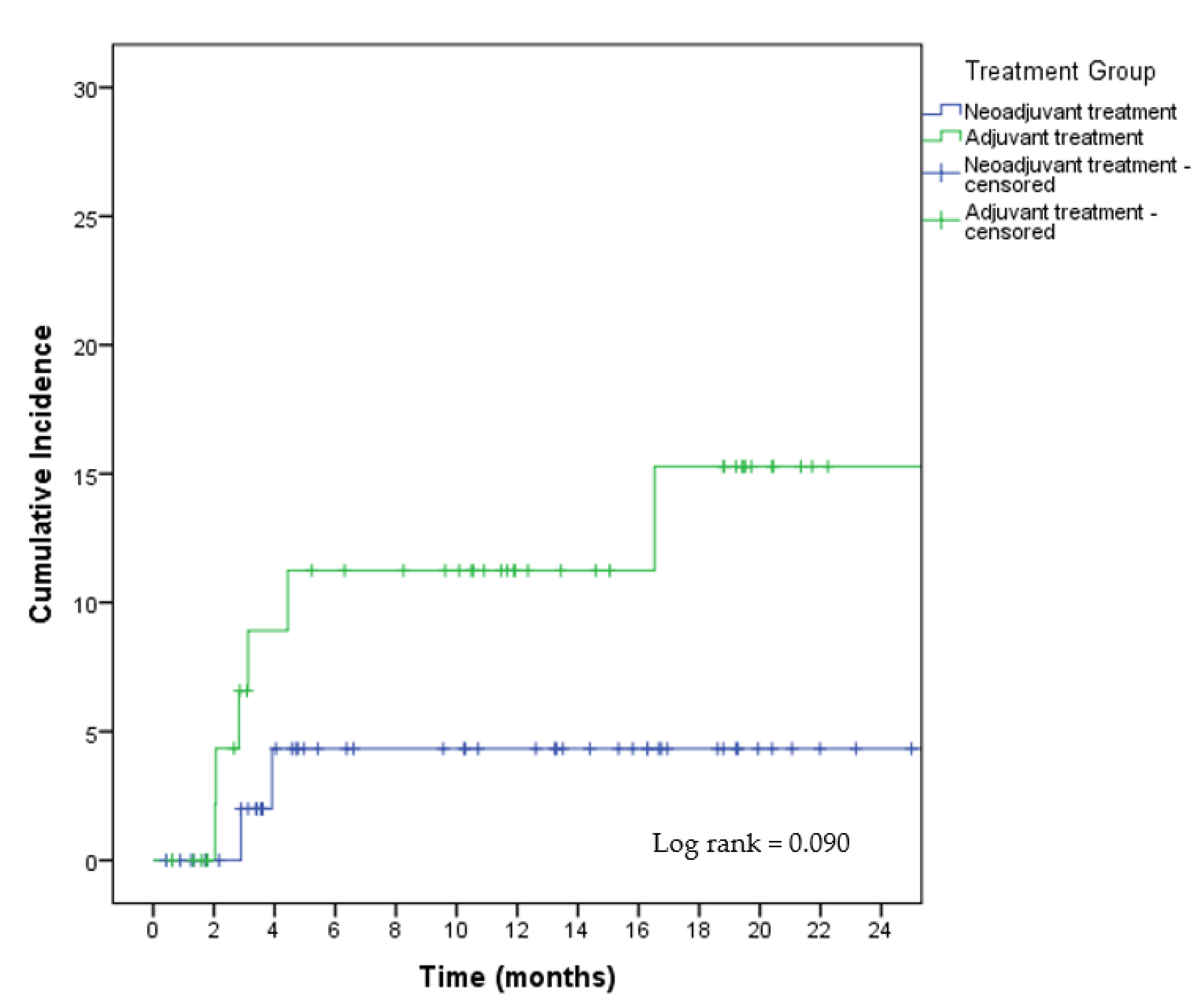
3.3. Oncologic Safety
3.4. Satisfaction and Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholl, S.M.; Fourquet, A.; Asselain, B.; Pierga, J.Y.; Vilcoq, J.R.; Durand, J.C.; Dorval, T.; Palangie, T.; Jouve, M.; Beuzeboc, P. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: Preliminary results of a randomized trial: S6. Eur. J. Cancer 1994, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998, 16, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Mauriac, L.; Macgrogan, G.; Avril, A.; Durand, M.; Floquet, A.; Debled, M.; Dilhuydy, J.M.; Bonichon, F.; Sein, I.B.B.G. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicenter randomized trial with a 124 month median follow up. Am. Oncol. 1999, 10, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, T.; Heidemann, L.N.; Möller, S.; Bille, C. Impact of neoadjuvant chemotherapy on surgical complications in breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 44–52. [Google Scholar] [CrossRef]
- Varghese, J.; Gohari, S.S.; Rizki, H.; Faheem, M.; Langridge, B.; Kümmel, S.; Johnson, L.; Schmid, P. A systematic review and meta-analysis on the effect of neoadjuvant chemotherapy on complications following immediate breast reconstruction. Breast 2021, 55, 55–62. [Google Scholar] [CrossRef]
- Song, J.; Zhang, X.; Liu, Q.; Peng, J.; Liang, X.; Shen, Y.; Liu, H.; Li, H. Impact of neoadjuvant chemotherapy on immediate breast reconstruction: A meta-analysis. PLoS ONE 2014, 9, e98225. [Google Scholar] [CrossRef]
- Adachi, Y.; Okumura, S.; Sawaki, M.; Hattori, M.; Yoshimura, A.; Gondo, N.; Kotani, H.; Iwase, M.; Kataoka, A.; Sugino, K.; et al. Effects of neoadjuvant chemotherapy on operative adverse events and chemotherapy and radiotherapy in patients undergoing immediate breast reconstruction. Breast Cancer 2020, 27, 716–723. [Google Scholar] [CrossRef]
- Ranisavljevic, M.; Selakovic, V.; Lukic, D.; Radovanovic, Z.; Vicko, F. Impact of neoadjuvant chemotherapy on wound complications after breast surgery. Arch. Oncol. 2013, 21, 105–108. [Google Scholar] [CrossRef]
- Frey, J.; Choi, M.; Karp, N. The effect of neoadjuvant chemotherapy compared to adjuvant chemotherapy in healing after nipple-sparing mastectomy. Plast. Reconstr. Surg. 2017, 139, 10e–19e. [Google Scholar] [CrossRef]
- Mégevand, V.; Scampa, M.; McEvoy, H.; Kalbermatten, D.F.; Oranges, C.M. Comparison of Outcomes Following Prepectoral and Subpectoral Implants for Breast Reconstruction: Systematic Review and Meta-Analysis. Cancers 2022, 14, 4223. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Xiu, B.; Huang, X.; Chi, W.; Hou, J.; Zhang, Y.; Tian, J.; Wang, J.; Wu, J. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: A systematic review and meta analysis. Eur. J. Surg. Oncol. 2019, 45, 1542–1550. [Google Scholar] [CrossRef]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Wimmer, K.; Fitzal, F. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann. Surg. Oncol. 2023, 30, 126–136. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04642508?term=PREQ&draw=2&rank=1 (accessed on 8 February 2023).
- Acea-Nebril, B.; García-Novoa, A.; García Jiménez, L. The PreQ-20 TRIAL: A prospective cohort study of the oncologic safety, quality of life and cosmetic outcomes of patients undergoing prepectoral breast reconstruction. PLoS ONE 2022, 17, e0269426. [Google Scholar] [CrossRef]
- Nava, M.B.; Catanuto, G.; Rocco, N. How to optimize aesthetic outcomes in implant-based breast reconstruction. Arch. Plast. Surg. 2018, 45, 4–13. [Google Scholar] [CrossRef]
- Carlson, G.W.; Bostwick, J., 3rd; Styblo, T.M.; Moore, B.; Bried, J.T.; Murray, D.R.; Wood, W.C. Skin-sparing Mastectomy. Oncologic and Reconstructive Considerations. Ann. Surg. 1997, 225, 570–575. [Google Scholar] [CrossRef]
- Rancati, A.; Angrigiani, C.; Hammond, D.; Nava, M.; Gonzalez, E.; Rostagno, R.; Gercovich, G. Preoperative digital mammography imaging in conservative mastectomy and immediate reconstruction. Gland Surg. 2016, 5, 9–14. [Google Scholar]
- Folli, S.; Curcio, A.; Buggi, F.; Mingozzi, M.; Asioli, S.; Nava, M.B. Improved sub-areolar breast tissue removal in nipple-sparing mastectomy using hydrodissection. Breast 2012, 21, 190–193. [Google Scholar] [CrossRef]
- eProvide. Available online: https://eprovide.mapi-trust.org/instruments/breast-q#basic_description (accessed on 8 February 2023).
- Schaverien, M.V.; Munnoch, D.A. Effect of neoadjuvant chemotherapy on outcomes of immediate free autologous breast reconstruction. Eur. J. Surg. Oncol. 2013, 39, 430–436. [Google Scholar] [CrossRef]
- Bertomeu, M.C.; Gallo, S.; Lauri, D.; Levine, M.N.; Orr, F.W.; Buchanan, M.R. Chemotherapy enhances endothelial cell reactivity to platelets. Clin. Exp. Metastasis 1990, 8, 511–518. [Google Scholar] [CrossRef]
- Haddad, T.C.; Greeno, E.W. Chemotherapy-induced thrombosis. Thromb. Res. 2006, 118, 555–568. [Google Scholar] [CrossRef]
- Ian, N.; Quah, G.S.; Graham, S.; Kanesalingam, K.; Meybodi, F.; Hsu, J.; Elder, E.E.; French, J. Immediate prepectoral implant reconstruction using TiLOOP Bra Pocket results in improved patient satisfaction over dual plane reconstruction. ANZ J. Surg. 2021, 91, 701–707. [Google Scholar]
- Nguyen-Sträuli, B.D.; Vorburger, D.; Frauchiger-Heuer, H.; Bringolf, L.; Maggi, N.; Talimi-Schnabel, J.; Dedes, K.J. Prepectoral implant-based breast reconstruction with TiLOOP® Bra Pocket—A single-centre retrospective study. J. Plast. Reconstr. Aesthetic Surg. 2021, 75, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.; Di Taranto, G.; Marcasciano, M.; Sordi, S.; Kothari, A.; Kovacs, T.; Lo Torto, F.; Cigna, E.; Calabrese, C.; Ribuffo, D. Evaluation of Prepectoral Implant Placement and Complete Coverage with TiLoop Bra Mesh for Breast Reconstruction: A Prospective Study on Long-Term and Patient-Reported BREAST-Q Outcomes. Plast. Reconstr. Surg. 2019, 143, 1e–9e. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.G.; Irri, R.; MacCallum, V.; Chattopadhyay, R.; Murphy, J.; Harvey, J.R. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast. Reconstr. Surg. 2018, 141, 1077–1084. [Google Scholar] [CrossRef]
- Urquia, L.N.; Hart, A.M.; Liu, D.Z.; Losken, A. Surgical Outcomes in Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2744. [Google Scholar] [CrossRef]
- Ribuffo, D.; Berna, G.; De Vita, R.; Di Benedetto, G.; Cigna, E.; Lo Torto, F.; Scalise, A. Dual-Plane Retro-pectoral Versus Pre-pectoral DTI Breast Reconstruction: An Italian Multicenter Experience. Aesthetic Plast. Surg. 2021, 45, 51–60. [Google Scholar] [CrossRef]
- Highton, L.; Johnson, R.; Kirwan, C.; Murphy, J. Prepectoral Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1488. [Google Scholar] [CrossRef]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef]
- Masiá, J.; iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J. Surg. Oncol. 2020, 122, 848–860. [Google Scholar] [CrossRef]
- Chandarana, M.; Harries, S.; National Braxon Audit Study Group. Multicentre study of prepectoral breast reconstruction using acellular dermal matrix. BJS Open 2020, 4, 71–77. [Google Scholar] [CrossRef]
- Casella, D.; Kaciulyte, J.; Lo Torto, F.; Mori, F.L.R.; Barellini, L.; Fausto, A.; Fanelli, B.; Greco, M.; Ribuffo, D.; Marcasciano, M. “To Pre or Not to Pre”: Introduction of a Prepectoral Breast Reconstruction Assessment Score to Help Surgeons Solving the Decision-Making Dilemma. Retrospective Results of a Multicenter Experience. Plast. Reconstr. Surg. 2021, 147, 1278–1286. [Google Scholar] [CrossRef]
- Kurian, A.W.; Lichtensztajn, D.Y.; Keegan, T.H.; Nelson, D.O.; Clarke, C.A.; Gomez, S.L. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA 2014, 312, 902–914. [Google Scholar] [CrossRef]
- Pimentel, F.; De Barros, P.; Alves, M. Trend in bilateral mastectomy for cases of unilateral breast cancer in a brazilian institute over a 10-year period. Mastology 2021, 31, e20210030. [Google Scholar]
- Lim, D.W.; Metcalfe, K.A.; Narod, S.A. Bilateral Mastectomy in Women with Unilateral Breast Cancer. A Review. JAMA Surg. 2021, 156, 569–576. [Google Scholar] [CrossRef]
- Pollon, E.; Oían, Y.; Chin, A.; Dirbas, F.; Asch, S.; Kurian, A. Rising rates of bilateral mastectomy with reconstruction following neoadjuvant chemotherapy. Int. J. Cancer 2018, 143, 3262–3272. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Dayan, E. Improving Communication with Millennial Patients. Plast. Reconstr. Surg. 2019, 144, 533–535. [Google Scholar] [CrossRef]
- Marmor, R.A.; Dai, W.; Jiang, X.; Wang, S.; Blair, S.L.; Huh, J. Increase in contralateral prophylactic mastectomy conversation online unrelated to decision-making. J. Surg. Res. 2017, 218, 253–260. [Google Scholar] [CrossRef]
- Marcasciano, M.; Frattaroli, J.; Mori, F.L.; Lo Torto, F.; Fioramonti, P.; Cavalieri, E.; Kaciulyte, J.; Greco, M.; Casella, D.; Ribuffo, D. The New Trend of Pre-pectoral Breast Reconstruction: An Objective Evaluation of the Quality of Online Information for Patients Undergoing Breast Reconstruction. Aesthetic Plast. Surg. 2019, 43, 593–599. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Acea-Nebril, B.; García-Novoa, A.; Cereijo-Garea, C.; Builes-Ramirez, S.; Bouzon-Alejandro, A.; Mosquera-Oses, J. Single-Incision Approach for Breast-Conserving Surgery: Effectiveness, Complications and Quality of Life. Ann. Surg. Oncol. 2019, 26, 2466–2474. [Google Scholar] [CrossRef]
- Acea Nebril, B.; García Novoa, A.; Polidorio, N.; Cereijo Garea, C.; Bouzón Alejandro, A.; Mosquera Oses, J. Extreme Oncoplasty: The Last Opportunity for Breast conservation-Analysis of Its Impact on Survival and Quality of Life. Breast J. 2019, 25, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Acea-Nebril, B.; Cereijo-Garea, C.; García-Novoa, A.; Bouzon-Alejandro, A.; Mosquera-Oses, J. Breast-Q 15 Prospective Study: Oncoplastic Breast Reduction Improve Quality of Live for Women with Macromastia. Breast J. 2020, 26, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Cereijo, C.; Pita-Fernández, S.; Acea Nebril, B.; García Novoa, A. Predictive factors of satisfaction and quality of life after immediate breast reconstruction using the BREAST-Q. J. Clin. Nurs. 2018, 27, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.H.; Lim, K.; Sze, P.W.; Ooi, A. Quality of life, pain of prepectoral and subpectoral implant-based breast reconstruction with a discussion on cost: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic. Surg. 2022, 75, 2550–2560. [Google Scholar] [CrossRef]
- Caputo, G.G.; Zingaretti, N.; Kiprianidis, I.; Zanfisi, C.; Domenici, L.; Parodi, P.C.; Governa, M. Quality of Life and Early Functional Evaluation in Direct-to-Implant Breast Reconstruction After Mastectomy: A Comparative Study Between Prepectoral Versus Dual-Plane Reconstruction. Clin. Breast Cancer 2021, 21, 344–351. [Google Scholar] [CrossRef]
| Patient Characteristics | Study Group n = 58 | Control Group 1 n = 53 | Control Group 2 n = 46 | p | TOTAL n = 157 |
|---|---|---|---|---|---|
| Age (years) | 43.3 (±7.6) (30–66) | 48.3 (±8.7) (29–69) | 50.9 (±9.3) (36–79) | <0.01 * | 47.2 (±9) (29–79) |
| Menstrual state | |||||
| Premenopausal | 49 (84.5%) | 39 (73.6%) | 34 (73.9%) | NS ** | 122 (77.7%) |
| Postmenopausal | 9 (15.5%) | 13 (26.4%) | 12 (26.1%) | 34 (33.3%) | |
| Laterality | |||||
| Right breast | 29 (50%) | 22 (41.5%) | 23 (50%) | NS ** | 74 (47.1%) |
| Left breast | 28 (48.3%) | 29 (54.7%) | 22 (47.8%) | 79 (50.3%) | |
| Bilateral | 1 (1.7%) | 2 (3.8%) | 1 (2.2%) | 4 (2.5%) | |
| Metachronic | |||||
| Tumour bed | 0 (0.0%) | 2 (3.8%) | 3 (6.5%) | 0.154 ** | 5 (3.2%) |
| Unscarred breast | 1 (1.7%) | 4 (7.5%) | 6 (13.0%) | 11 (7.0%) | |
| Contralateral breast | 2 (3.4%) | 1 (1.9%) | 1 (2.2%) | 4 (2.5%) | |
| Genetic study | |||||
| Not requested | 7 (12.1%) | 27 (50.9%) | 32 (69.6%) | 66 (42.0%) | |
| Pending | 5 (8.6%) | 16 (30.2%) | 5 (10.9%) | 26 (16.6%) | |
| BRCA1 | 11 (19.0%) | 2 (3.8%) | 0 (0.0%) | 13 (8.3%) | |
| BRCA2 | 4 (6.9%) | 1 (1.9%) | 1 (2.2%) | 6 (3.8%) | |
| Li–Fraumeni syndrome | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) | |
| PALB2 | 2 (3.4%) | 1 (1.9%) | 1 (2.2%) | 4 (2.5%) | |
| RAD51 | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) | |
| BRIP1 | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6% | |
| ATM | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.3%) | |
| Negative study | 24 (41.4%) | 6 (11.4%) | 7 (15.2%) | <0.01 ** | 37 (23.5%) |
| BMI, kg/m2 | 24.3 (±4.8) | 24.6 (±5.1) | 23.2 (±3.8) | NS * | 24.2 (±4.7) |
| Sternum–NAC distance | 19.0 (±3.2) | 19.2 (±3.4) | 19.9 (±2.7) | NS * | 20.2 (±3.4) |
| Surgical Features | Study Group | Control Group 1 | Control Group 2 | p | TOTAL |
|---|---|---|---|---|---|
| n = 58 | n = 53 | n = 46 | n = 157 | ||
| Bilateral Mastectomy | 40 (69.0%) | 16 (30.2%) | 16 (34.8%) | <0.01 ** | 72 (45.9%) |
| Type of mastectomy | |||||
| Stewart lower pole | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 0.026 ** | 1 (0.6%) |
| SSM I | 7 (12.1%) | 5 (9.4%) | 2 (4.3%) | 14 (8.9%) | |
| SSM II | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 1 (0.6%) | |
| SSM IV | 12 (20.7%) | 5 (9.4%) | 4 (8.7%) | 21 (13.4%) | |
| NSM inframammary | 34 (58.6%) | 24 (45.3%) | 23 (50%) | 81 (51.6%) | |
| NSM vertical | 5 (8.6%) | 17 (32.1%) | 17 (36.9%) | 39 (24.8%) | |
| Axillary surgery | |||||
| No study | 1 (1.7%) | 3 (5.7%) | 5 (10.9%) | <0.01 ** | 9 (5.7%) |
| SLNB | 43 (74.1%) | 27 (50.9%) | 41 (89.1%) | 111 (70.7%) | |
| Lymphadenectomy | 14 (24.1%) | 23 (43.4%) | 0 (0.0%) | 39 (24.8%) | |
| Oncologic breast weight (g) | 308.4 (±201.0) | 349.4 (±281.3) | 243.5 (±183.8) | 0.055 * | 301.7 (±228.9) |
| Contralateral breast weight (g) | 326.6 (±220.9) | 278.6 (±307.0) | 249.1 (±263.8) | 0.206 * | 297.9 (±252.9) |
| Surgical time (min) | 182.5 (±53.7) | 169.8 (±47.1) | 139.0 (±48.0) | <0.01 * | 164.4 (±52.7) |
| Unilateral m. | 148.1 (±32.1) | 157.9 (±44.2) | 123.5 (±31.3) | 0.02 * | 143.1 (±40.2) |
| Bilateral m. | 196.3 (±54.9) | 194.3 (±44.2) | 168.1 (±60.3) | 0.213* | 189.1 (±54.6) |
| Mean stay | 1.9 ± 0.8 | 1.5 ± 0.6 | 1.6 ± 0.5 | 0.30 * | 1.7 ± 0.7 |
| Histological type | |||||
| DCIS | 0 (0.0%) | 0 (0.0%) | 14 (30.4%) | <0.01 ** | 14 (8.9%) |
| IDC | 56 (96.6%) | 41 (77.4%) | 24 (52.2%) | 121 (77.1%) | |
| ILC | 2 (3.4%) | 10 (18.9%) | 7 (15.2%) | 19 (12.1%) | |
| Other | 0 (0.0%) | 2 (3.8%) | 1 (2.2%) | 3 (1.9%) | |
| Tumour subtype | |||||
| Luminal A | 0 (0.0%) | 16 (30.2%) | 15 (32.6%) | <0.01 ** | 31 (19.7%) |
| Luminal B Her2- | 22 (37.9%) | 22 (41.5%) | 15 (32.6%) | 59 (37.6%) | |
| Luminal B Her2+ | 12 (20.7%) | 8 (15.1%) | 0 (0.0%) | 20 (12.7%) | |
| Her2+ | 2 (3.4%) | 3 (5.7%) | 0 (0.0%) | 5 (3.2%) | |
| Triple Negative | 22 (37.9%) | 4 (7.5%) | 0 (0.0%) | 26 (16.6%) | |
| Not valid | 0 (0.0%) | 0 (0.0%) | 16 (34.8%) | 16 (10.2%) | |
| Tumour size (pT) | |||||
| Tis | 0 (0.0%) | 0 (0.0%) | 14 (30.4%) | <0.01 ** | 0 (0.0%) |
| T1mic | 0 (0.0%) | 0 (0.0%) | 2 (4.3%) | 2 (1.3%) | |
| T1a | 10 (17.2%) | 2 (3.8%) | 1 (2.2%) | 13 (8.3%) | |
| T1b | 5 (8.6%) | 9 (17%) | 4 (8.7%) | 18 (11.5%) | |
| T1c | 9 (15.5%) | 14 (26.4%) | 14 (30.4%) | 37 (23.6%) | |
| T2 | 8 (13.8%) | 20 (37.7%) | 10 (21.7%) | 38 (24.2%) | |
| T3 | 1 (1.7%) | 8 (15.1%) | 1 (2.2%) | 10 (6.4%) | |
| Axillary staging (pN) | |||||
| Nx | 1 (1.7%) | 3 (5.7%) | 5 (10.9%) | <0.01 ** | 9 (5.7%) |
| N0 | 41 (70.7%) | 17 (32.1%) | 36 (78.2%) | 94 (59.9%) | |
| N1mic | 1 (1.7%) | 6 (11.3%) | 4 (8.7%) | 11 (7.0%) | |
| N1 | 10 (17.2%) | 17 (32.1%) | 1 (2.1%) | 28 (17.8%) | |
| N2 | 3 (5.2%) | 8 (15.1%) | 0 (0.0%) | 11 (7.0%) | |
| N3 | 2 (3.4%) | 2 (3.8%) | 0 (0.0%) | 4 (2.5%) | |
| Time from the surgery to the next treatment (days) | 49.8 ± 115.1 | 42 ± 17.8 | 66.0 ± 14.6 | 0.004 * | 47.1 ± 18.4 |
| Radiation therapy | 36 (62.1%) | 33 (62.3%) | 13 (28.3%) | <0.01 ** | 82 (52.2%) |
| Complication Type | Study Group n = 58 | Control Group 1 n = 53 | Control Group 2 n = 46 | p | Total n = 157 |
|---|---|---|---|---|---|
| Postoperative complications | 8 (13.8%) | 9 (16.9%) | 5 (10.9%) | 0.681 ** | 22 (14.0%) |
| Haematoma | 2 (3.4%) | 1 (1.9%) | 2 (4.3%) | 4 (2.5%) | |
| Abscess | 3 (5.2%) | 0 (0.0%) | 0 (0.0%) | 3 (1.9%) | |
| Breast seroma | 2 (3.4%) | 2 (3.8%) | 1 (2.2%) | 5 (3.2%) | |
| Wound dehiscence | 0 (0.0%) | 4 (7.5%) | 0 (0.0%) | 4 (2.5%) | |
| Partial necrosis of the NAC | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 1 (0.6%) | |
| Skin necrosis | 2 (3.4%) | 2 (3.8%) | 1 (2.2%) | 5 (3.2%) | |
| Rash | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 1 (0.6%) | |
| Readmission | 3 (5.2%) | 11 (20.8%) | 3 (6.5%) | 0.014 ** | 17 (5.7%) |
| Reoperation | 2 (3.4%) | 10 (18.9%) | 3 (6.5%) | 0.014 ** | 15 (3.8%) |
| Implant Loss | 2 (3.4%) | 7 (13.2%) | 1 (2.2%) | 0.042 ** | 10 (6.4%) |
| Unilateral or Bilateral Mastectomy | Study Group | Control Group 1 | Control Group 2 | p | Total |
|---|---|---|---|---|---|
| n = 58 | n = 53 | n = 46 | n = 157 | ||
| Bilateral mastectomy | |||||
| Genetic mutation | |||||
| Yes | 22 (37.9%) | 3 (5.7%) | 1 (2.2%) | <0.01 ** | 26 (16.6%) |
| No | 18 (31.0%) | 13 (24.5%) | 15 (32.6%) | 46 (29.3%) | |
| TOTAL | 40 (69.0%) | 16 (30.2%) | 16 (34.8%) | 72 (45.9%) | |
| Unilateral mastectomy | |||||
| Genetic mutation | |||||
| Yes | 0 (0.0%) | 1 (1.9%) | 1 (2.2%) | 0.749 ** | 2 (1.3%) |
| No | 18 (31.0%) | 36 (67.9%) | 29 (63.0%) | 83 (52.9%) | |
| TOTAL | 18 (31.0%) | 37 (69.8%) | 30 (65.2%) | 30 (65.2%) | 85 (54.1%) |
| Events | Study Group n = 46 | Control Group 2 n = 58 | Control Group 3 n = 53 | NO Systemic Therapy n = 46 | p | Total n = 157 |
|---|---|---|---|---|---|---|
| Mean follow-up (months) | 16.8 ± 10.8 | 10.9 ± 10.2 | 15.1 ± 11.5 | 16.8 ± 10.8 | 0.012 * | 14.0 ± 11.0 |
| Locoregional relapses | 1 (2.2%) | 2 (3.4%) | 0 (0.0%) | 1 (2.2%) | - | 3 (1.9%) |
| Distant metastasis | 0 (0.0%) | 3 (5.2%) | 1 (1.9%) | 0 (0.0%) | - | 4 (2.5%) |
| Deaths | 0 (0.0%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | - | 1 (0.6%) |
| Preoperative Satisfaction | Study Group n = 49 | Control Group 1 n = 41 | Control Group 2 n = 37 | p | Total n = 127 |
|---|---|---|---|---|---|
| Satisfaction with the breast | 66.7 ± 20.9 | 66.9 ± 22.8 | 68.9 ± 23.1 | 0.911 * | 67.4 ± 22.0 |
| Psychosocial well-being | 68.7 ± 19.6 | 70.4 ± 18.2 | 73.5 ± 19.3 | 0.571 * | 70.7 ± 19.0 |
| Physical well-being | 74.4 ± 14.6 | 68.7 ± 14.4 | 72.8 ± 12.3 | 0.116 ** | 72.1 ± 14.0 |
| Abdomen assessment | 77.8 ± 15.8 | 80.3 ± 21.2 | 83.1 ± 17.0 | 0.406 * | 80.1 ± 18.0 |
| Sexual satisfaction | 56.1 ± 20.7 | 61.1 ± 21.9 | 71.0 ± 22.3 | 0.007 * | 62.1 ± 22.3 |
| Postoperative Satisfaction | Study Group n = 16 | Control Group 1 n = 9 | Control Group 2 n = 14 | p | Total n = 39 |
|---|---|---|---|---|---|
| Satisfaction with the breast | 66.4 ± 19.6 | 60.3 ± 13.6 | 71.5 ± 19.2 | 0.369* | 66.9 ± 18.3 |
| Outcome | 83.8 ± 15.3 | 74,0 ± 21.1 | 81.6 ± 16.8 | 0.398 * | 80.7 ± 17.3 |
| Psychosocial well-being | 79.9 ± 20.5 | 68.4 ± 18.8 | 76.6 ± 16.1 | 0.398 * | 76.1 ± 18.7 |
| Physical well-being | 64.9 ± 19.9 | 68.0 ± 9.7 | 69.9 ± 11.5 | 0.672 * | 67.5 ± 14.9 |
| Abdomen assessment | 79.5 ± 28.9 | 79.3 ± 20.5 | 84.3 ± 17.6 | 0.959 * | 81.6 ± 18.2 |
| Sexual satisfaction | 63.3 ± 24.6 | 53.3 ± 13.6 | 66.3 ± 18.1 | 0.473 * | 63.1 ± 20.2 |
| Nipple | 97.3 ± 5.5 | 50 | 100 | 0.112 * | 93.4 ± 17.5 |
| Information | 86.1 ± 17.1 | 77.7 ± 20.2 | 81.9 ± 16,2 | 0.518 * | 82.7 ± 17.4 |
| Surgeon | 94.4 ± 15.4 | 98.3 ± 5.0 | 91.0 ± 17.3 | 0.221 * | 94.2 ± 14.3 |
| Medical team | 96.9 ± 5.9 | 94.6 ± 16.3 | 100 | 0.165 * | 97.4 ± 8.7 |
| Office equipment | 96.3 ± 12.8 | 95.3 ± 14.0 | 100 | 0.436 * | 97.3 ± 10.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acea-Nebril, B.; García-Novoa, A.; Cereijo-Garea, C.; Conde Iglesias, C.; Bouzón Alejandro, A.; Díaz Carballada, C. Safety and Quality of Life in Women with Immediate Reconstruction with Polyurethane Implants after Neoadjuvant Chemotherapy: Outcomes from The Preq-20 Trial. Cancers 2023, 15, 1113. https://doi.org/10.3390/cancers15041113
Acea-Nebril B, García-Novoa A, Cereijo-Garea C, Conde Iglesias C, Bouzón Alejandro A, Díaz Carballada C. Safety and Quality of Life in Women with Immediate Reconstruction with Polyurethane Implants after Neoadjuvant Chemotherapy: Outcomes from The Preq-20 Trial. Cancers. 2023; 15(4):1113. https://doi.org/10.3390/cancers15041113
Chicago/Turabian StyleAcea-Nebril, Benigno, Alejandra García-Novoa, Carmen Cereijo-Garea, Carmen Conde Iglesias, Alberto Bouzón Alejandro, and Carlota Díaz Carballada. 2023. "Safety and Quality of Life in Women with Immediate Reconstruction with Polyurethane Implants after Neoadjuvant Chemotherapy: Outcomes from The Preq-20 Trial" Cancers 15, no. 4: 1113. https://doi.org/10.3390/cancers15041113
APA StyleAcea-Nebril, B., García-Novoa, A., Cereijo-Garea, C., Conde Iglesias, C., Bouzón Alejandro, A., & Díaz Carballada, C. (2023). Safety and Quality of Life in Women with Immediate Reconstruction with Polyurethane Implants after Neoadjuvant Chemotherapy: Outcomes from The Preq-20 Trial. Cancers, 15(4), 1113. https://doi.org/10.3390/cancers15041113





