A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population
2.2. Data Collection and Treatment
2.3. Variable Declaration
2.4. Follow-Up and Outcome
2.5. Statistical Analyses
3. Results
3.1. Clinical Characteristics of Patients
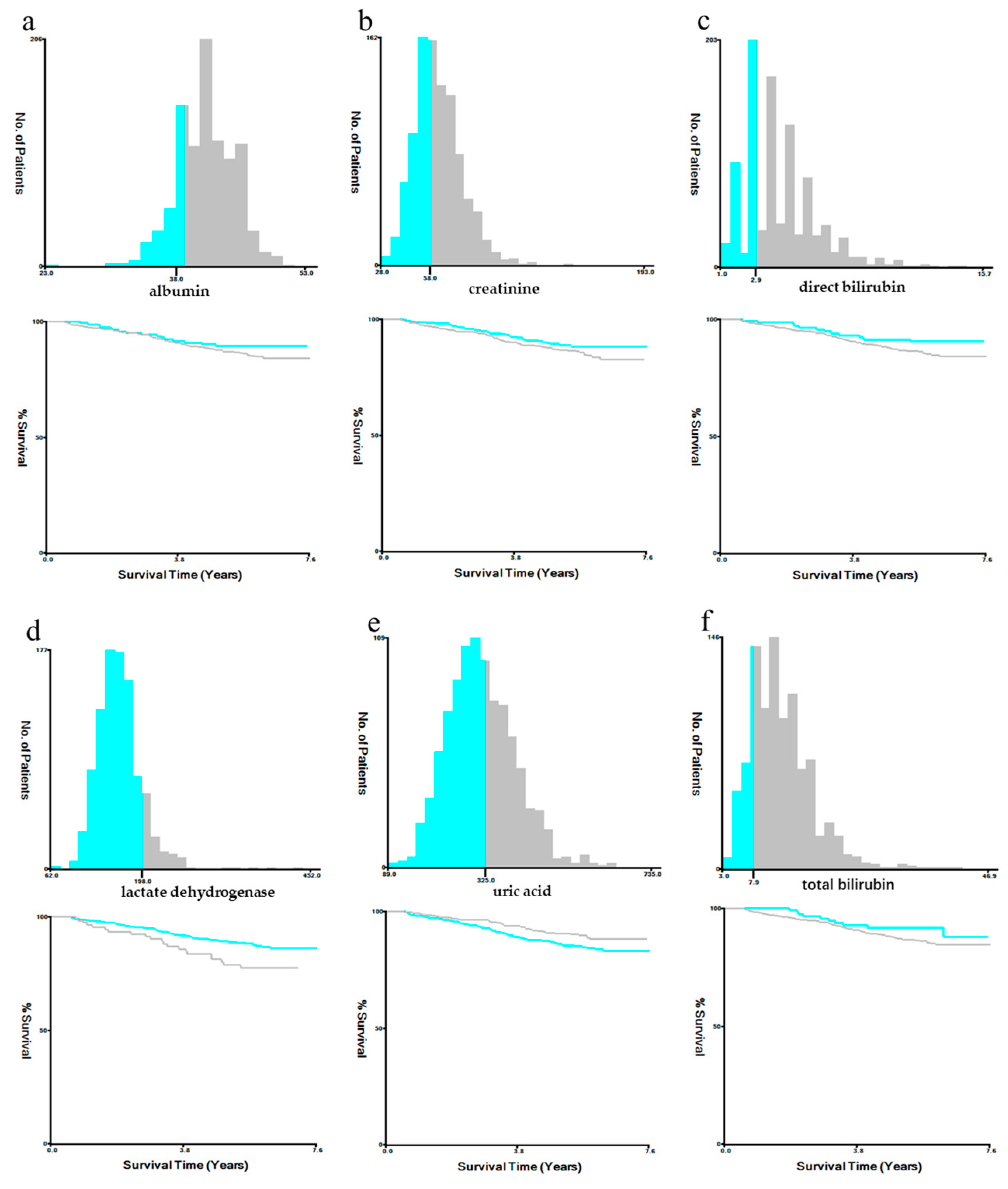
3.2. Construction of the Systematic Oxidative Stress Score
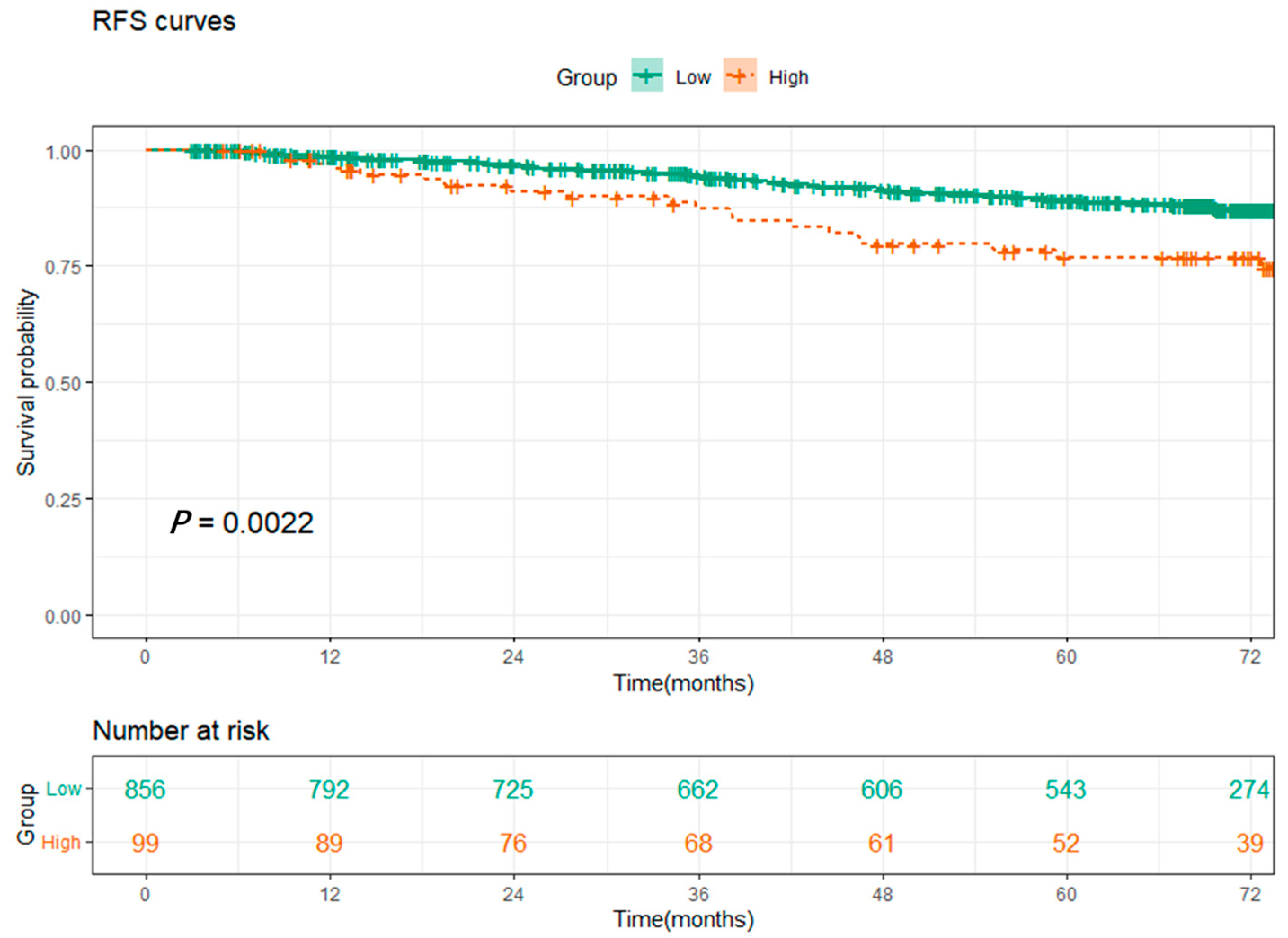
3.3. Survival Analysis and the Relationship between SOS and Clinical Characteristics
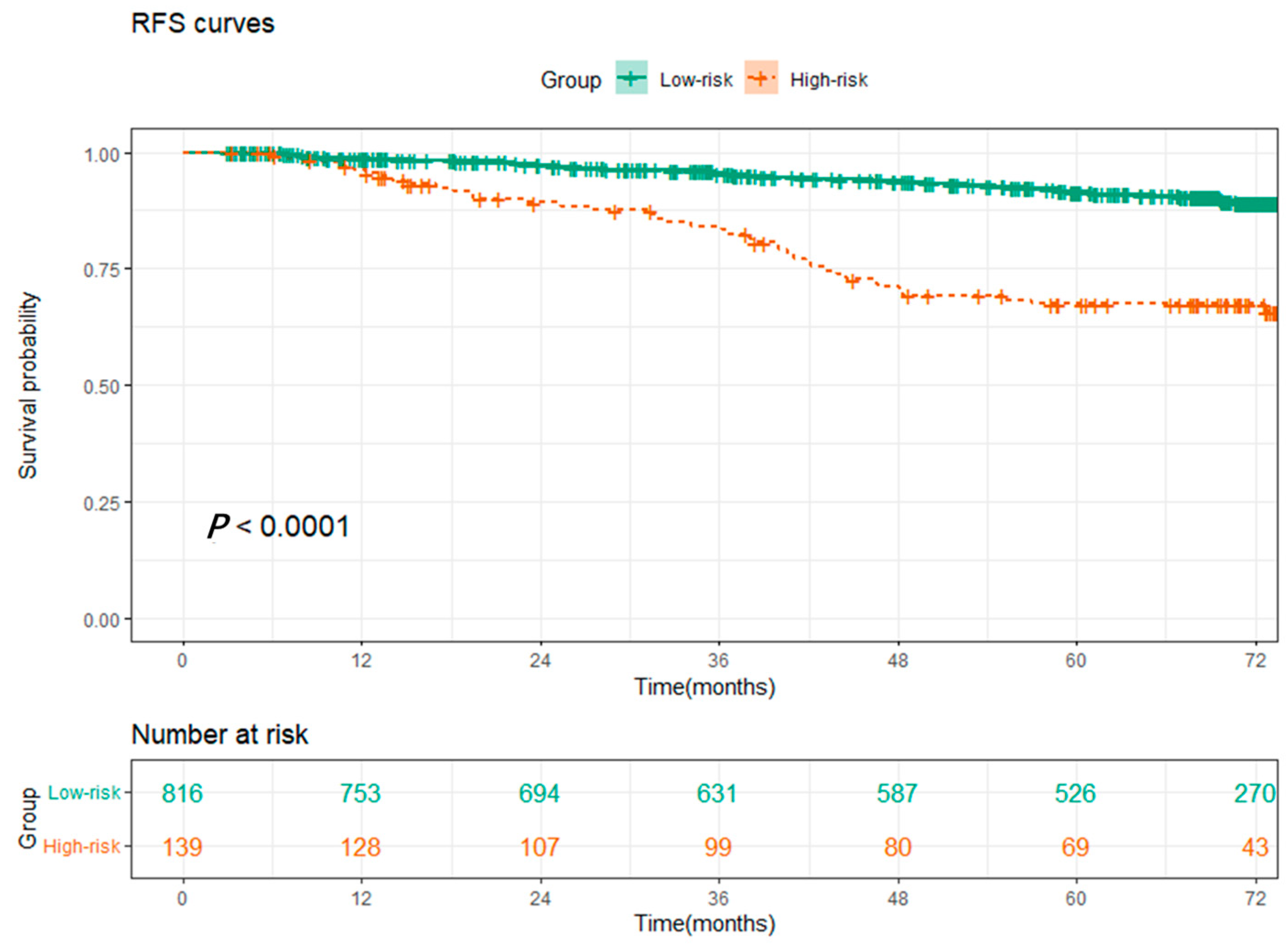
3.4. SOS Was an Independent Prognostic Indicator of RFS for NSCLC Patients
3.5. Construction and Validation of the Prognostic Nomogram for NSCLC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.-J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung cancer LDCT screening and mortality reduction—Evidence, pitfalls and future perspectives. Nat. Rev. Clin. Oncol. 2021, 18, 135–151. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Bai, C.; Wang, X.; Powell, C.A. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2019, 468, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Deboever, N.; Mitchell, K.G.; Feldman, H.A.; Cascone, T.; Sepesi, B. Current Surgical Indications for Non–Small-Cell Lung Cancer. Cancers 2022, 14, 1263. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Rami-Porta, R.; Asamura, H. The 8th TNM edition for lung cancer: A critical analysis. Ann. Transl. Med. 2018, 6, 87. [Google Scholar] [CrossRef]
- Guerrera, F.; Lococo, F.; Evangelista, A.; Rena, O.; Ampollini, L.; Vannucci, J.; Errico, L.; Lausi, P.O.; Ventura, L.; Marchese, V.; et al. Risk of recurrence in stage I adenocarcinoma of the lung: A multi-institutional study on synergism between type of surgery and type of nodal staging. J. Thorac. Dis. 2019, 11, 564–572. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Rami-Porta, R.; Vallières, E.; Tsuboi, M.; Rusch, V.; Goldstraw, P. Visceral Pleural Invasion: Pathologic Criteria and Use of Elastic Stains: Proposal for the 7th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2008, 3, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, C.; Tian, T.; Chen, Q. A risk classification system predicting the cancer-specific survival for postoperative stage IB non-small-cell lung cancer patients without lymphovascular and visceral pleural invasion. Lung Cancer 2021, 161, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Y.; Shao, J.; Liu, D.; Li, W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer 2020, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2016, 387, 95–105. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini-Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef]
- Kim, S.-M.; Hwang, K.-A.; Choi, K.-C. Potential roles of reactive oxygen species derived from chemical substances involved in cancer development in the female reproductive system. BMB Rep. 2018, 51, 557–562. [Google Scholar] [CrossRef]
- Sandesc, M.; Rogobete, A.F.; Bedreag, O.H.; Dinu, A.; Papurica, M.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Bratu, L.M.; Bratu, T.; et al. Analysis of oxidative stress-related markers in critically ill polytrauma patients: An observational prospective single-center study. Bosn. J. Basic Med. Sci. 2018, 18, 191–197. [Google Scholar] [CrossRef]
- Claps, G.; Faouzi, S.; Quidville, V.; Chehade, F.; Shen, S.; Vagner, S.; Robert, C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 749–762. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2015, 5, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Huitzil-Melendez, F.D.; Capanu, M.; O’Reilly, E.M.; Duffy, A.; Gansukh, B.; Saltz, L.L.; Abou-Alfa, G.K. Advanced hepatocellular carcinoma: Which staging systems best predict prognosis? J. Clin. Oncol. 2010, 28, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxidative Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ping, L.; Du, T.; Wang, Y.; Sun, Y.; Liang, G.; Wang, X.; Xie, X.; Wei, W.; Xiao, X.; et al. A Novel Systematic Oxidative Stress Score Predicts the Prognosis of Patients with Operable Breast Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 9441896. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef]
- Petrelli, F.; Ardito, R.; Merelli, B.; Lonati, V.; Cabiddu, M.; Seghezzi, S.; Barni, S.; Ghidini, A. Prognostic and predictive role of elevated lactate dehy-drogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: A systematic review and me-ta-analysis. Melanoma Res. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- LaFleur, J.; Hefler-Frischmuth, K.; Grimm, C.; Schwameis, R.; Gensthaler, L.; Reiser, E.; Hefler, L.A. Prognostic Value of Serum Creatinine Levels in Patients with Epithelial Ovarian Cancer. Anticancer Res. 2018, 38, 5127–5130. [Google Scholar] [CrossRef]
- Schwameis, R.; Postl, M.; Bekos, C.; Hefler, L.; Reinthaller, A.; Seebacher, V.; Grimm, C.; Polterauer, S.; Helmy-Bader, S. Prognostic value of serum creatine level in patients with vulvar cancer. Sci. Rep. 2019, 9, 11129. [Google Scholar] [CrossRef]
- Garinet, S.; Wang, P.; Mansuet-Lupo, A.; Fournel, L.; Wislez, M.; Blons, H. Updated Prognostic Factors in Localized NSCLC. Cancers 2022, 14, 1400. [Google Scholar] [CrossRef]
- Qian, J.; Xu, J.; Wang, S.; Qian, F.; Yang, W.; Zhang, B.; Zhang, Y.; Nie, W.; Lou, Y.; Lu, J.; et al. Adjuvant Chemotherapy Candidates in Stage I Lung Adenocarcinomas Following Complete Lobectomy. Ann. Surg. Oncol. 2019, 26, 2392–2400. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Que, S.-J.; Chen, J.-Y.; Zhong, Q.; Liu, Z.-Y.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; Lin, M.; et al. Development and validation of metabolic scoring to individually predict prognosis and monitor recurrence early in gastric cancer: A large-sample analysis. Eur. J. Surg. Oncol. (EJSO) 2022, 48, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Fang, Z.; Hang, D.; Wang, F.; Polychronidis, G.; Wang, L.; Lo, C.; Wang, K.; Zhong, R.; Knudsen, M.D.; et al. Circulating liver function markers and colorectal cancer risk: A prospective cohort study in the UK Biobank. Int. J. Cancer 2020, 148, 1867–1878. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, S.; Yan, L.; Gu, J.; Yang, J.; Yang, M.; Liu, L.; Cai, K. A nomogram based on pretreatment levels of serum bilirubin and total bile acid levels predicts survival in colorectal cancer patients. BMC Cancer 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Lou, Y.; Hu, S.; Yu, K.; Li, R.; Zhang, X.; Jin, B.; Han, B. Pretreatment direct bilirubin and total cholesterol are significant predictors of overall survival in advanced non-small-cell lung cancer patients with EGFR mutations. Int. J. Cancer 2017, 140, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, S.; Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Wulaningsih, W. Serum inflammatory markers and colorectal cancer risk and survival. Br. J. Cancer 2017, 116, 1358–1365. [Google Scholar] [CrossRef]
- Merriel, S.W.; Carroll, R.; Hamilton, F.; Hamilton, W. Association between unexplained hypoalbuminaemia and new cancer di-agnoses in UK primary care patients. Fam. Pract. 2016, 33, 449–452. [Google Scholar] [CrossRef]
- Sprague, B.L.; Trentham-Dietz, A.; Klein, B.E.; Klein, R.; Cruickshanks, K.J.; Lee, K.E.; Hampton, J.M. Physical Activity, White Blood Cell Count, and Lung Cancer Risk in a Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2714–2722. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Wu, Z.; Wen, Y.; Wang, G.; Chen, S.; Tan, F.; Li, J.; Wu, S.; Dai, M.; et al. Association between pre-diagnostic serum albumin and cancer risk: Results from a prospective population-based study. Cancer Med. 2021, 10, 4054–4065. [Google Scholar] [CrossRef]
- Chen, S.; Ye, T.; Yang, S.; Zhao, Y.; Zhang, Y.; Huang, Q.; Wu, H.; Hu, H.; Sun, Y.; Zhang, Y.; et al. Prognostic Implication of Tumor Spread Through Air Spaces in Patients with Pathologic N0 Lung Adenocarcinoma. Lung Cancer 2022, 161, 33–38. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Zhang, F.; Han, R.; Ding, Q.; Xu, X.; Shu, J.; Ye, F.; Shi, L.; Mao, Y. Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920978147. [Google Scholar] [CrossRef]
- Jia, M.; Yu, S.; Gao, H.; Sun, P.-L. Spread Through Air Spaces (STAS) in Lung Cancer: A Multiple-Perspective and Update Review. Cancer Manag. Res. 2020, 12, 2743–2752. [Google Scholar] [CrossRef]
- Huang, L.; Tang, L.; Dai, L.; Shi, Y. The prognostic significance of tumor spread through air space in stage I lung adenocarcinoma. Thorac. Cancer 2022, 13, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, E.; Blaauwgeers, H.J.L.G.; de Cuba, E.M.V.; Yick, C.Y.; Flieder, D.B. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch. Pathol. Lab. Med. 2016, 140, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Toki, M.I.; Harrington, K.; Syrigos, K.N. The role of spread through air spaces (STAS) in lung adenocarcinoma prognosis and therapeutic decision making. Lung Cancer 2020, 146, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, G.; Xu, J.; Cui, L.; Dong, W.; Ni, Y.; Qu, X.; Du, J. Sublobectomy versus lobectomy for stage I non-small cell lung cancer in the elderly. Int. J. Surg. 2017, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kamigaichi, A.; Tsutani, Y.; Kagimoto, A.; Fujiwara, M.; Mimae, T.; Miyata, Y.; Okada, M. Comparing Segmentectomy and Lobectomy for Clinical Stage IA Solid-dominant Lung Cancer Measuring 2.1 to 3 cm. Clin. Lung Cancer 2020, 21, e528–e538. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, X.; Dai, J.; Guo, Y.; Jin, K.; Zhu, Y.; Wang, H.; Jiang, G. Propensity-matched Comparison of VATS Left Upper Trisegmentectomy and Lobectomy. Ann. Thorac. Surg. 2021, 114, 1007–1014. [Google Scholar] [CrossRef]
- Huang, C.; Hsu, P.; Chen, C.; Yeh, Y.; Hsu, H.; Shih, C.; Huang, B. Surgeons’ preference sublobar resection for stage I NSCLC less than 3 cm. Thorac. Cancer 2020, 11, 907–917. [Google Scholar] [CrossRef]


| Variables | n = 955 |
|---|---|
| Gender | |
| Male | 407 (42.6%) |
| Female | 548 (57.4%) |
| Age at surgery, years (IQR) | 61 (56–66) |
| ≤61 | 491 (51.4%) |
| >61 | 464 (48.6%) |
| Smoking history | |
| No | 792 (82.9%) |
| Yes | 163 (17.1%) |
| Extent of surgery | |
| Lobectomy | 863 (90.4%) |
| Sub-lobectomy | 91 (9.5%) |
| Pneumonectomy | 1 (0.1%) |
| Predominant pattern | |
| Lepidic | 302 (31.6%) |
| Acinar/Papillary | 562 (58.8%) |
| Micropapillary/Solid | 59 (6.2%) |
| Others | 32 (3.4%) |
| Tumor size, cm | |
| ≤1.0 | 72 (7.5%) |
| 1.1–2.0 | 468 (49.0%) |
| 2.1–3.0 | 316 (33.1%) |
| 3.1–4.0 | 99 (10.4%) |
| Visceral pleural invasion | |
| Absent | 861 (90.2%) |
| Present | 94 (9.8%) |
| Lymphovascular invasion | |
| Absent | 945 (99.0%) |
| Present | 10 (1.0%) |
| Spread through air space | |
| Absent | 925 (96.9%) |
| Present | 30 (3.1%) |
| Epidermal growth factor receptor mutation | |
| Without | 358 (37.5%) |
| 19-del | 266 (27.9%) |
| L858R | 279 (29.2%) |
| Others | 52 (5.4%) |
| Adjuvant chemotherapy | |
| No | 683 (71.5%) |
| Yes | 272 (28.5%) |
| Pathological stage | |
| IA | 775 (81.2%) |
| IB | 180 (18.8%) |
| Total bilirubin | |
| ≤7.9 | 128 (13.4%) |
| >7.9 | 827 (86.6%) |
| Direct bilirubin | |
| ≤2.9 | 150 (15.7%) |
| >2.9 | 805 (84.3%) |
| Albumin | |
| ≤38 | 175 (18.3%) |
| >38 | 780 (81.7%) |
| Uric acid | |
| ≤325 | 560 (58.6%) |
| >325 | 395 (41.4%) |
| Creatinine | |
| ≤58 | 395 (41.4%) |
| >58 | 560 (58.6%) |
| Lactate dehydrogenase | |
| ≤198 | 838 (87.7%) |
| >198 | 117 (12.3%) |
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Total bilirubin | ||||||
| ≤7.9 | 1 | |||||
| >7.9 | 1.636 | 0.854–3.136 | 0.138 | |||
| Direct bilirubin | ||||||
| ≤2.9 | 1 | 1 | ||||
| >2.9 | 1.586 | 0.871–2.890 | 0.132 | 1.681 | 0.918–3.077 | 0.093 |
| Albumin | ||||||
| ≤38 | 1 | |||||
| >38 | 1.384 | 0.814–2.352 | 0.230 | |||
| Uric acid | ||||||
| ≤325 | 1 | 1 | ||||
| >325 | 0.674 | 0.452–1.004 | 0.053 | 0.566 | 0.373–0.858 | 0.007 |
| Creatinine | ||||||
| ≤58 | 1 | 1 | ||||
| >58 | 1.378 | 0.929–2.043 | 0.111 | 1.587 | 1.051–2.395 | 0.028 |
| Lactate dehydrogenase | ||||||
| ≤198 | 1 | 1 | ||||
| >198 | 1.855 | 1.152–2.986 | 0.011 | 1.991 | 1.233–3.214 | 0.005 |
| Multivariable Analysis | ||||
|---|---|---|---|---|
| Variables | Coef | Exponential (Coef) | 95% CI | p-Value |
| Uric acid | −0.562 | 0.570 | 0.376–0.865 | 0.008 |
| Creatinine | 0.490 | 1.632 | 1.081–2.461 | 0.020 |
| Lactate dehydrogenase | 0.636 | 1.888 | 1.173–3.041 | 0.009 |
| Variables | Total (n = 955) | High-Level (n = 99) | Low-Level (n = 856) | p-Value |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Male | 407 | 15 (15.2%) | 392 (45.8%) | |
| Female | 548 | 84 (84.8%) | 464 (54.2%) | |
| Age at surgery, years | 0.408 | |||
| ≤61 | 491 | 47 (47.5%) | 444 (51.9%) | |
| >61 | 464 | 52 (52.5%) | 412 (48.1%) | |
| Smoking history | 0.005 | |||
| No | 792 | 92 (92.9%) | 700 (81.8%) | |
| Yes | 163 | 7 (7.1%) | 156 (18.2%) | |
| Predominant pattern | 0.942 | |||
| Lepidic | 302 | 34 (34.3%) | 268 (31.3%) | |
| Acinar/Papillary | 562 | 56 (56.6%) | 506 (59.1%) | |
| Micropapillary/Solid | 59 | 6 (6.1%) | 53 (6.2%) | |
| Others | 32 | 3 (3.0%) | 29 (3.4%) | |
| Tumor size, cm | 0.012 | |||
| ≤1.0 | 72 | 3 (3.0%) | 69 (8.1%) | |
| 1.1–2.0 | 468 | 39 (39.4%) | 429 (50.1%) | |
| 2.1–3.0 | 316 | 41 (41.4%) | 275 (32.1%) | |
| 3.1–4.0 | 99 | 16 (16.2%) | 83 (9.7%) | |
| Visceral pleural invasion | 0.246 | |||
| Absent | 861 | 86 (86.9%) | 775 (90.5%) | |
| Present | 94 | 13 (13.1%) | 81 (9.5%) | |
| Lymphovascular invasion | 0.127 | |||
| Absent | 945 | 96 (97.0%) | 849 (99.2%) | |
| Present | 10 | 3 (3.0%) | 7 (0.8%) | |
| Spread through air space | 1 | |||
| Absent | 925 | 96 (97.0%) | 829 (96.8%) | |
| Present | 30 | 3 (3.0%) | 27 (3.2%) | |
| Epidermal growth factor receptor mutation | 0.806 | |||
| Without | 358 | 36 (36.4%) | 322 (37.6%) | |
| 19-del | 266 | 25 (25.2%) | 241 (28.2%) | |
| L858R | 279 | 33 (33.3%) | 246 (28.7%) | |
| Others | 52 | 5 (5.1%) | 47 (5.5%) | |
| Extent of surgery | 0.841 | |||
| Lobectomy | 863 | 89 (89.9%) | 774 (90.4%) | |
| Sub-lobectomy | 91 | 10 (10.1%) | 81 (9.5%) | |
| Adjuvant chemotherapy | 0.110 | |||
| No | 683 | 64 (64.6%) | 619 (72.3%) | |
| Yes | 272 | 35 (35.4%) | 237 (27.7%) |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.796 | 0.546–1.159 | 0.233 | |||
| Age at surgery, years (IQR) | ||||||
| ≤61 | 1 | 1 | ||||
| >61 | 1.931 | 1.308–2.850 | 0.001 | 2.003 | 1.350–2.971 | 0.001 |
| Smoking history | ||||||
| No | 1 | |||||
| Yes | 0.992 | 0.599–1.645 | 0.976 | |||
| Extent of surgery | ||||||
| Lobectomy | 1 | |||||
| Sub-lobectomy | 1.021 | 0.533–1.958 | 0.950 | |||
| Predominant pattern | ||||||
| Lepidic | 1 | 1 | ||||
| Acinar/Papillary | 2.206 | 1.318–3.692 | 0.003 | 2.103 | 1.247–3.548 | 0.005 |
| Micropapillary/Solid | 4.713 | 2.375–9.352 | <0.001 | 4.022 | 1.961–8.250 | <0.001 |
| Others | 0.874 | 0.203–3.769 | 0.857 | 0.997 | 0.231–4.305 | 0.996 |
| Tumor size, cm | ||||||
| ≤1.0 | 1 | |||||
| 1.1–2.0 | 2.126 | 0.659–6.859 | 0.207 | |||
| 2.1–3.0 | 3.880 | 1.209–12.449 | 0.023 | |||
| 3.1–4.0 | 3.650 | 1.057–12.611 | 0.041 | |||
| Visceral pleural invasion | ||||||
| Absent | 1 | 1 | ||||
| Present | 2.839 | 1.816–4.440 | <0.001 | 2.198 | 1.384–3.489 | 0.001 |
| Lymphovascular invasion | ||||||
| Absent | 1 | |||||
| Present | 5.858 | 2.384–14.393 | <0.001 | |||
| Spread through air space | ||||||
| Absent | 1 | |||||
| Present | 2.045 | 0.898–4.660 | 0.089 | |||
| Epidermal growth factor receptor mutation | ||||||
| Without | 1 | |||||
| 19-del | 0.966 | 0.610–1.529 | 0.882 | |||
| L858R | 0.835 | 0.520–1.341 | 0.455 | |||
| Others | 0.972 | 0.414–2.280 | 0.947 | |||
| Adjuvant chemotherapy | ||||||
| No | 1 | |||||
| Yes | 1.274 | 0.857–1.895 | 0.231 | |||
| Systematic oxidative stress score | ||||||
| Low | 1 | 1 | ||||
| High | 2.097 | 1.291–3.407 | 0.003 | 2.015 | 1.229–3.303 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.-Y.; Hao, Y.; Yu, H.-H.; Wu, L.-L.; Liu, Z.-Y.; Peng, Q.; Li, Z.-X.; Li, K.; Liu, Y.; Wang, R.-R.; et al. A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma. Cancers 2023, 15, 1718. https://doi.org/10.3390/cancers15061718
Qian J-Y, Hao Y, Yu H-H, Wu L-L, Liu Z-Y, Peng Q, Li Z-X, Li K, Liu Y, Wang R-R, et al. A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma. Cancers. 2023; 15(6):1718. https://doi.org/10.3390/cancers15061718
Chicago/Turabian StyleQian, Jia-Yi, Yun Hao, Hai-Hong Yu, Lei-Lei Wu, Zhi-Yuan Liu, Qiao Peng, Zhi-Xin Li, Kun Li, Yu’e Liu, Rang-Rang Wang, and et al. 2023. "A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma" Cancers 15, no. 6: 1718. https://doi.org/10.3390/cancers15061718
APA StyleQian, J.-Y., Hao, Y., Yu, H.-H., Wu, L.-L., Liu, Z.-Y., Peng, Q., Li, Z.-X., Li, K., Liu, Y., Wang, R.-R., & Xie, D. (2023). A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma. Cancers, 15(6), 1718. https://doi.org/10.3390/cancers15061718







