Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings
Abstract
Simple Summary
Abstract
1. Introduction
2. 2020 WHO Classification of Chondrosarcomas
2.1. First Stage: Histological Grading
2.2. Second Stage: Primary vs. Secondary
2.3. Third Stage: Central vs. Peripheral vs. Periosteal
2.4. Fourth Stage: Conventional vs. Subtypes
3. Diagnostic Dilemma of Chondrosarcoma Classification
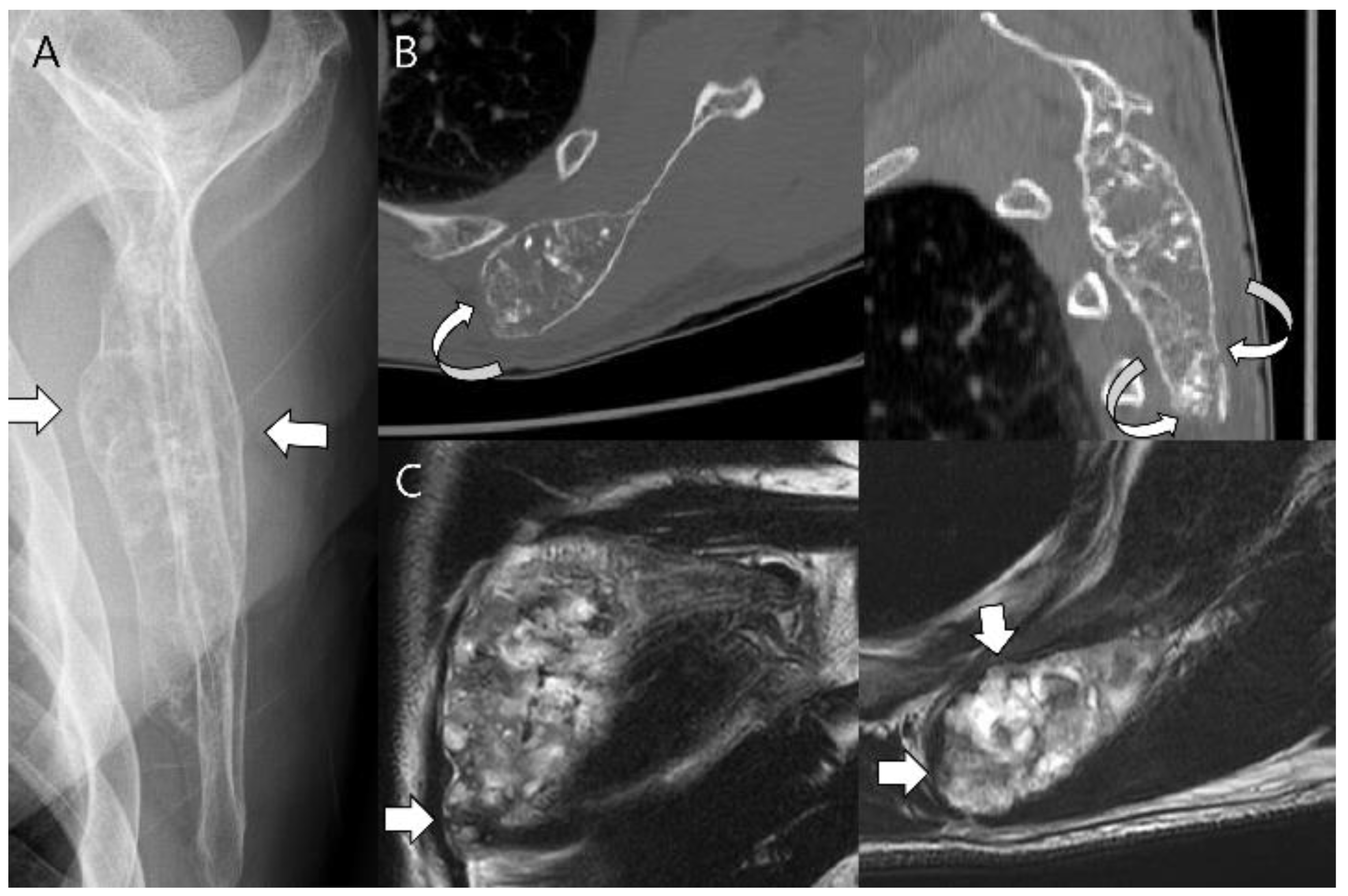
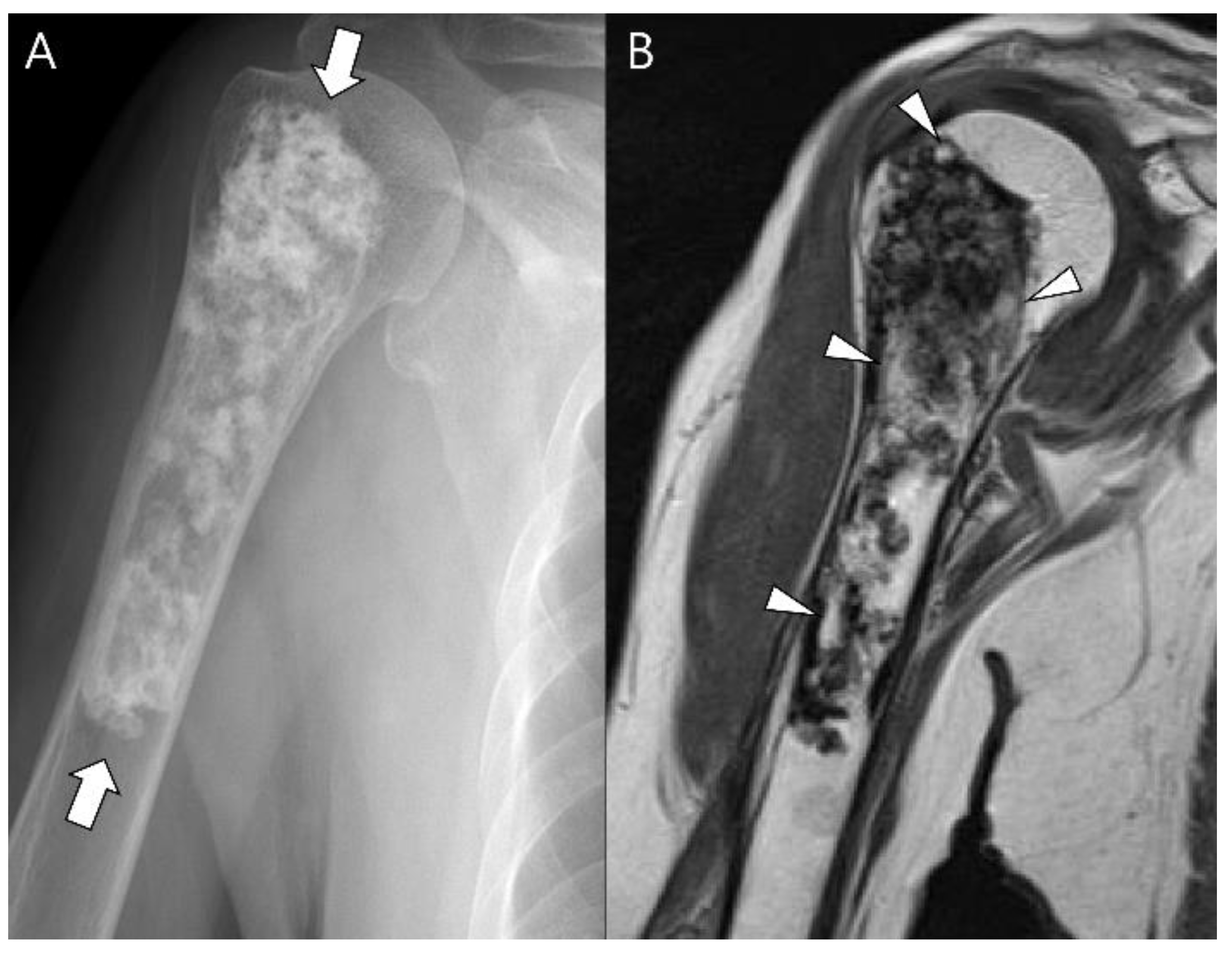
3.1. Distinction between Enchondroma and ACT
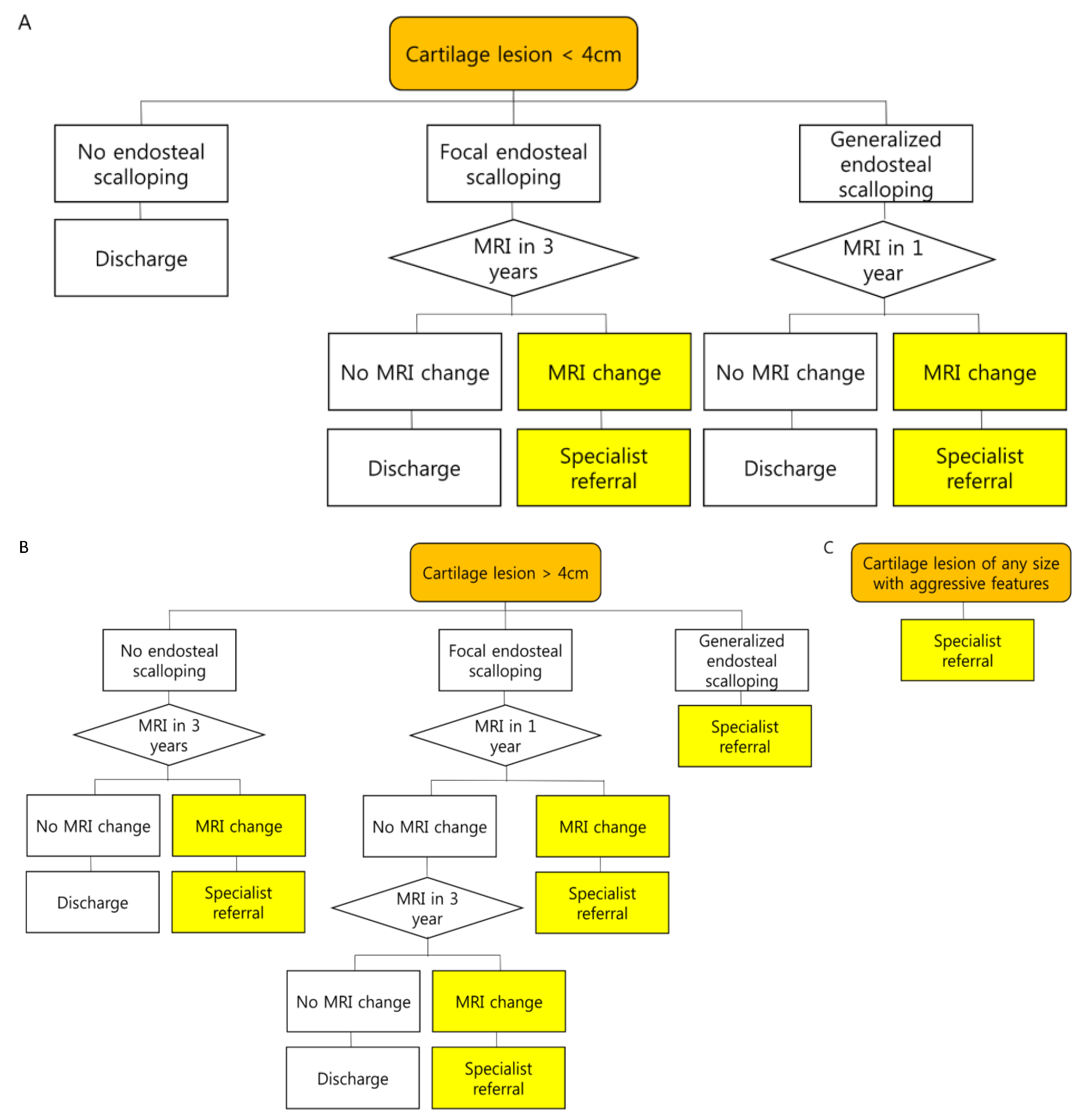
3.2. Biopsy or Follow-Up? Questions for Incidental Cartilage Lesions in the Long Bones
3.3. Distinction between ACT/CS1 and High-Grade Chondrosarcoma
4. Current Treatments and Management
5. Targets and Novel Treatment Options
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphey, M.D.; Walker, E.A.; Wilson, A.J.; Kransdorf, M.J.; Temple, H.T.; Gannon, F.H. From the archives of the AFIP: Imaging of primary chondrosarcoma: Radiologic-pathologic correlation. Radiographics 2003, 23, 1245–1278. [Google Scholar] [CrossRef]
- Marco, R.A.W.; Gitelis, S.; Brebach, G.T.; Healey, J.H. Cartilage tumors: Evaluation and treatment. Am. Acad. Orthop. Surg. 2000, 8, 292–304. [Google Scholar] [CrossRef]
- Ahmed, A.R.; Tan, T.-S.; Unni, K.K.; Collins, M.S.; Wenger, D.E.; Sim, F.H. Secondary chondrosarcoma in osteochondroma: Report of 107 patients. Clin. Orthop. Relat. Res. 2003, 411, 193–206. [Google Scholar] [CrossRef]
- Altay, M.; Bayrakci, K.; Yildiz, Y.; Erekul, S.; Saglik, Y. Secondary chondrosarcoma in cartilage bone tumors: Report of 32 patients. J. Orthop. Sci. 2007, 12, 415–423. [Google Scholar] [CrossRef]
- Douis, H.; Saifuddin, A. The imaging of cartilaginous bone tumours. II. Chondrosarcoma. Skelet. Radiol. 2013, 42, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T. Towards a new understanding and classification of chondrogenic neoplasias of the skeleton--biochemistry and cell biology of chondrosarcoma and its variants. Virchows Arch. 2002, 441, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Douis, H.; Parry, M.; Vaiyapui, S.; Davies, A.M. What are the differentiating clinical and MRI-features of enchondromas from low-grade chondrosarcomas? Eur. Radiol. 2018, 28, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.E.; Childs, B.R.; Eckhoff, M.D.; Rajani, R.; Potter, B.K.; Polfer, E.M. Atypical Cartilaginous Tumors: Trends in Management. JAAOS Glob. Res. Rev. 2021, 5, e21.00277. [Google Scholar] [CrossRef]
- Bindiganavile, S.; Han, I.; Yun, J.Y.; Kim, H.-S. Long-term Outcome of Chondrosarcoma: A Single Institutional Experience. Cancer Res. Treat. 2015, 47, 897–903. [Google Scholar] [CrossRef]
- Fromm, J.; Klein, A.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Birkenmaier, C.; Roeder, F.; Jansson, V.; Dürr, H.R. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer 2018, 18, 849. [Google Scholar] [CrossRef] [PubMed]
- van Praag, V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; Study group Bone and Soft tissue tumours (WeBot); Fiocco, M.; van de Sande, M.A.J. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Engel, H.; Herget, G.W.; Füllgraf, H.; Sutter, R.; Benndorf, M.; Bamberg, F.; Jungmann, P.M. Chondrogenic Bone Tumors: The Importance of Imaging Characteristics. Rofo 2020, 193, 262–275. [Google Scholar] [CrossRef]
- Kim, M.-J.; Cho, K.-J.; Ayala, A.G.; Ro, J.Y. Chondrosarcoma: With Updates on Molecular Genetics. Sarcoma 2011, 2011, 405437. [Google Scholar] [CrossRef]
- Murphey, M.D.; Flemming, D.J.; Boyea, S.R.; Bojescul, J.A.; Sweet, D.E.; Temple, H.T. Enchondroma versus chondrosarcoma in the appendicular skeleton: Differentiating features. Radiographics 1998, 18, 1213–1237. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, J.; McLeod, R.A.; Unni, K.K.; Ilstrup, D.M.; Pritchard, D.J. Primary chondrosarcoma of long bones and limb girdles. Cancer 1998, 83, 2105–2119. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Gambarotti, M.; Angelini, A.; Palmerini, E.; Staals, E.L.; Ruggieri, P.; Papagelopoulos, P.J. Chondrosarcomas Revisited. Orthopedics 2012, 35, e379–e390. [Google Scholar] [CrossRef]
- Brien, E.W.; Mirra, J.M.; Kerr, R. Benign and malignant cartilage tumors of bone and joint: Their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skelet. Radiol. 1997, 26, 325–353. [Google Scholar] [CrossRef]
- Flemming, D.J.; Murphey, M.D. Enchondroma and Chondrosarcoma. Semin. Musculoskelet. Radiol. 2000, 4, 59–71. [Google Scholar] [CrossRef]
- Bloem, J.L.; Reidsma, I.I. Bone and soft tissue tumors of hip and pelvis. Eur. J. Radiol. 2012, 81, 3793–3801. [Google Scholar] [CrossRef]
- Hudson, T.M.; Manaster, B.J.; Springfield, D.S.; Spanier, S.S.; Enneking, W.F.; Hawkins, I.F. Radiology of medullary chondrosarcoma: Preoperative treatment planning. Skelet. Radiol. 1983, 10, 69–78. [Google Scholar] [CrossRef]
- Reiter, F.B.; Ackerman, L.V.; Staple, T.W. Central Chondrosarcoma of the Appendicular Skeleton. Radiology 1972, 105, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Schiller, A.L.; Mankin, H.J. Chondrosarcoma: Correlation of radiological and histological grade. Radiology 1984, 150, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Logie, C.I.; Walker, E.A.; Forsberg, J.A.; Potter, B.K.; Murphey, M.D. Chondrosarcoma: A Diagnostic Imager’s Guide to Decision Making and Patient Management. Semin. Musculoskelet. Radiol. 2013, 17, 101–115. [Google Scholar] [CrossRef]
- Mayes, G.B.; Wallace, S.; Bernardino, M.E. Computed tomography of chondrosarcoma. J. Comput. Tomogr. 1981, 5, 345–348. [Google Scholar] [CrossRef]
- Wuisman, P.I.J.M.; Jutte, P.C.; Ozaki, T. Secondary chondrosarcoma in osteochondromas Medullary extension in 15 of 45 cases. Acta Orthop. 1997, 68, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Pierz, K.A.; Stieber, J.R.; Kusumi, K.; Dormans, J.P. Hereditary multiple exostoses: One center’s experience and review of etiology. Clin. Orthop. Relat. Res. 2002, 401, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, H.S.; Zimmerman, N.B.; Simon, M.A.; Wroble, R.R.; Millar, E.A.; Bonfiglio, M. The malignant potential of enchondromatosis. J. Bone Jt. Surg. 1987, 69, 269–274. [Google Scholar] [CrossRef]
- Liu, J.; Hudkins, P.G.; Swee, R.G.; Unni, K.K. Bone sarcomas associated with Ollier’s disease. Cancer 1987, 59, 1376–1385. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Papagelopoulos, P.J.; Soucacos, P.N. Skeletal Osteochondromas Revisited. Orthopedics 2008, 31, 1018–1028. [Google Scholar] [CrossRef]
- Lin, P.P.; Moussallem, C.D.; Deavers, M.T. Secondary Chondrosarcoma. J. Am. Acad. Orthop. Surg. 2010, 18, 608–615. [Google Scholar] [CrossRef]
- Mandahl, N.; Gustafson, P.; Mertens, F.; Åkerman, M.; Baldetorp, B.; Gisselsson, D.; Knuutila, S.; Bauer, H.C.F.; Larsson, O. Cytogenetic aberrations and their prognostic impact in chondrosarcoma. Genes Chromosom. Cancer 2002, 33, 188–200. [Google Scholar] [CrossRef]
- Murphey, M.D.; Choi, J.J.; Kransdorf, M.J.; Flemming, D.J.; Gannon, F.H. Imaging of Osteochondroma: Variants and Complications with Radiologic-Pathologic Correlation. Radiographics 2000, 20, 1407–1434. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Davies, A.; Cassar-Pullicino, V. Imaging the Complications of Osteochondromas. Clin. Radiol. 2002, 57, 18–28. [Google Scholar] [CrossRef]
- Woertler, K.; Lindner, N.; Gosheger, G.; Brinkschmidt, C.; Heindel, W. Osteochondroma: MR imaging of tumor-related complications. Eur. Radiol. 2000, 10, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.; Sissons, H.A. Radiographic hallmarks of peripheral chondrosarcoma. Radiology 1984, 151, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Murphey, M.D.; Flemming, D.J.; Kransdorf, M.J. Improved differentiation of benign osteochondromas from secondary chondrosarcomas with standardized measurement of cartilage cap at CT and MR imaging. Radiology 2010, 255, 857–865. [Google Scholar] [CrossRef]
- Herget, G.W.; Strohm, P.; Rottenburger, C.; Kontny, U.; Krauß, T.; Bohm, J.; Sudkamp, N.; Uhl, M. Insights into Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma 2014, 61, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Verdegaal, S.H.; Bovée, J.V.; Pansuriya, T.C.; Grimer, R.J.; Ozger, H.; Jutte, P.C.; Julian, M.S.; Biau, D.J.; Geest, I.C.; Leithner, A.; et al. Incidence, Predictive Factors, and Prognosis of Chondrosarcoma in Patients with Ollier Disease and Maffucci Syndrome: An International Multicenter Study of 161 Patients. Oncology 2011, 16, 1771–1779. [Google Scholar] [CrossRef]
- Robinson, P.; White, L.M.; Sundaram, M.; Kandel, R.; Wunder, J.; McDonald, D.J.; Janney, C.; Bell, R.S. Periosteal chondroid tumors: Radiologic evaluation with pathologic correlation. AJR Am. J. Roentgenol. 2001, 177, 1183–1188. [Google Scholar] [CrossRef]
- Vanel, D.; De Paolis, M.; Monti, C.; Mercuri, M.; Picci, P. Radiological features of 24 periosteal chondrosarcomas. Skelet. Radiol. 2001, 30, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Papagelopoulos, P.J.; Galanis, E.C.; Mavrogenis, A.F.; Savvidou, O.D.; Bond, J.R.; Unni, K.K.; Sim, F.H. Survivorship Analysis in Patients with Periosteal Chondrosarcoma. Clin. Orthop. Relat. Res. 2006, 448, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Boriani, S.; Laus, M.; Campanacci, M. Periosteal chondrosarcoma and periosteal osteosarcoma. Two distinct entities. J. Bone Jt. Surg. 1982, 64, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.M.; Unni, K.K.M.; McLeod, R.A.M.; Pritchard, D.J.M. Periosteal chondroma and periosteal chondrosarcoma. Am. J. Surg. Pathol. 1985, 9, 666–677. [Google Scholar] [CrossRef]
- Collins, M.S.; Koyama, T.; Swee, R.G.; Inwards, C.Y. Clear cell chondrosarcoma: Radiographic, computed tomographic, and magnetic resonance findings in 34 patients with pathologic correlation. Skelet. Radiol. 2003, 32, 687–694. [Google Scholar] [CrossRef]
- Kumar, R.; David, R.; Cierney, G., 3rd. Clear cell chondrosarcoma. Radiology 1985, 154, 45–48. [Google Scholar] [CrossRef]
- Bagley, L.; Kneeland, J.B.; Dalinka, M.K.; Bullough, P.; Brooks, J. Unusual behavior of clear cell chondrosarcoma. Skelet. Radiol. 1993, 22, 279–282. [Google Scholar] [CrossRef]
- PPresent, D.; Bacchini, P.; Pignatti, G.; Picci, P.; Bertoni, F.; Campanacci, M. Clear cell chondrosarcoma of bone. Skelet. Radiol. 1991, 20, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.E.; Lillemoe, T.J.; Manivel, J.C.; Wick, M.R. Mesenchymal chondrosarcoma. An immunohistochemical study. Arch. Pathol. Lab. Med. 1990, 114, 943–948. [Google Scholar]
- Hoang, M.P.; Suarez, P.A.; Donner, L.R.; Ro, J.Y.; Ordñez, N.G.; Ayala, A.G.; Czerniak, B. Mesenchymal Chondrosarcoma: A Small Cell Neoplasm with Polyphenotypic Differentiation. Int. J. Surg. Pathol. 2000, 8, 291–301. [Google Scholar] [CrossRef]
- Nakashima, Y.; Unni, K.K.; Shives, T.C.; Swee, R.G.; Dahlin, D.C. Mesenchymal chondrosarcoma of bone and soft tissue. A review of 111 cases. Cancer 1986, 57, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.Q. Mesenchymal chondrosarcoma of the maxilla. AJR Am. J. Roentgenol. 2002, 179, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Shinaver, C.N.; Mafee, M.F.; Choi, K.H. MRI of mesenchymal chondrosarcoma of the orbit: Case report and review of the literature. Neuroradiology 1997, 39, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Capanna, R.; Bertoni, F.; Bettelli, G.; Picci, P.; Bacchini, P.; Present, D.; Giunti, A.; Campanacci, M. Dedifferentiated chondrosarcoma. J. Bone Jt. Surg. Am. 1988, 70, 60–69. [Google Scholar] [CrossRef]
- Daly, P.J.; Sim, F.H.; Wold, L.E. Dedifferentiated chondrosarcoma of bone. Orthopedics 1989, 12, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.; Picci, P.; Campanacci, L.; Rulli, E. Dedifferentiated chondrosarcoma. Skelet. Radiol. 1995, 24, 409–416. [Google Scholar] [CrossRef]
- Grimer, R.J.; Gosheger, G.; Taminiau, A.; Biau, D.; Matejovsky, Z.; Kollender, Y.; San-Julian, M.; Gherlinzoni, F.; Ferrari, C. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur. J. Cancer 2007, 43, 2060–2065. [Google Scholar] [CrossRef]
- Littrell, L.A.; Wenger, D.E.; Wold, L.E.; Bertoni, F.; Unni, K.K.; White, L.; Kandel, R.; Sundaram, M. Radiographic, CT, and MR Imaging Features of Dedifferentiated Chondrosarcomas: A Retrospective Review of 174 De Novo Cases. Radiographics 2004, 24, 1397–1409. [Google Scholar] [CrossRef]
- Saifuddin, A.; Mann, B.; Mahroof, S.; Pringle, J.; Briggs, T.; Cannon, S. Dedifferentiated chondrosarcoma: Use of MRI to guide needle biopsy. Clin. Radiol. 2004, 59, 268–272. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Wada, T.; Nagoya, S.; Ikeda, T.; Isu, K.; Yamashiro, K.; Kawai, A.; Ishii, T.; Araki, N.; Myoui, A.; et al. Extraskeletal myxoid chondrosarcoma: A Multi-Institutional Study of 42 Cases in Japan. Cancer 2003, 97, 1285–1292. [Google Scholar] [CrossRef]
- Drilon, A.D.; Popat, S.; Bhuchar, G.; D’Adamo, D.R.; Keohan, M.L.; Fisher, C.; Antonescu, C.R.; Singer, S.; Brennan, M.F.; Judson, I.; et al. Extraskeletal myxoid chondrosarcoma: A retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 2008, 113, 3364–3371. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Argani, P.; Erlandson, R.A.; Healey, J.H.; Ladanyi, M.; Huvos, A.G. Skeletal and extraskeletal myxoid chondrosarcoma: A comparative clinicopathologic, ultrastructural, and molecular study. Cancer 1998, 83, 1504–1521. [Google Scholar] [CrossRef]
- Aigner, T.; Oliveira, A.M.; Nascimento, A.G. Extraskeletal myxoid chondrosarcomas do not show a chondrocytic phenotype. Mod. Pathol. 2004, 17, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.E. WHO Classification of Soft Tissue and Bone, fourth edition: Summary and commentary. Curr. Opin. Oncol. 2013, 25, 571–573. [Google Scholar]
- Gassert, F.G.; Breden, S.; Neumann, J.; Gassert, F.T.; Bollwein, C.; Knebel, C.; Lenze, U.; von Eisenhart-Rothe, R.; Mogler, C.; Makowski, M.R.; et al. Differentiating Enchondromas and Atypical Cartilaginous Tumors in Long Bones with Computed Tomography and Magnetic Resonance Imaging. Diagnostics 2022, 12, 2186. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-B.; Jee, W.-H.; Sunwoo, H.-J.; Cho, J.-H.; Kim, J.-Y.; Chun, K.-A.; Hong, S.-J.; Chung, H.W.; Sung, M.-S.; Lee, Y.-S.; et al. MR differentiation of low-grade chondrosarcoma from enchondroma. Clin. Imaging 2013, 37, 542–547. [Google Scholar] [CrossRef]
- Crim, J.; Schmidt, R.; Layfield, L.; Hanrehan, C.; Manaster, B.J. Can imaging criteria distinguish enchondroma from grade 1 chondrosarcoma? Eur. J. Radiol. 2015, 84, 2222–2230. [Google Scholar] [CrossRef]
- Lisson, C.S.; Lisson, C.G.; Flosdorf, K.; Mayer-Steinacker, R.; Schulthesis, M.; von Baer, A.; Barth, T.F.E.; Beer, A.J.; Baumhauer, M.; Meier, R.; et al. Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: A pilot study. Eur. Radiol. 2018, 28, 468–477. [Google Scholar] [CrossRef]
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J. Bone Jt. Surg. Am. 2007, 89, 2113–2123. [Google Scholar] [CrossRef]
- Eefting, D.; Schrage, Y.M.; Geirnaerdt, M.J.A.; Le Cessie, S.; Taminiau, A.H.M.; Bovée, J.V.M.G.; Hogendoorn, P.C.W. Assessment of Interobserver Variability and Histologic Parameters to Improve Reliability in Classification and Grading of Central Cartilaginous Tumors. Am. J. Surg. Pathol. 2009, 33, 50–57. [Google Scholar] [CrossRef]
- De Coninck, T.; Jans, L.; Sys, G.; Verstraeten, T.; Forsyth, R.; Poffyn, B.; Verstraete, K. Dynamic contrast-enhanced MR imaging for differentiation between enchondroma and chondrosarcoma. Eur. Radiol. 2013, 23, 3140–3152. [Google Scholar] [CrossRef]
- Douis, H.; Jeys, L.; Grimer, R.; Vaiyapuri, S.; Davies, A.M. Is there a role for diffusion-weighted MRI (DWI) in the diagnosis of central cartilage tumors? Skelet. Radiol. 2015, 44, 963–969. [Google Scholar] [CrossRef]
- Fritz, B.; Muller, D.A.; Sutter, R.; Wurnig, M.C.; Wagner, M.W.; Pfirrmann, C.W.A.; Fischer, M.A. Magnetic Resonance Imaging-Based Grading of Cartilaginous Bone Tumors: Added Value of Quantitative Texture Analysis. Investig. Radiol. 2018, 53, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, K.; Le, H.; Jiang, Y.; Li, W.; Geng, Y.; Li, S.; Hong, G. Radiomics Nomograms Based on Non-enhanced MRI and Clinical Risk Factors for the Differentiation of Chondrosarcoma from Enchondroma. J. Magn. Reson. Imaging 2021, 54, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Tyrrell, P.N.M.; Singh, J.; Gregory, J.; Cribb, G.L.; Cool, P. Surveillance of intramedullary cartilage tumours in long bones. Bone Jt. J. 2016, 98-B, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Deckers, C.; Schreuder, B.H.; Hannink, G.; de Rooy, J.W.; van der Geest, I. Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J. Surg. Oncol. 2016, 114, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Parlier-Cuau, C.; Bousson, V.; Ogilvie, C.M.; Lackman, R.D.; Laredo, J.-D. When should we biopsy a solitary central cartilaginous tumor of long bones? Literature review and management proposal. Eur. J. Radiol. 2011, 77, 6–12. [Google Scholar] [CrossRef]
- Vanel, D.; Ruggieri, P.; Ferrari, S.; Picci, P.; Gambarotti, M.; Staals, E.; Alberghini, M. The incidental skeletal lesion: Ignore or explore? Cancer Imaging 2009, 9, S38–S43. [Google Scholar] [CrossRef]
- Patel, A.; Davies, A.; Botchu, R.; James, S. A pragmatic approach to the imaging and follow-up of solitary central cartilage tumours of the proximal humerus and knee. Clin. Radiol. 2019, 74, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Deckers, C.; de Rooy, J.W.J.; Flucke, U.; Schreuder, H.W.B.; Dierselhuis, E.F.; van der Geest, I.C.M. Midterm MRI Follow-Up of Untreated Enchondroma and Atypical Cartilaginous Tumors in the Long Bones. Cancers 2021, 13, 4093. [Google Scholar] [CrossRef]
- van de Sande, M.A.J.; van der Wal, R.J.P.; Canete, A.N.; van Rijswijk, C.S.P.; Kroon, H.M.; Dijkstra, P.D.S.; Bloem, J.L.H. Radiologic differentiation of enchondromas, atypical cartilaginous tumors, and high-grade chondrosarcomas-Improving tumor-specific treatment: A paradigm in transit? Cancer 2019, 125, 3288–3291. [Google Scholar] [CrossRef]
- Sullivan, C.W.; Kazley, J.M.; Murtaza, H.; Cooley, M.; Jones, D.; DiCaprio, M.R. Team Approach: Evaluation and Management of Low-Grade Cartilaginous Lesions. JBJS Rev. 2020, 8, e0054. [Google Scholar] [CrossRef]
- Omlor, G.W.; Lohnherr, V.; Lange, J.; Gantz, S.; Mechtersheimer, G.; Merle, C.; Raiss, P.; Fellenberg, J.; Lehner, B. Outcome of conservative and surgical treatment of enchondromas and atypical cartilaginous tumors of the long bones: Retrospective analysis of 228 patients. BMC Musculoskelet. Disord. 2019, 20, 134. [Google Scholar] [CrossRef]
- Akoh, C.C.; Craig, E.; Troester, A.M.; Miller, B.J. Radiographic Enchondroma Surveillance: Assessing Clinical Outcomes and Costs Effectiveness. Iowa Orthop. J. 2019, 39, 185–193. [Google Scholar] [PubMed]
- Deckers, C.; Steyvers, M.J.; Hannink, G.; Schreuder, H.W.B.; de Rooy, J.W.J.; Van Der Geest, I.C.M. Can MRI differentiate between atypical cartilaginous tumors and high-grade chondrosarcoma? A systematic review. Acta Orthop. 2020, 91, 471–478. [Google Scholar] [CrossRef]
- Laitinen, M.; Stevenson, J.D.; Parry, M.C.; Sumathi, V.; Grimer, R.J.; Jeys, L. The role of grade in local recurrence and the disease-specific survival in chondrosarcomas. Bone Jt. J. 2018, 100-B, 662–666. [Google Scholar] [CrossRef]
- Jain, V.; Oliveira, I.; Chavda, A.; Khoo, M.; Saifuddin, A. MRI differentiation of low-grade and high-grade chondrosarcoma of the shoulder girdle, chest wall and pelvis: A pictorial review based on 111 consecutive cases. Br. J. Radiol. 2021, 94, 20201404. [Google Scholar] [CrossRef] [PubMed]
- Douis, H.; Singh, L.; Saifuddin, A. MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur. Radiol. 2013, 24, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.M.; Iv, A.A.; Aljubair, H. Magnetic Resonance Imaging Role in the Differentiation Between Atypical Cartilaginous Tumors and High-Grade Chondrosarcoma: An Updated Systematic Review. Cureus 2020, 12, e11237. [Google Scholar] [CrossRef]
- MacSweeney, F.; Darby, A.; Saifuddin, A. Dedifferentiated chondrosarcoma of the appendicular skeleton: MRI-pathological correlation. Skelet. Radiol. 2003, 32, 671–678. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Choi, J.-Y.; Moon, K.C.; Kim, H.-S.; Kang, H.S. Differentiating high-grade from low-grade chondrosarcoma with MR imaging. Eur. Radiol. 2009, 19, 3008–3014. [Google Scholar] [CrossRef]
- Geirnaerdt, M.J.A.; Hogendoorn, P.; Bloem, J.L.; Taminiau, A.H.M.; Van Der Woude, H.-J. Cartilaginous Tumors: Fast Contrast-enhanced MR Imaging. Radiology 2000, 214, 539–546. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Chen, H.-Y.; Yu, J.-N.; Zhu, X.-L.; Chen, J.-Y.; Shao, G.-L.; Yu, R.-S. Diagnostic Value of CT- and MRI-Based Texture Analysis and Imaging Findings for Grading Cartilaginous Tumors in Long Bones. Front. Oncol. 2021, 11, 700204. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Albano, D.; Chianca, V.; Messina, C.; Gambino, A.; Ugga, L.; Cortese, M.C.; Lazzara, A.; Ricci, D.; et al. MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur. J. Radiol. 2020, 128, 109043. [Google Scholar] [CrossRef]
- Zhong, J.; Hu, Y.; Ge, X.; Xing, Y.; Ding, D.; Zhang, G.; Zhang, H.; Yang, Q.; Yao, W. A systematic review of radiomics in chondrosarcoma: Assessment of study quality and clinical value needs handy tools. Eur. Radiol. 2022, 33, 1433–1444. [Google Scholar] [CrossRef]
- Leerapun, T.; Hugate, R.R.; Inwards, C.Y.; Scully, S.P.; Sim, F.H. Surgical Management of Conventional Grade I Chondrosarcoma of Long Bones. Clin. Orthop. Relat. Res. 2007, 463, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Hogendoorn, P.C.W.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.M.; Bovee, J.V.M.G. The clinical approach towards chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Bovee, J.; Cleton-Jansen, A.-M.; Taminiau, A.H.; Hogendoorn, P. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005, 6, 599–607. [Google Scholar] [CrossRef]
- Dickey, I.D.; Rose, P.S.; Fuchs, B.; Wold, L.E.; Okuno, S.H.; Sim, F.H.; Scully, S.P. Dedifferentiated Chondrosarcoma: The Role of Chemotherapy with Updated Outcomes. J. Bone Jt. Surg. 2004, 86, 2412–2418. [Google Scholar] [CrossRef]
- van Maldegem, A.M.; Bovée, J.V.; Gelderblom, H. Comprehensive analysis of published studies involving systemic treatment for chondrosarcoma of bone between 2000 and 2013. Clin. Sarcoma Res. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Frezza, A.M.; Cesari, M.; Baumhoer, D.; Biau, D.; Bielack, S.; Campanacci, D.A.; Casanova, J.; Esler, C.; Ferrari, S.; Funovics, P.T.; et al. Mesenchymal chondrosarcoma: Prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur. J. Cancer 2015, 51, 374–381. [Google Scholar] [CrossRef]
- Suit, H.D.; Goitein, M.; Munzenrider, J.; Verhey, L.; Davis, K.R.; Koehler, A.; Linggood, R.; Ojemann, R.G. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J. Neurosurg. 1982, 56, 377–385. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Lin, C.-Y.; Kuo, S.-J.; Su, C.-M.; Tang, C.-H. An update on current and future treatment options for chondrosarcoma. Expert Rev. Anticancer. Ther. 2019, 19, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Mery, B.; Espenel, S.; Guy, J.-B.; Rancoule, C.; Vallard, A.; Aloy, M.-T.; Rodriguez-Lafrasse, C.; Magné, N. Biological aspects of chondrosarcoma: Leaps and hurdles. Crit. Rev. Oncol. 2018, 126, 32–36. [Google Scholar] [CrossRef]
- Tlemsani, C.; Larousserie, F.; De Percin, S.; Audard, V.; Hadjadj, D.; Chen, J.; Biau, D.; Anract, P.; Terris, B.; Goldwasser, F.; et al. Biology and Management of High-Grade Chondrosarcoma: An Update on Targets and Treatment Options. Int. J. Mol. Sci. 2023, 24, 1361. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, R.; Ayadi, M.; Gomez-Brouchet, A.; Armenoult, L.; Banneau, G.; Elarouci, N.; Tallegas, M.; Decouvelaere, A.-V.; Aubert, S.; Redini, F.; et al. Integrated molecular characterization of chondrosarcoma reveals critical determinants of disease progression. Nat. Commun. 2019, 10, 4622. [Google Scholar] [CrossRef]
- Chen, J.-C.; Shih, H.-C.; Lin, C.-Y.; Guo, J.-H.; Huang, C.; Huang, H.-C.; Chong, Z.-Y.; Tang, C.-H. MicroRNA-631 Resensitizes Doxorubicin-Resistant Chondrosarcoma Cells by Targeting Apelin. Int. J. Mol. Sci. 2023, 24, 839. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets and Emerging Treatments in Advanced Chondrosarcoma. Int. J. Mol. Sci. 2022, 23, 1096. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, E.; Savadkoohi, M.G.; Majidzadeh-A, K.; Esmaeili, R. Chondrosarcoma: An overview of clinical behavior, molecular mechanisms mediated drug resistance and potential therapeutic targets. Crit. Rev. Oncol. 2018, 131, 102–109. [Google Scholar] [CrossRef]
- Thoenen, E.; Curl, A.; Iwakuma, T. TP53 in bone and soft tissue sarcomas. Pharmacol. Ther. 2019, 202, 149–164. [Google Scholar] [CrossRef]
- Su, C.-M.; Fong, Y.-C.; Tang, C.-H. An overview of current and future treatment options for chondrosarcoma. Expert Opin. Orphan. Drugs 2014, 2, 217–227. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, H.-J. Biomarkers of chondrosarcoma. J. Clin. Pathol. 2018, 71, 579–583. [Google Scholar] [CrossRef] [PubMed]

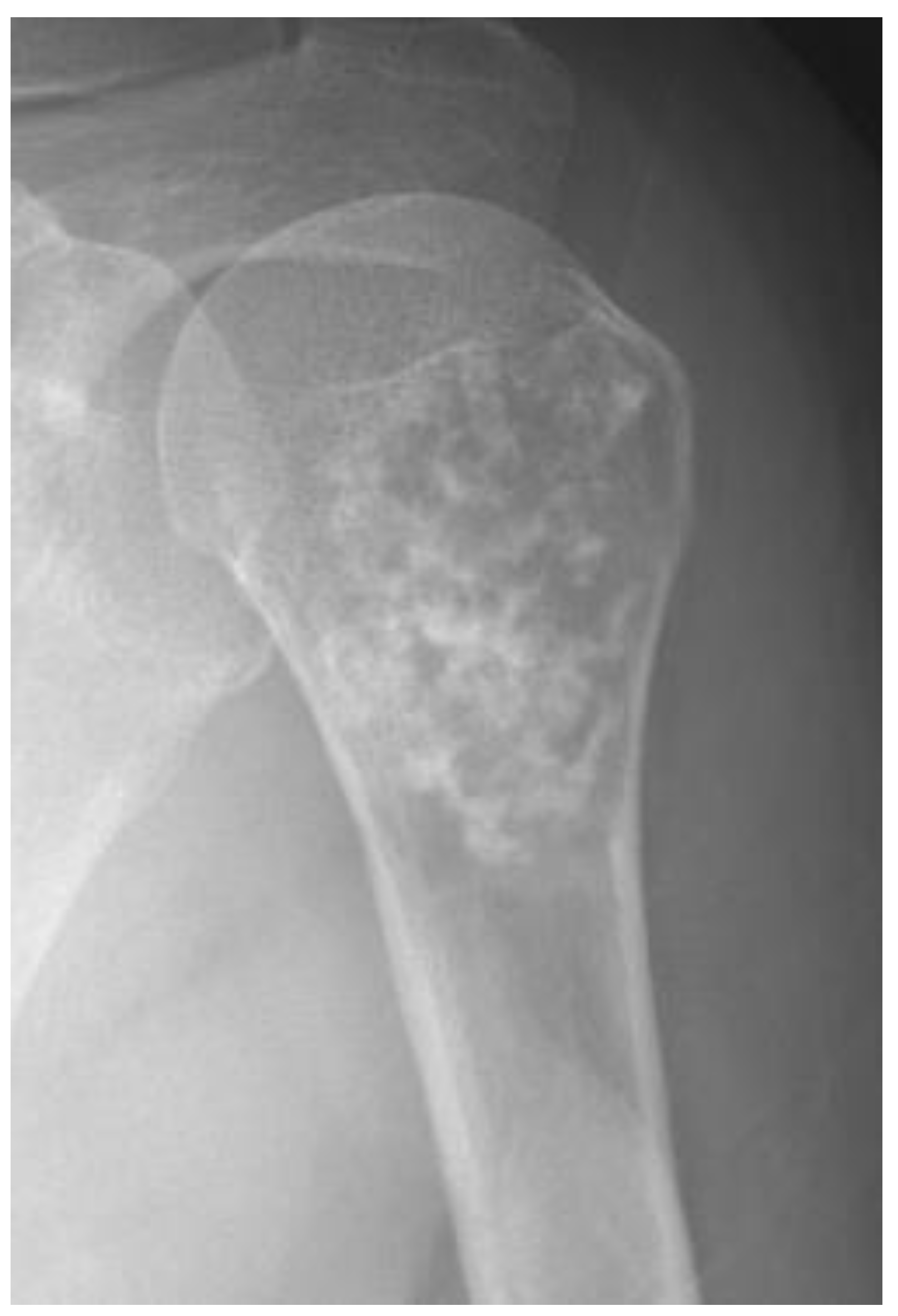
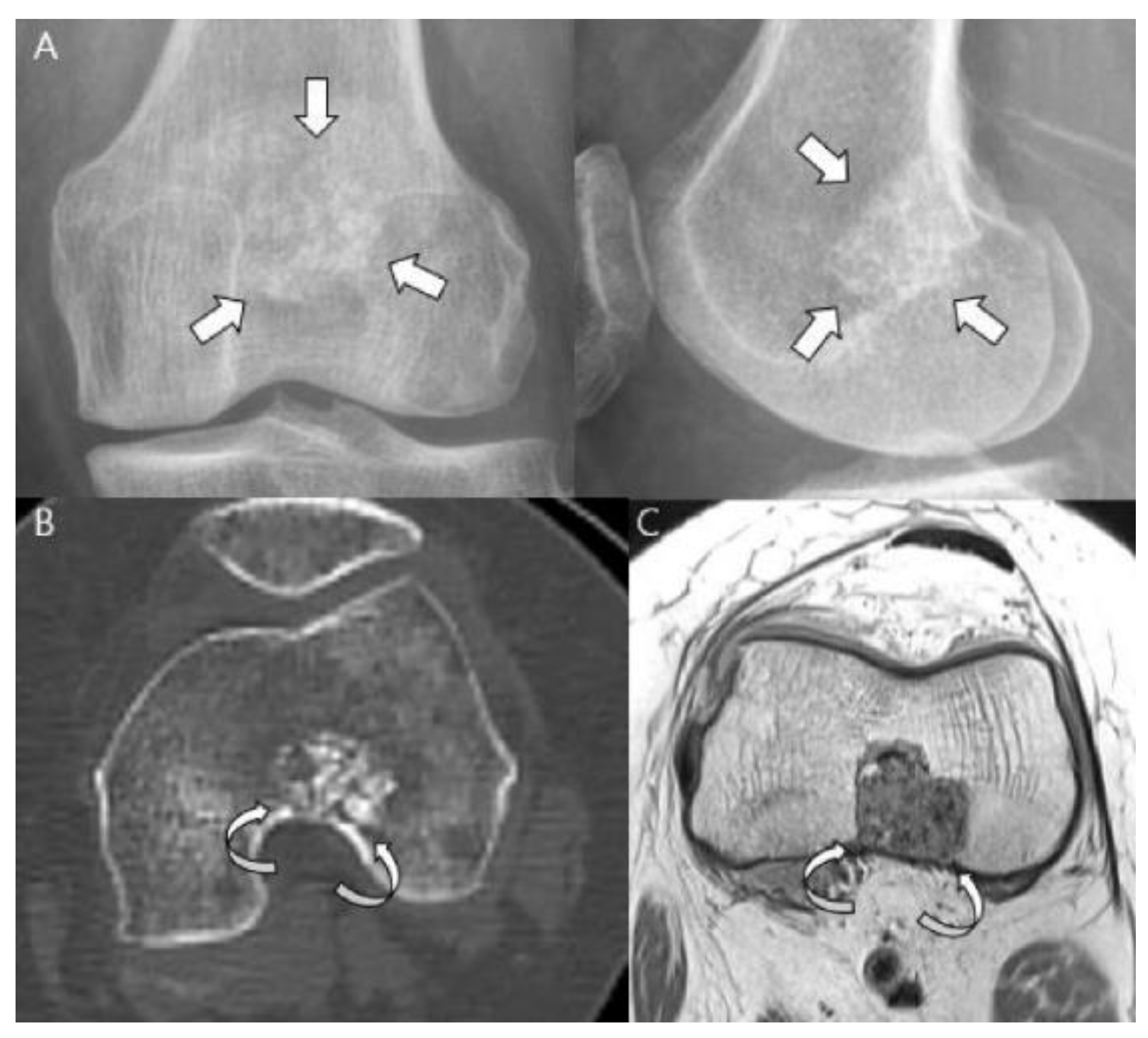
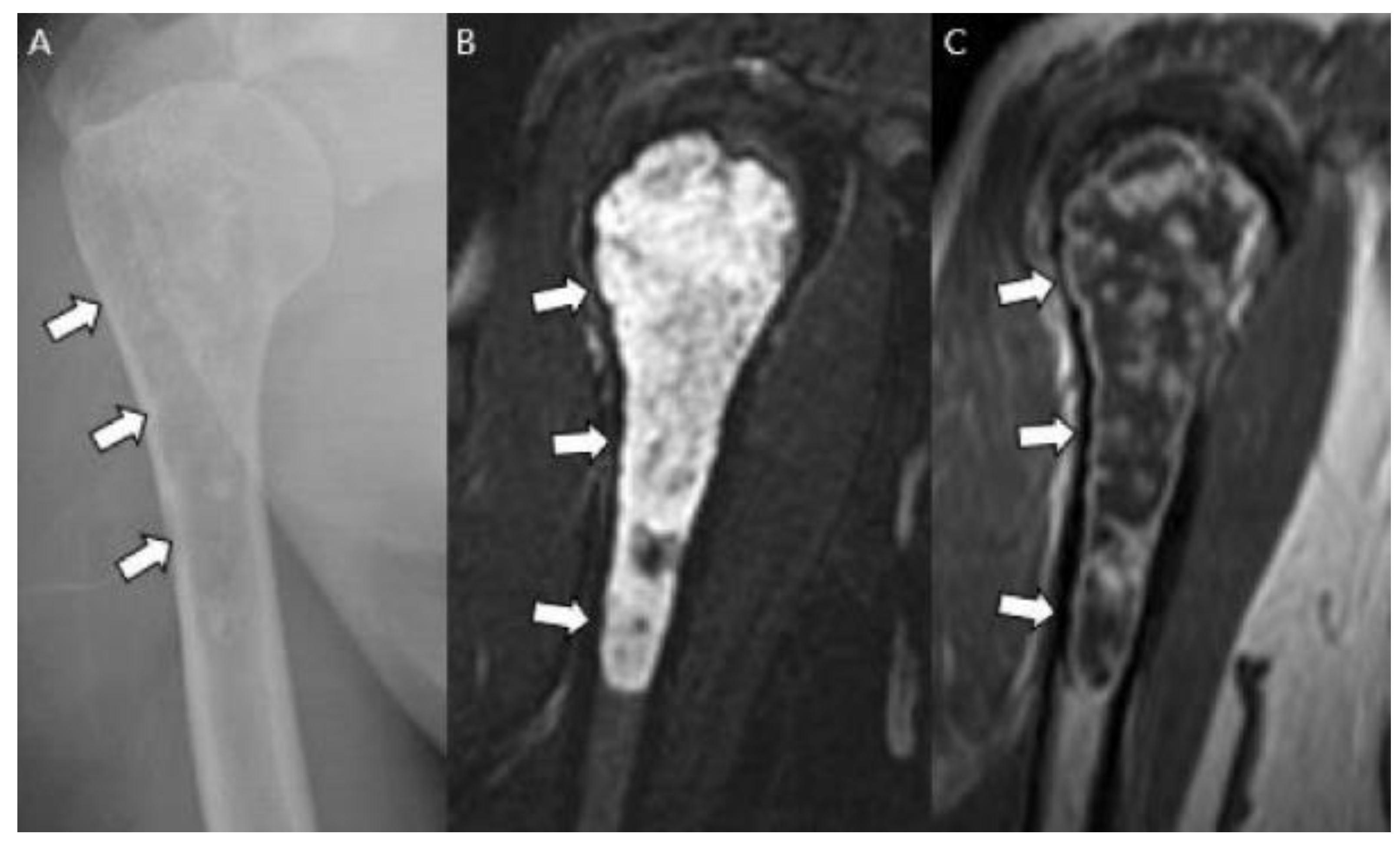
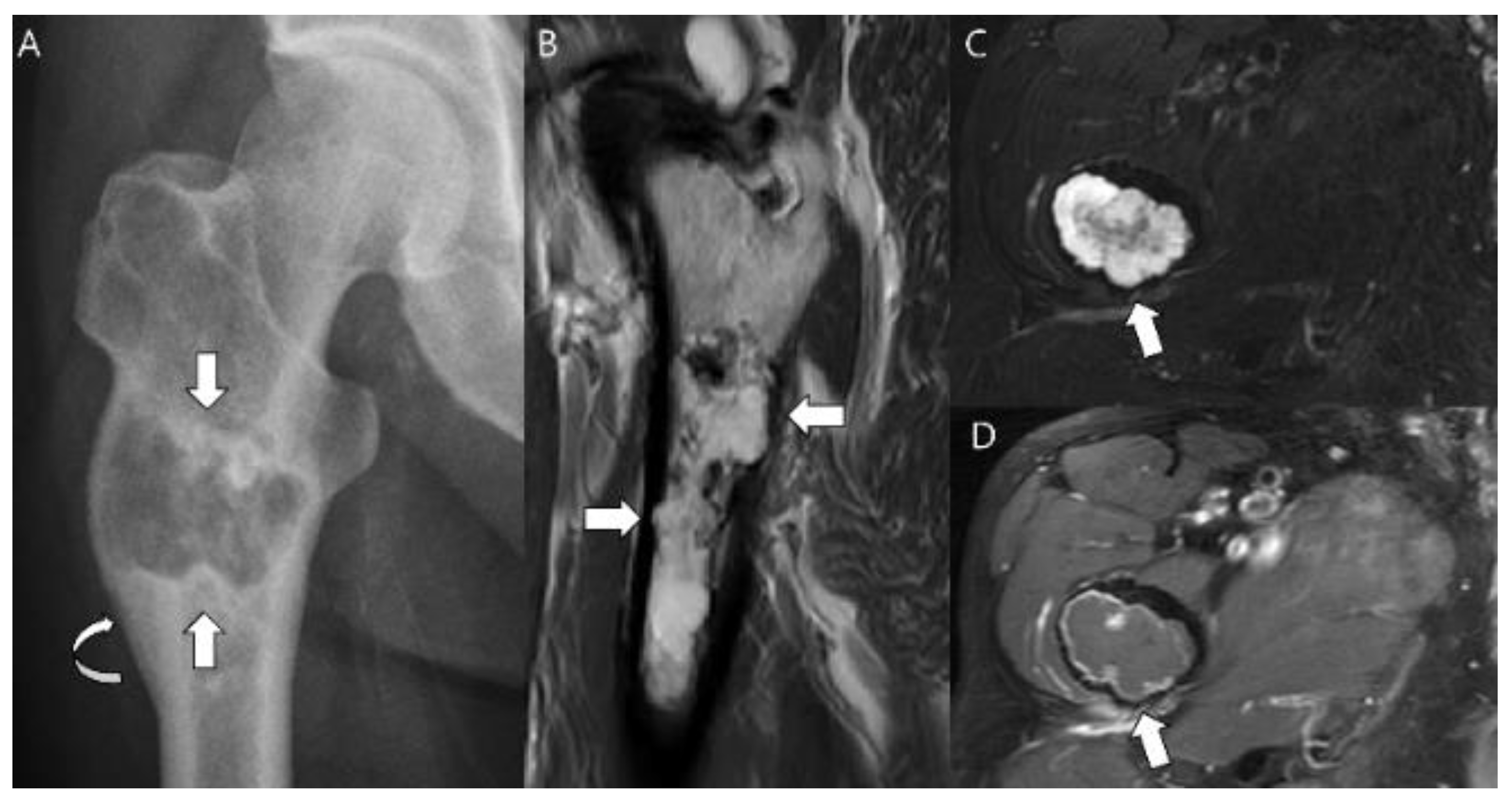
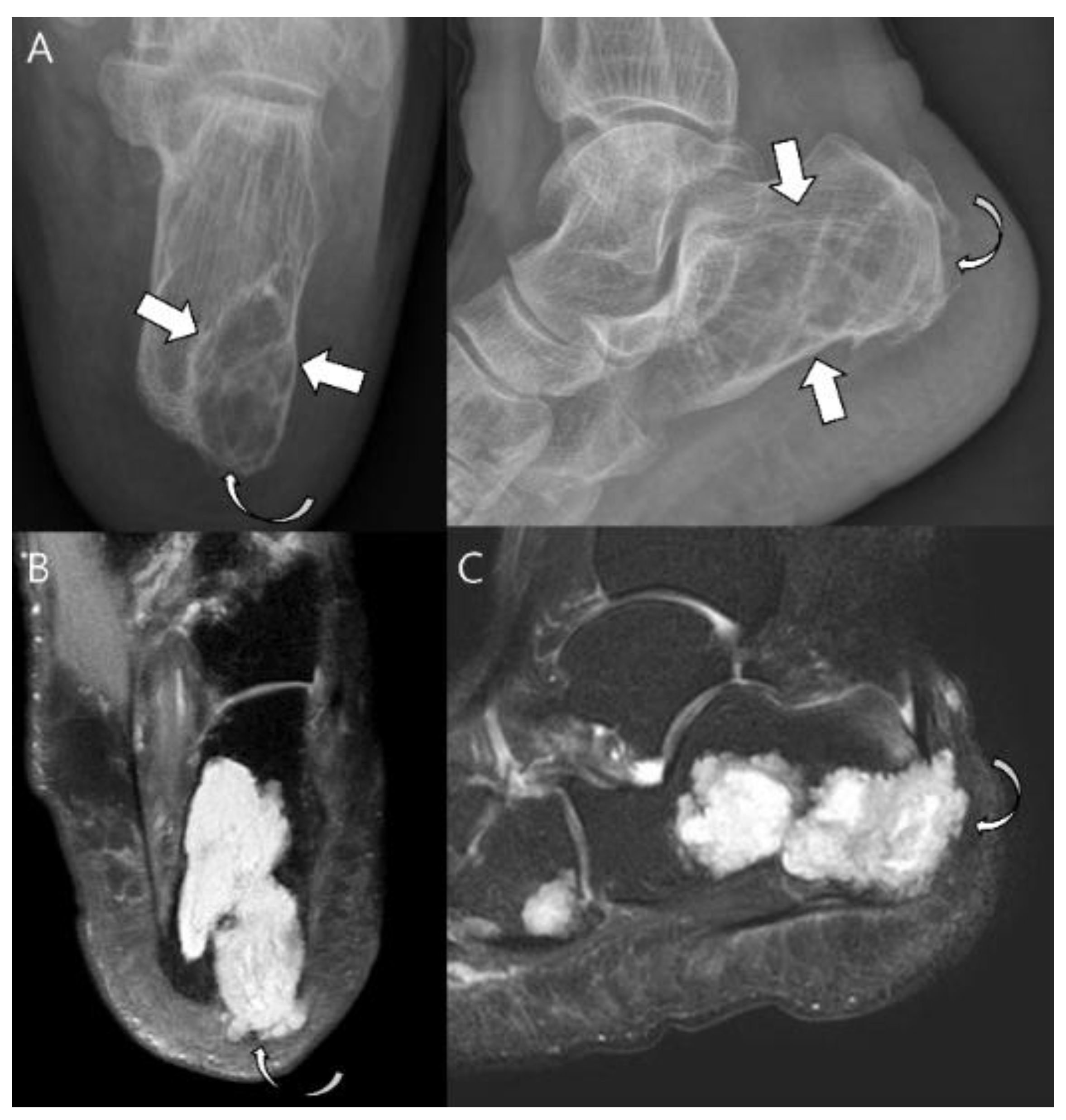
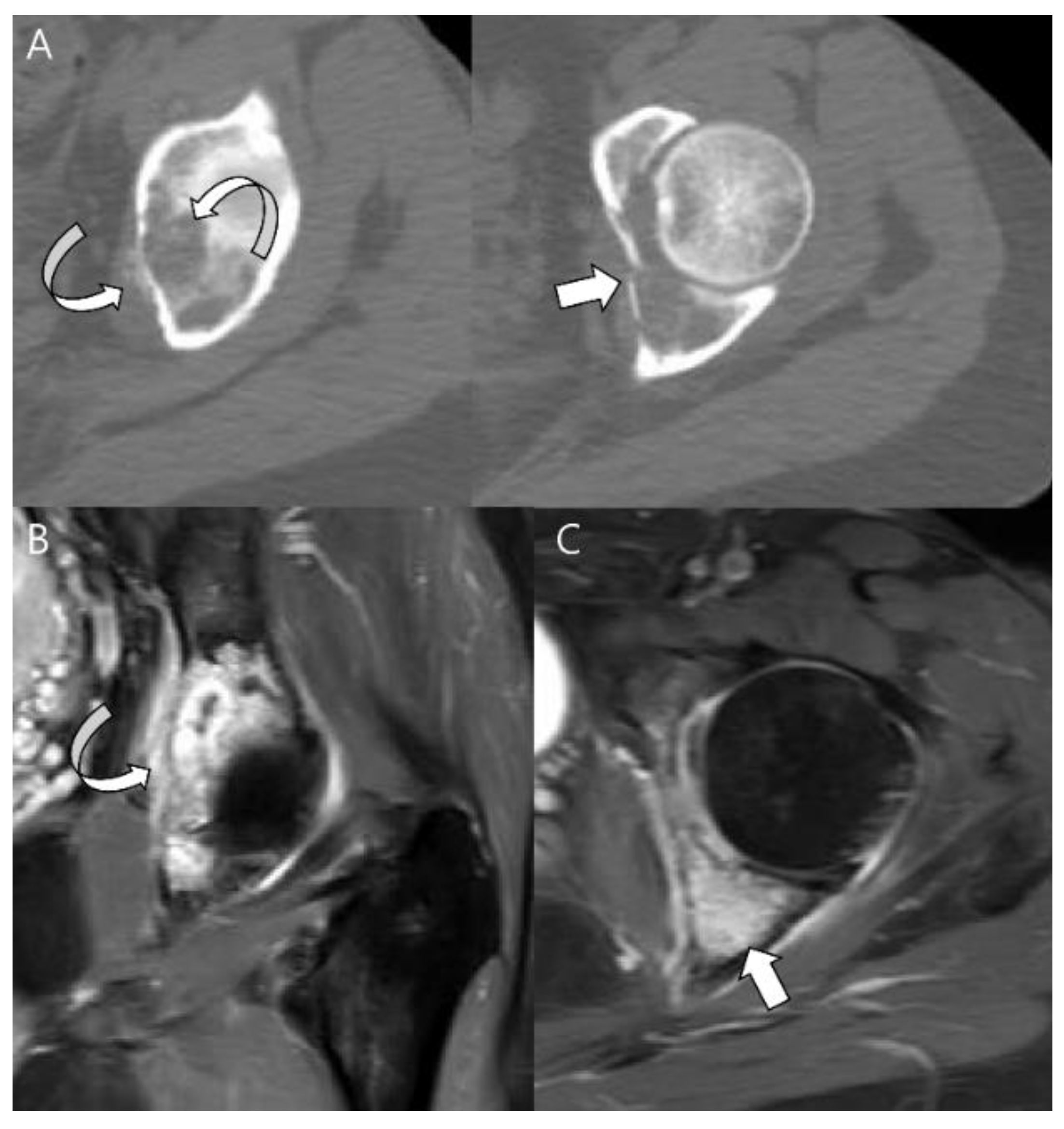
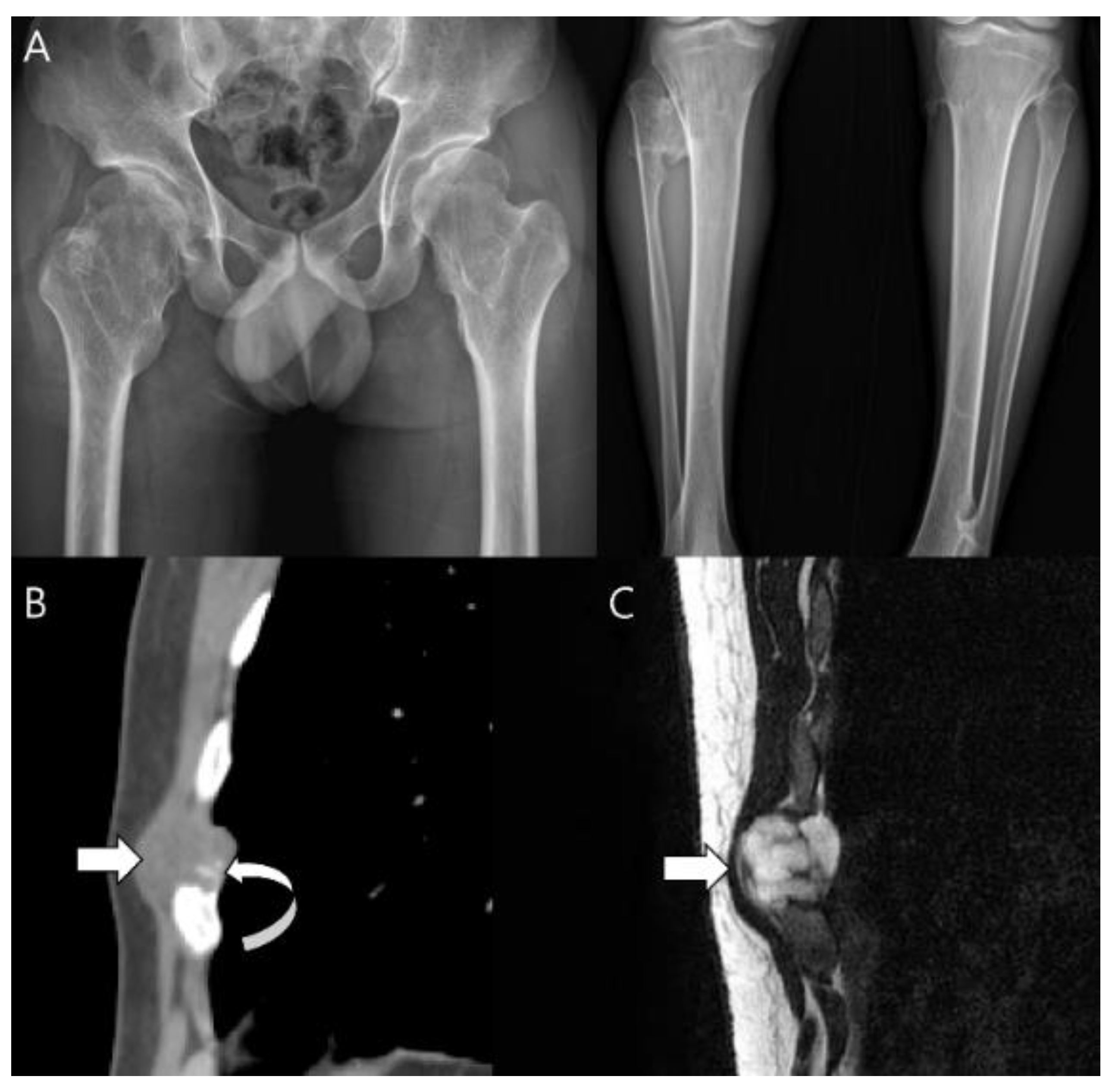
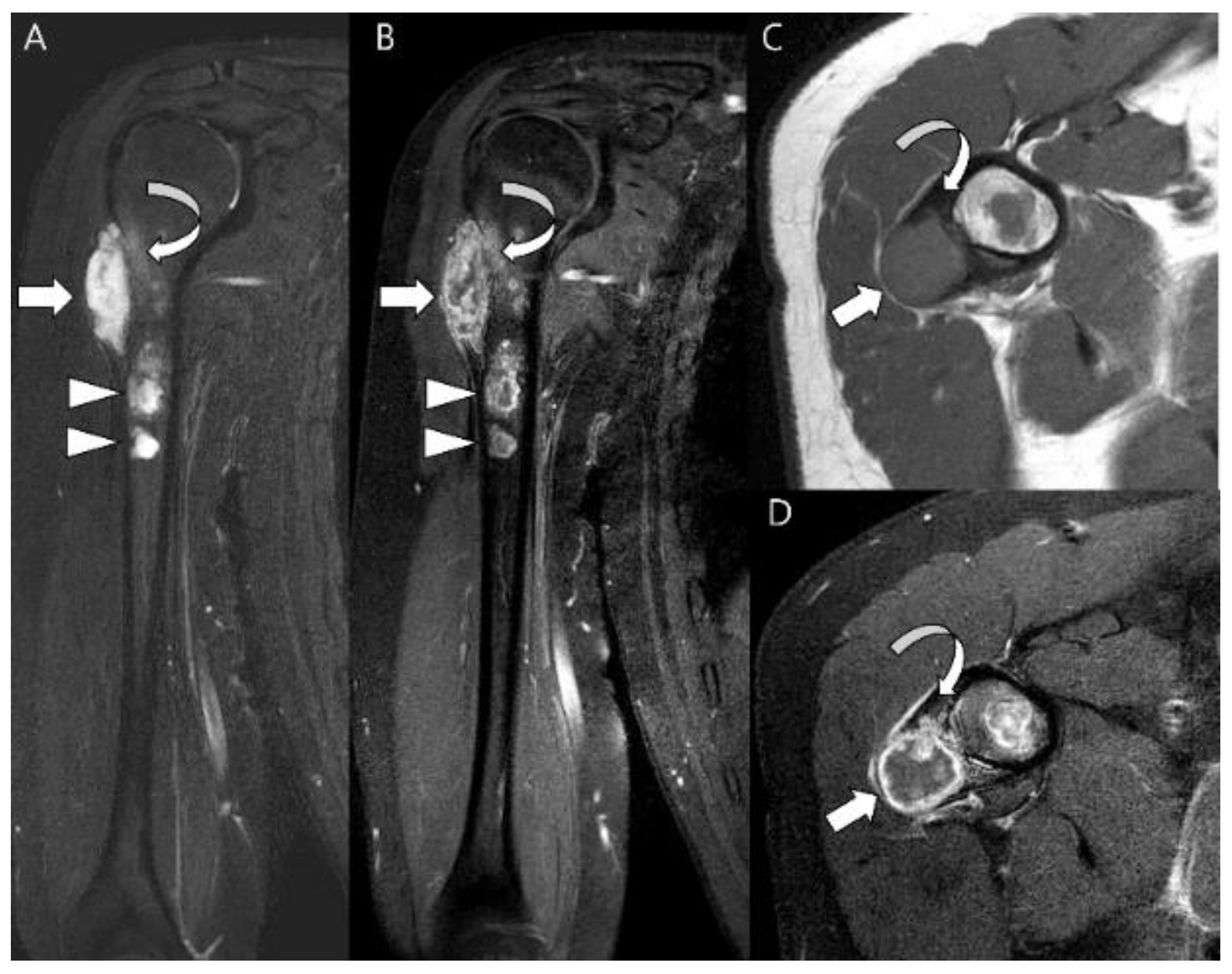
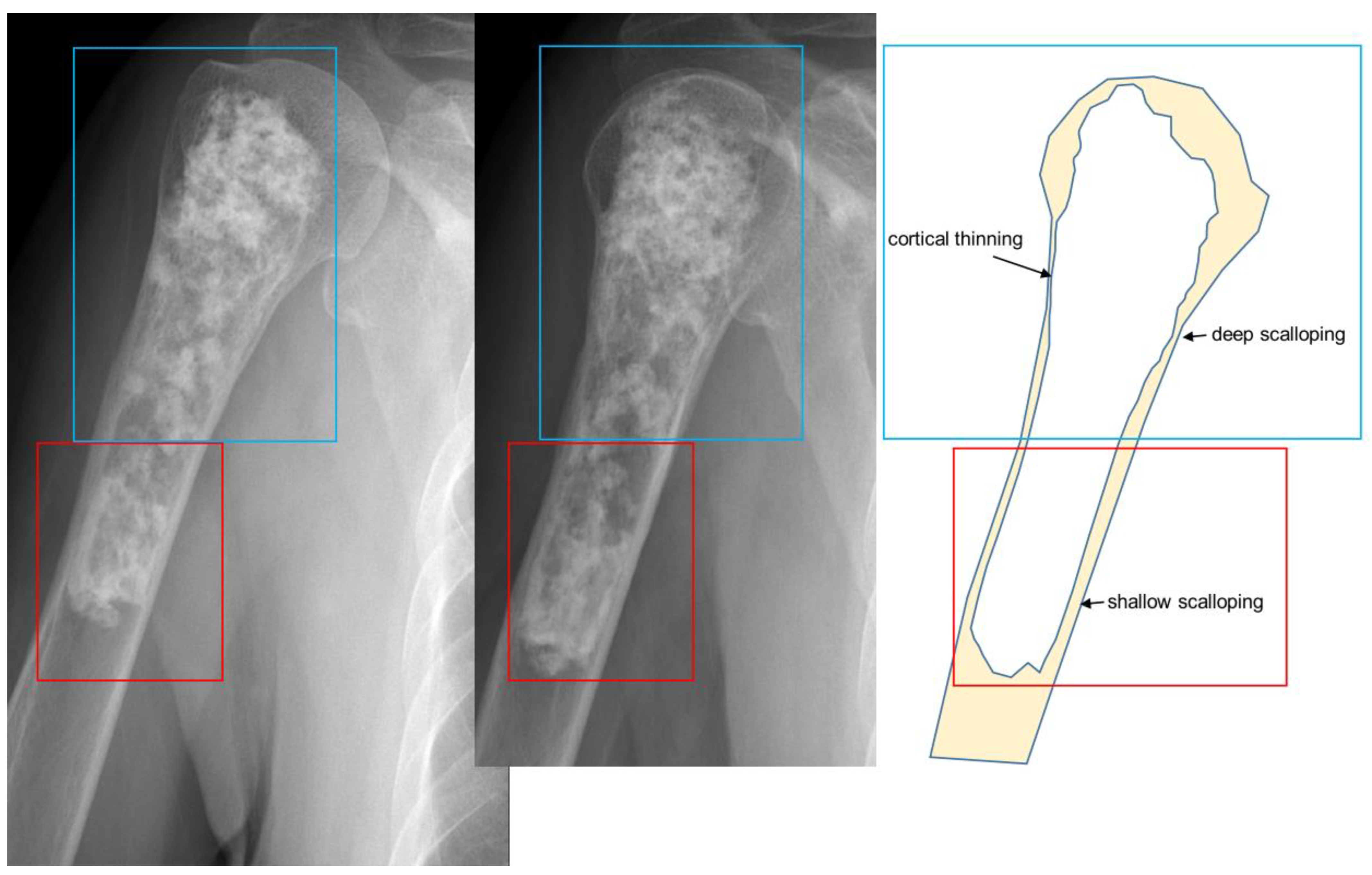

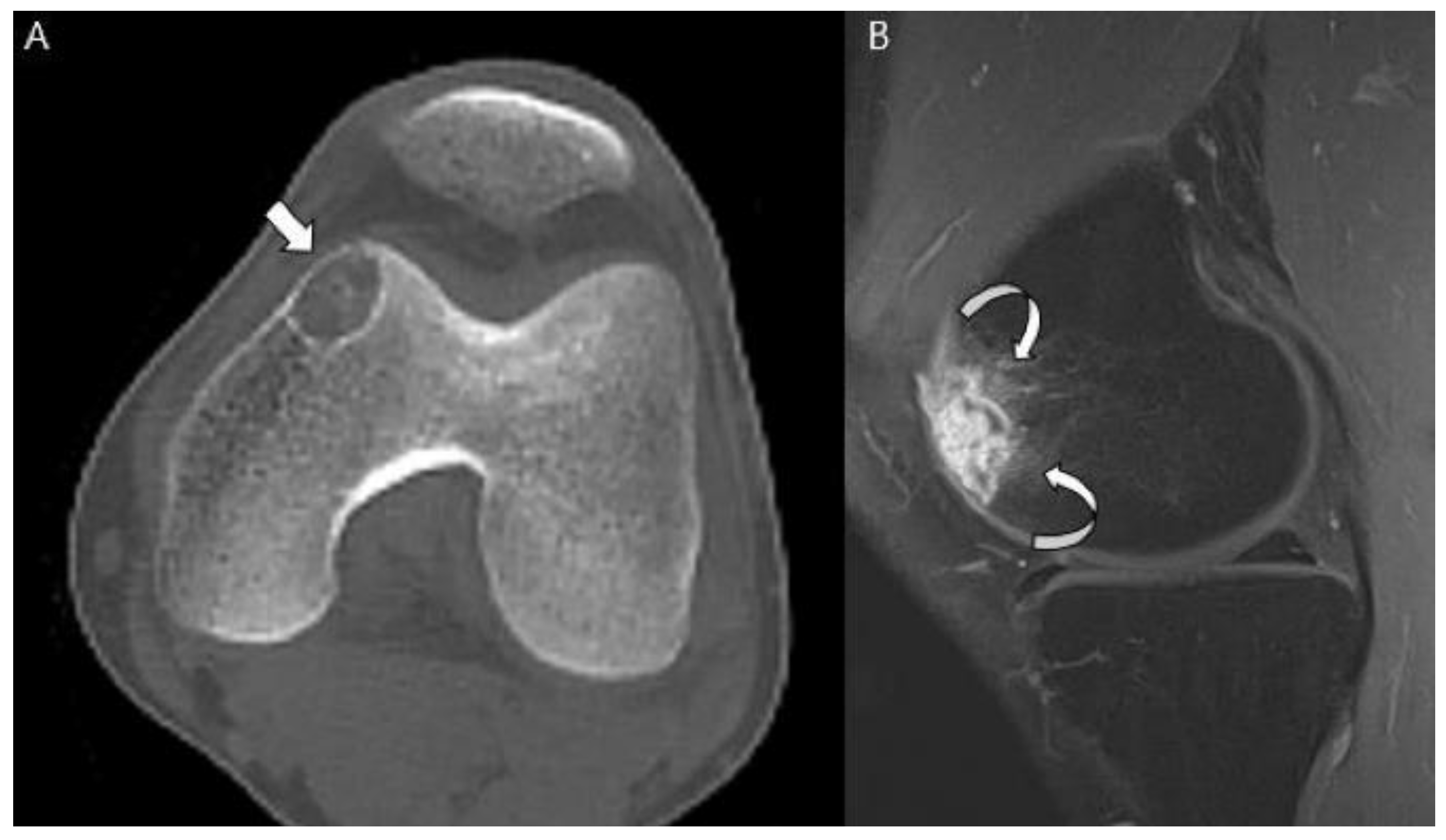
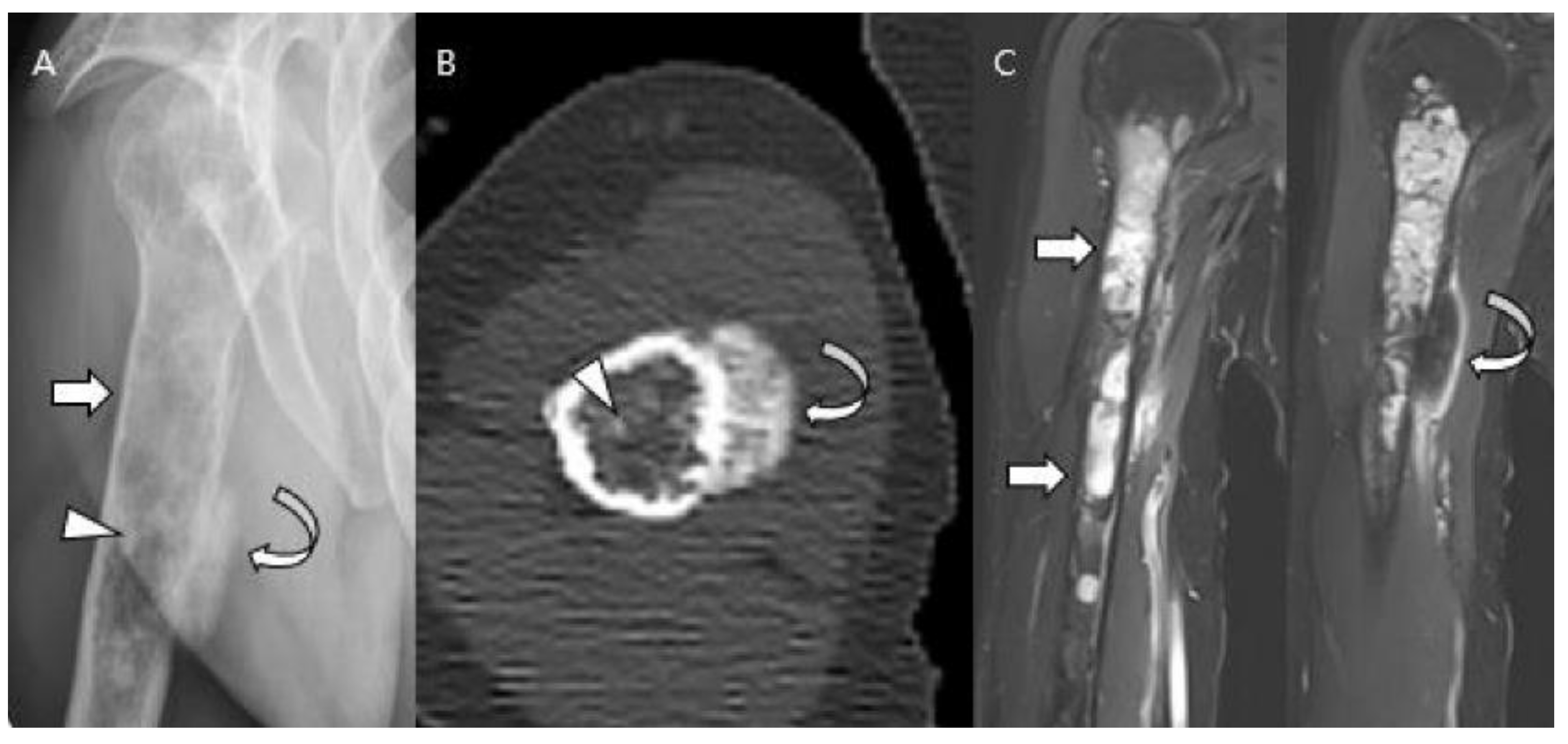

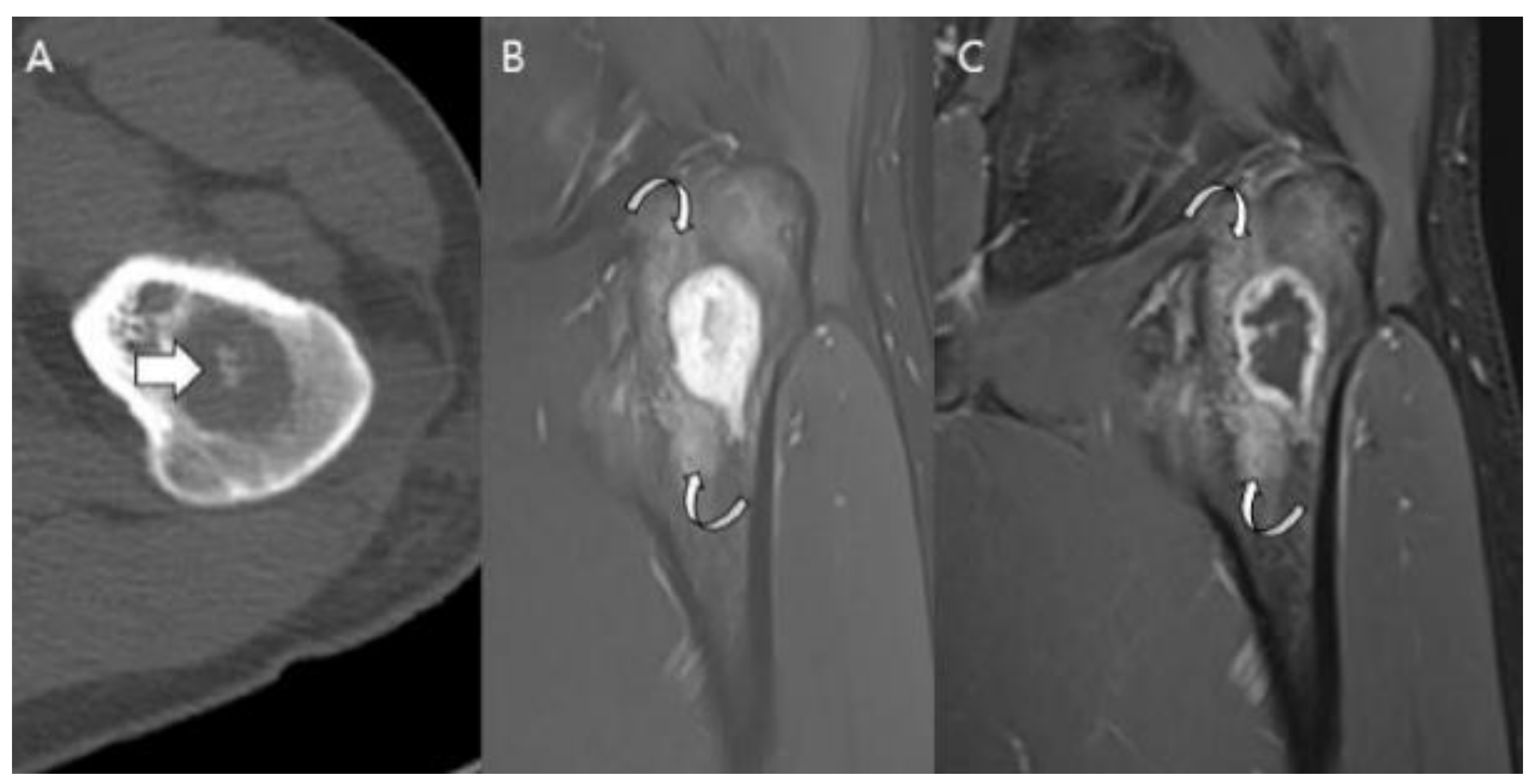

| Entity | Remarks | |
|---|---|---|
| Conventional chondrosarcomas | Central atypical cartilaginous tumor (ACT)/chondrosarcoma grade 1 (CS1) | De novo or secondary (possible precursor: enchondroma) |
| Secondary peripheral ACT/CS1 | Precursor: osteochondroma | |
| Central chondrosarcoma grades 2 and 3 (CS2,3) | De novo or secondary (possible precursor: enchondroma) | |
| Secondary peripheral CS2,3 | Precursor: osteochondroma | |
| Periosteal chondrosarcoma | ||
| Rare subtypes | Dedifferentiated chondrosarcoma | Precursor: conventional chondrosarcoma |
| Mesenchymal chondrosarcoma | ||
| Clear cell chondrosarcoma | ||
| Chondrosarcoma (CS) Type | Molecular Features |
|---|---|
| Conventional central CS | IDH1/2 mutations COL2A1 mutations CDKN2A/B deletions |
| Conventional peripheral CS | EXT1/2 mutations |
| Conventional periosteal CS | Hedgehog pathway |
| Dedifferentiated CS | IDH1/2 mutations TP53 mutations PD-L1 expression |
| Mesenchymal CS | HEY1–NCOA2 fusion |
| Clear cell CS | No evidence of mutations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, S.K. Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers 2023, 15, 1703. https://doi.org/10.3390/cancers15061703
Kim J-H, Lee SK. Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers. 2023; 15(6):1703. https://doi.org/10.3390/cancers15061703
Chicago/Turabian StyleKim, Jun-Ho, and Seul Ki Lee. 2023. "Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings" Cancers 15, no. 6: 1703. https://doi.org/10.3390/cancers15061703
APA StyleKim, J.-H., & Lee, S. K. (2023). Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers, 15(6), 1703. https://doi.org/10.3390/cancers15061703





