Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Nucleic Acid Isolation
2.3. Next-Generation Sequencing Studies
2.4. Statistical Analyses
3. Results
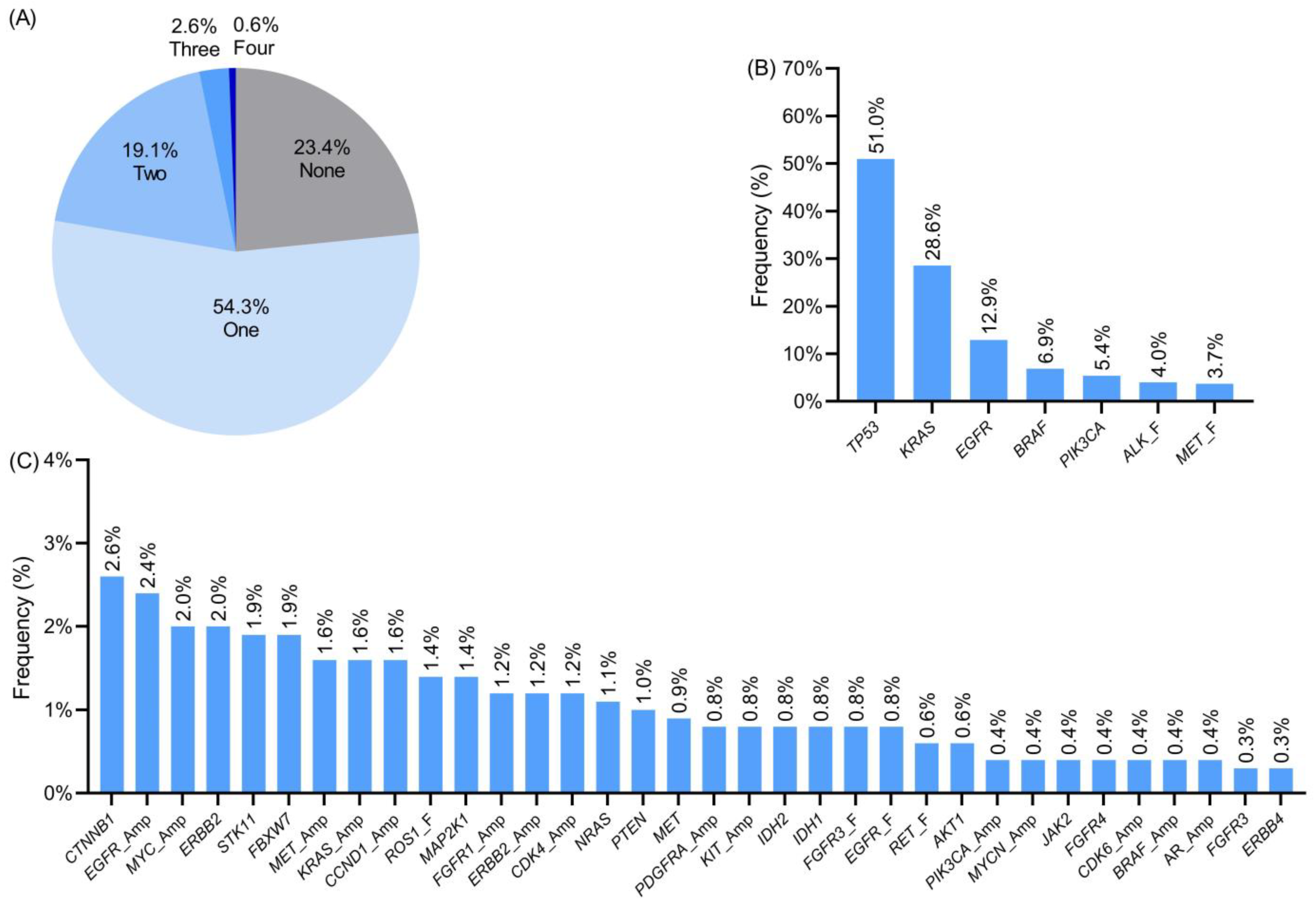
3.1. Molecular Alterations Detected Using Next-Generation Sequencing
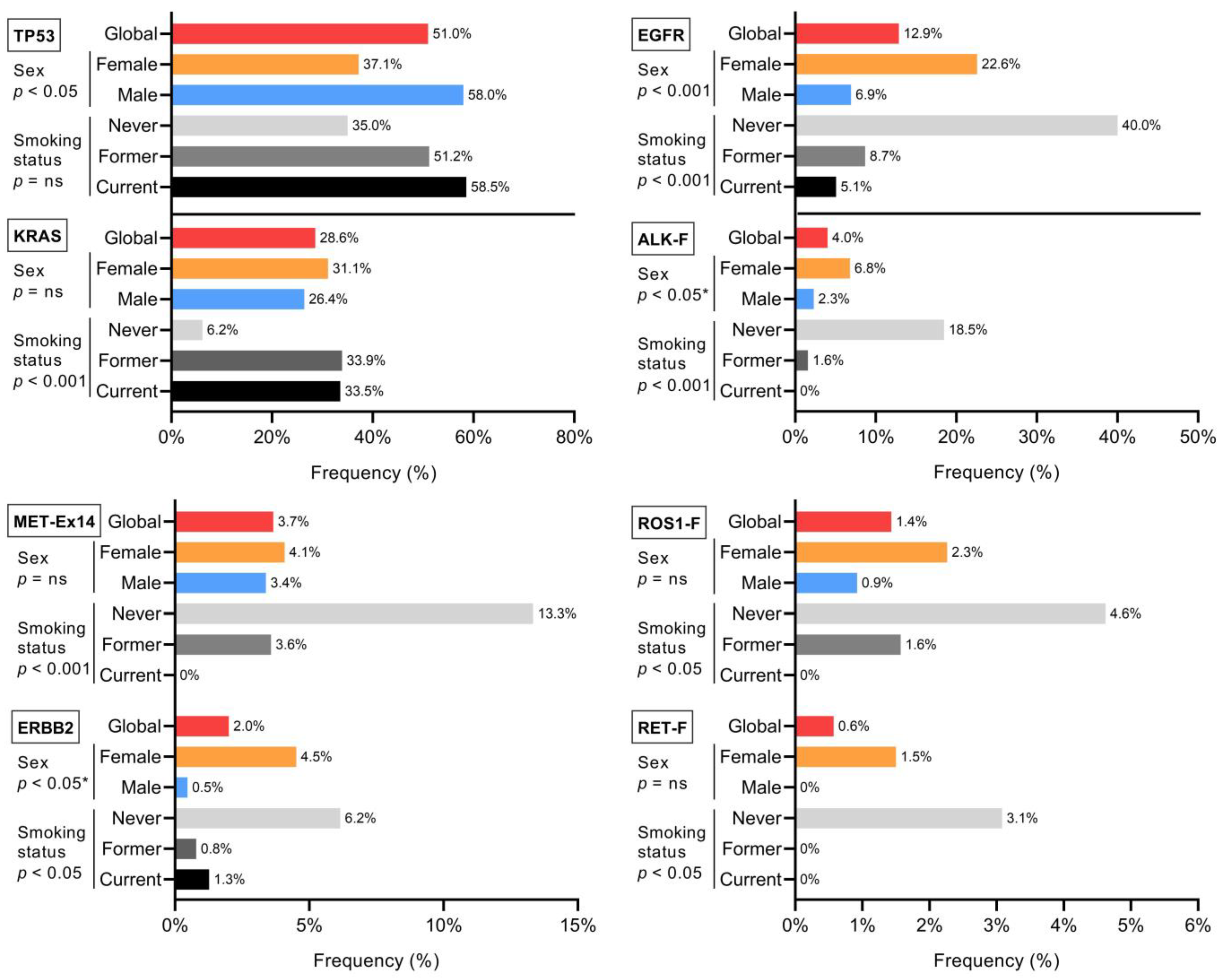
3.2. Clinical–Pathological Associations with Molecular Alterations
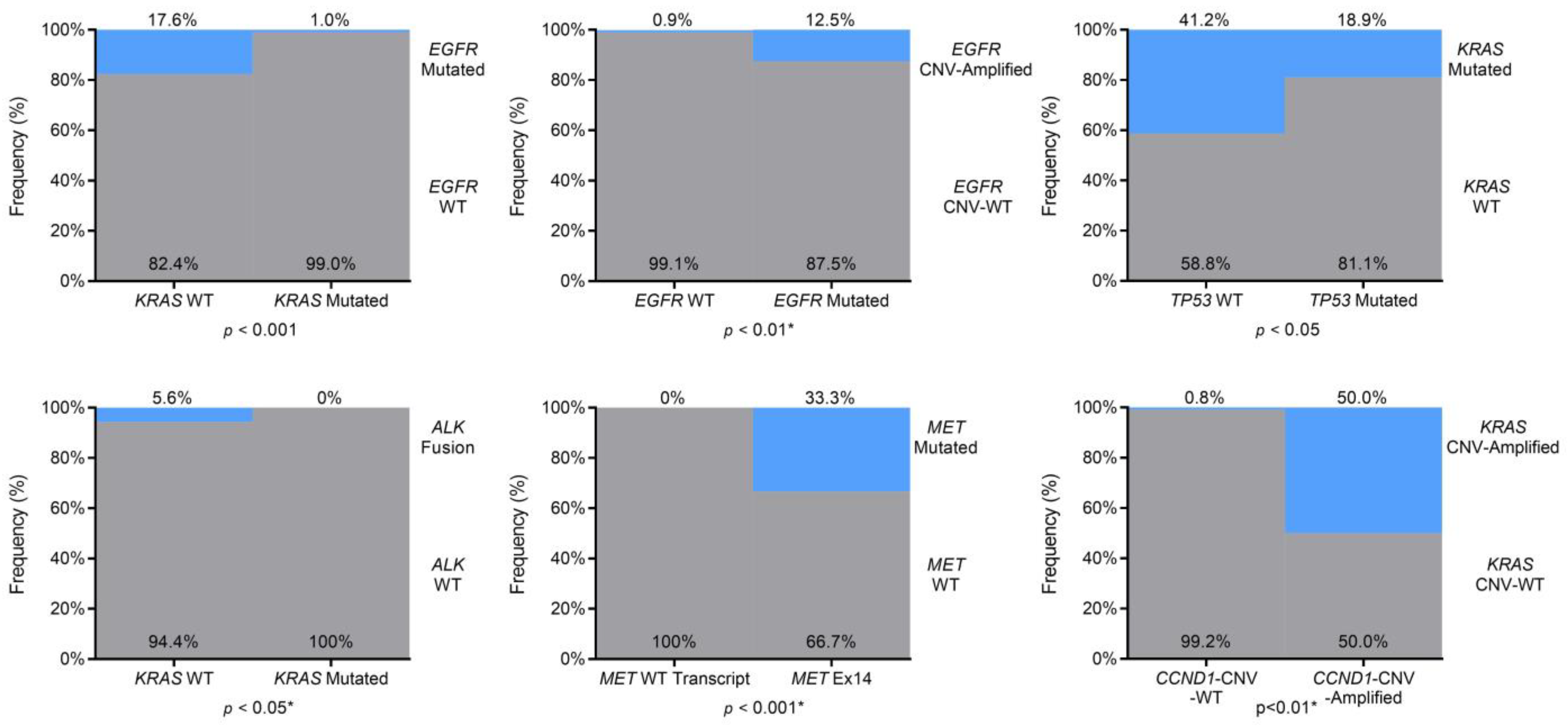
3.3. Co-Occurring or Mutually Exclusive Genetic Alterations
3.4. Clinically Relevant Genetic Variants
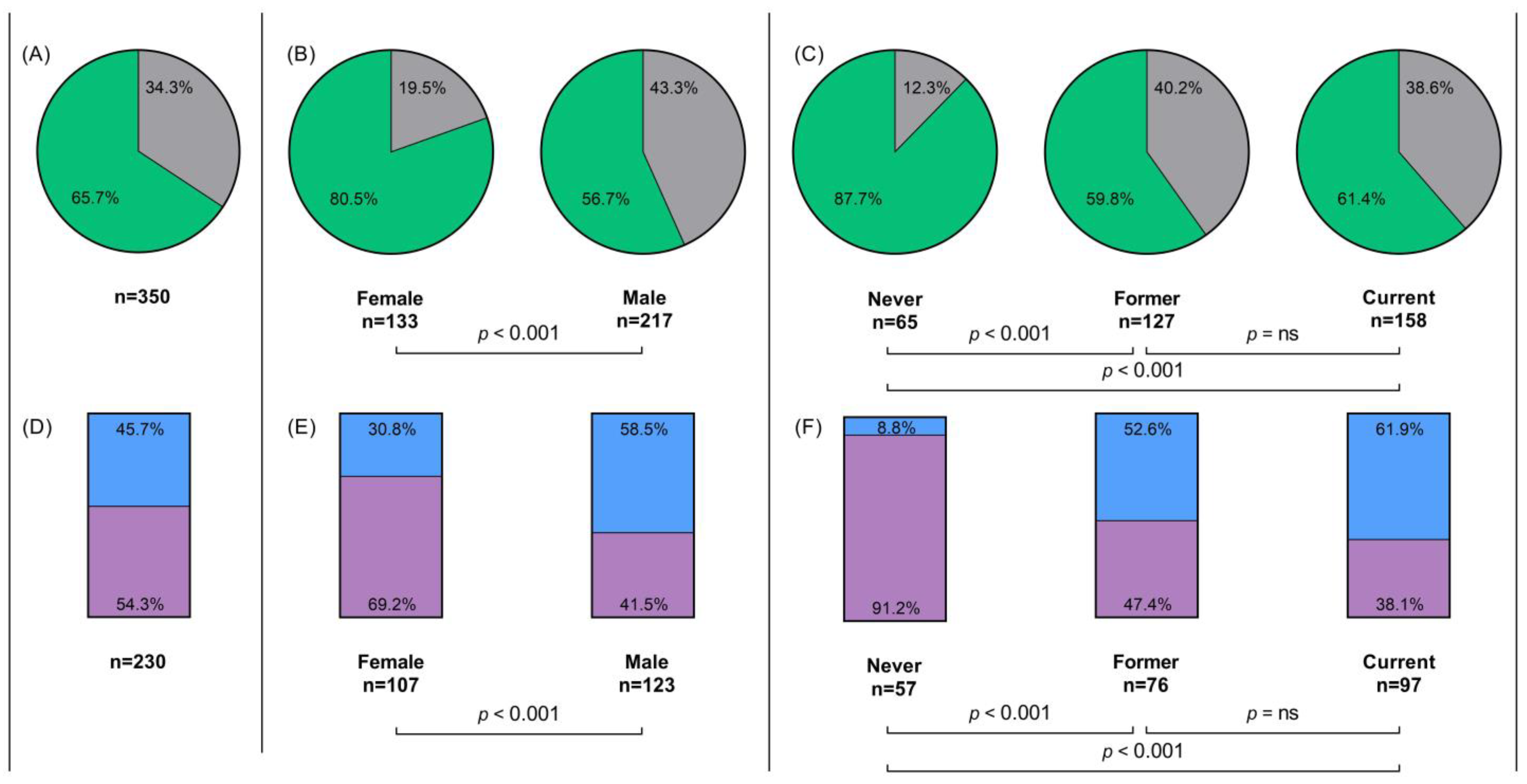
3.5. First-Line Treatment Analyses
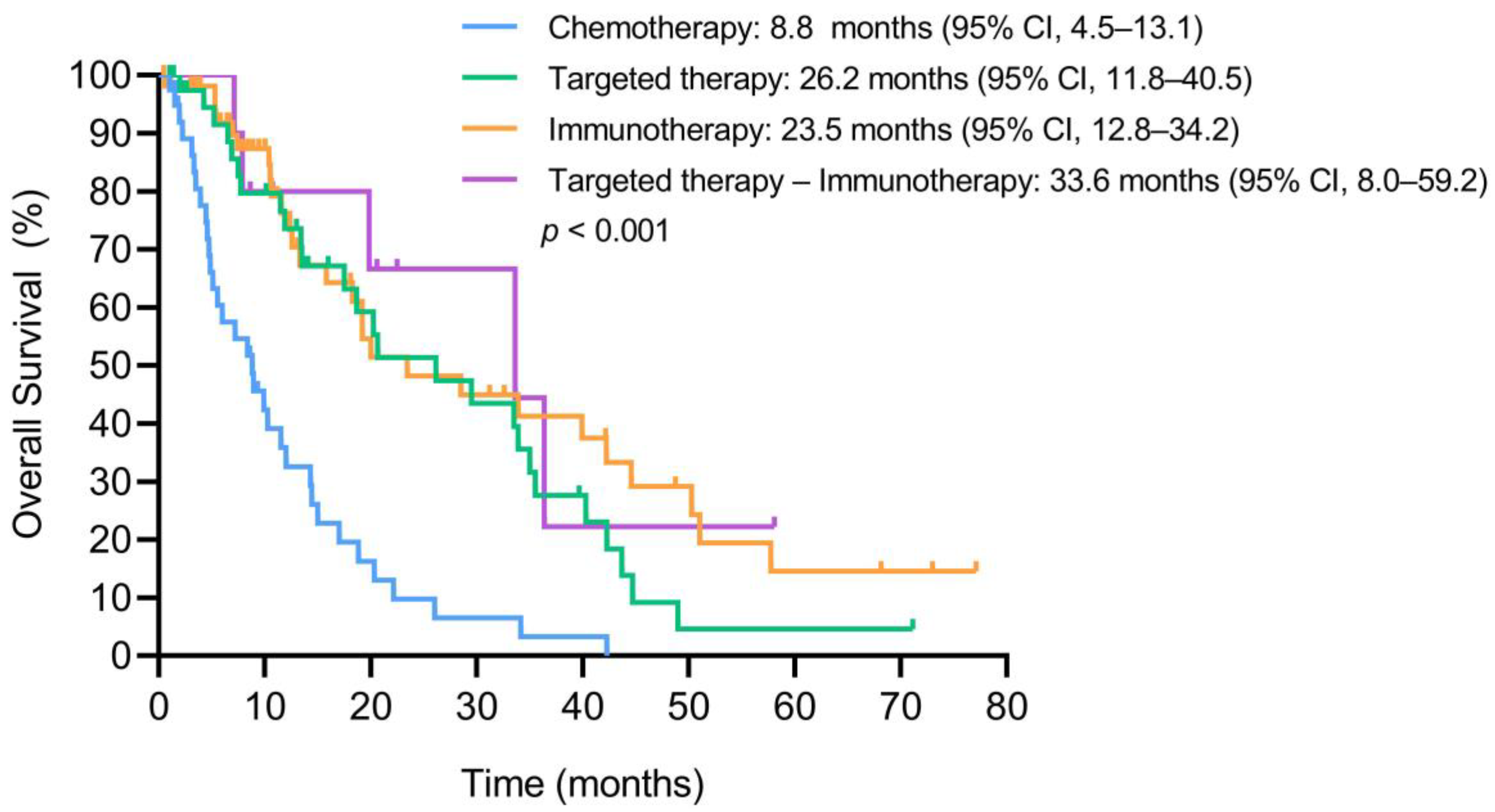
3.6. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.Y.; Yang, J.C.H.; Yang, P.C. Precision Management of Advanced Non-Small Cell Lung Cancer; Annual Reviews Inc.: San Mateo, CA, USA, 2020; Volume 71, pp. 117–136. [Google Scholar]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular Profiling for Precision Cancer Therapies. Genome Med. 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients with Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Arreaza, G.; Qiu, P.; Pang, L.; Albright, A.; Hong, L.Z.; Marton, M.J.; Levitan, D. Pre-Analytical Considerations for Successful Next-Generation Sequencing (NGS): Challenges and Opportunities for Formalin-Fixed and Paraffin-Embedded Tumor Tissue (FFPE) Samples. Int. J. Mol. Sci. 2016, 17, 1579. [Google Scholar] [CrossRef] [PubMed]
- Isla, D.; Lozano, M.D.; Paz-Ares, L.; Salas, C.; de Castro, J.; Conde, E.; Felip, E.; Gómez-Román, J.; Garrido, P.; Enguita, A.B. New Update to the Guidelines on Testing Predictive Biomarkers in Non-Small-Cell Lung Cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2022. [Google Scholar] [CrossRef]
- Schneider, F.; Maurer, C.; Friedberg, R.C. International Organization for Standardization (ISO) 15189. Ann. Lab. Med. 2017, 37, 365–370. [Google Scholar] [CrossRef]
- ISO-ISO 15189:2012-Medical Laboratories—Requirements for Quality and Competence. Available online: https://www.iso.org/standard/56115.html (accessed on 4 August 2022).
- Smolle, E.; Pichler, M. Non-Smoking-Associated Lung Cancer: A Distinct Entity in Terms of Tumor Biology, Patient Characteristics and Impact of Hereditary Cancer Predisposition. Cancers 2019, 11, 204. [Google Scholar] [CrossRef]
- Zhang, T.; Joubert, P.; Ansari-Pour, N.; Zhao, W.; Hoang, P.H.; Lokanga, R.; Moye, A.L.; Rosenbaum, J.; Gonzalez-Perez, A.; Martínez-Jiménez, F.; et al. Genomic and Evolutionary Classification of Lung Cancer in Never Smokers. Nat. Genet. 2021, 53, 1348–1359. [Google Scholar] [CrossRef]
- Garrido, P.; Conde, E.; de Castro, J.; Gómez-Román, J.J.; Felip, E.; Pijuan, L.; Isla, D.; Sanz, J.; Paz-Ares, L.; López-Ríos, F. Updated Guidelines for Predictive Biomarker Testing in Advanced Non-Small-Cell Lung Cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Penault-Llorca, F.; Kerr, K.M.; Garrido, P.; Thunnissen, E.; Dequeker, E.; Normanno, N.; Patton, S.J.; Fairley, J.; Kapp, J.; de Ridder, D.; et al. Expert Opinion on NSCLC Small Specimen Biomarker Testing-Part 2: Analysis, Reporting, and Quality Assessment. Virchows Arch. 2022, 481, 351–366. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Simarro, J.; Murria, R.; Pérez-Simó, G.; Llop, M.; Mancheño, N.; Ramos, D.; de Juan, I.; Barragán, E.; Laiz, B.; Cases, E.; et al. Development, Implementation and Assessment of Molecular Diagnostics by next Generation Sequencing in Personalized Treatment of Cancer: Experience of a Public Reference Healthcare Hospital. Cancers 2019, 11, 1196. [Google Scholar] [CrossRef]
- Jornada Medicina de Precisión | El Acceso a Determinaciones Moleculares Debe Estar Disponible En El SNS Para Aumentar La Supervivencia de Los Pacientes Con Cáncer | SEOM: Sociedad Española de Oncología Médica. Available online: https://seom.org/notas-prensa/209177-jornada-medicina-de-precision-el-acceso-a-determinaciones-moleculares-debe-estar-disponible-en-el-sns-para-aumentar-la-supervivencia-de-los-pacientes-con-cancer (accessed on 5 January 2023).
- Provencio, M.; Carcereny, E.; Rodríguez-Abreu, D.; López-Castro, R.; Guirado, M.; Camps, C.; Bosch-Barrera, J.; García-Campelo, R.; Ortega-Granados, A.L.; González-Larriba, J.L.; et al. Lung Cancer in Spain: Information from the Thoracic Tumors Registry (TTR Study). Transl. Lung Cancer Res. 2019, 8, 461–475. [Google Scholar] [CrossRef]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Barlesi, F.; Mazieres, J.; Merlio, J.P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine Molecular Profiling of Patients with Advanced Non-Small-Cell Lung Cancer: Results of a 1-Year Nationwide Programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Tsoulos, N.; Papadopoulou, E.; Metaxa-Mariatou, V.; Tsaousis, G.; Efstathiadou, C.; Tounta, G.; Scapeti, A.; Bourkoula, E.; Zarogoulidis, P.; Pentheroudakis, G.; et al. Tumor Molecular Profiling of NSCLC Patients Using next Generation Sequencing. Oncol. Rep. 2017, 38, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Isla, D.; Majem, M.; Viñolas, N.; Artal, A.; Blasco, A.; Felip, E.; Garrido, P.; Remón, J.; Baquedano, M.; Borrás, J.M.; et al. A Consensus Statement on the Gender Perspective in Lung Cancer. Clin Transl. Oncol. 2017, 19, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-Specificity in Lung Cancer Risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef]
- Ragavan, M.; Patel, M.I. The Evolving Landscape of Sex-Based Differences in Lung Cancer: A Distinct Disease in Women. Eur. Respir. Rev. 2022, 31, 210100. [Google Scholar] [CrossRef]
- Yu, X.Q.; Yap, M.L.; Cheng, E.S.; Ngo, P.J.; Vaneckova, P.; Karikios, D.; Canfell, K.; Weber, M.F. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings from a Large Australian Cohort. J. Thorac. Oncol. 2022, 17, 688–699. [Google Scholar] [CrossRef]
- Ye, Y.; Jing, Y.; Li, L.; Mills, G.B.; Diao, L.; Liu, H.; Han, L. Sex-Associated Molecular Differences for Cancer Immunotherapy. Nat. Commun. 2020, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yuan, J.Q.; Wang, K.F.; Fu, X.H.; Han, X.R.; Threapleton, D.; Yang, Z.Y.; Mao, C.; Tang, J.L. The Prevalence of EGFR Mutation in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Feng, Y.; Wan, H.; Shi, G.; Niu, W. Clinicopathological and Demographical Characteristics of Non-Small Cell Lung Cancer Patients with ALK Rearrangements: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e100866. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Herschtal, A.; Soussi, T.; Lozano, G.; Chen, H.; Liang, H.; Speed, T.P.; Haupt, Y. Identification of Cancer Sex-Disparity in the Functional Integrity of P53 and Its X Chromosome Network. Nat. Commun. 2019, 10, 5385. [Google Scholar] [CrossRef]
- Arcila, M.E.; Chaft, J.E.; Nafa, K.; Roy-Chowdhuri, S.; Lau, C.; Zaidinski, M.; Paik, P.K.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. Prevalence, Clinicopathologic Associations, and Molecular Spectrum of ERBB2 (HER2) Tyrosine Kinase Mutations in Lung Adenocarcinomas. Clin. Cancer Res. 2012, 18, 4910–4918. [Google Scholar] [CrossRef]
- Bu, S.; Wang, R.; Pan, Y.; Yu, S.; Shen, X.; Li, Y.; Sun, Y.; Chen, H. Clinicopathologic Characteristics of Patients with HER2 Insertions in Non-Small Cell Lung Cancer. Ann. Surg. Oncol. 2017, 24, 291–297. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Yang, J.; Yu, G.; Zhu, P.; Jiang, Z.; Feng, K.; Lu, Y.; Bao, B.; Zhong, F. Smoking Alters the Evolutionary Trajectory of Non-Small Cell Lung Cancer. Exp. Ther. Med. 2019, 18, 3315–3324. [Google Scholar] [CrossRef]
- Li, X.; Huang, C.; Xie, X.; Wu, Z.; Tian, X.; Wu, Y.; Du, X.; Shi, L. The Impact of Smoking Status on the Progression-Free Survival of Non-Small Cell Lung Cancer Patients Receiving Molecularly Target Therapy or Immunotherapy versus Chemotherapy: A Meta-Analysis. J. Clin. Pharm. Ther. 2021, 46, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.M.; Sun, K.Y.; Ruestow, P.; Cowan, D.M.; Madl, A.K. Lung Cancer Mutation Profile of EGFR, ALK, and KRAS: Meta-Analysis and Comparison of Never and Ever Smokers. Lung Cancer 2016, 102, 122–134. [Google Scholar] [CrossRef]
- Dias, M.; Linhas, R.; Campainha, S.; Conde, S.; Barroso, A. Lung Cancer in Never-Smokers-What Are the Differences? Acta Oncol. 2017, 56, 931–935. [Google Scholar] [CrossRef]
- Wei, X.W.; Gao, X.; Zhang, X.C.; Yang, J.J.; Chen, Z.H.; Wu, Y.L.; Zhou, Q. Mutational Landscape and Characteristics of ERBB2 in Non-Small Cell Lung Cancer. Thorac. Cancer 2020, 11, 1512–1521. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhan, P.; Zhang, X.; Lv, T.; Song, Y. Clinicopathologic Characteristics of Patients with ROS1 Fusion Gene in Non-Small Cell Lung Cancer: A Meta-Analysis. Transl. Lung Cancer Res. 2015, 4, 300–309. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET Fusions Define a Unique Molecular and Clinicopathologic Subtype of Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef]
- Seoane, J.; de Mattos-Arruda, L. The Challenge of Intratumour Heterogeneity in Precision Medicine. J. Intern. Med. 2014, 276, 41–51. [Google Scholar] [CrossRef]
- Zhang, J.; Späth, S.S.; Marjani, S.L.; Zhang, W.; Pan, X. Characterization of Cancer Genomic Heterogeneity by Next-Generation Sequencing Advances Precision Medicine in Cancer Treatment. Precis. Clin. Med. 2018, 1, 29–48. [Google Scholar] [CrossRef]
- Sholl, L.M.; Yeap, B.Y.; Iafrate, A.J.; Holmes-Tisch, A.J.; Chou, Y.P.; Wu, M.T.; Goan, Y.G.; Su, L.; Benedettini, E.; Yu, J.; et al. Lung Adenocarcinoma with EGFR Amplification Has Distinct Clinicopathologic and Molecular Features in Never-Smokers. Cancer Res. 2009, 69, 8341–8348. [Google Scholar] [CrossRef]
- Ruiz-Patiño, A.; Castro, C.D.; Ricaurte, L.M.; Cardona, A.F.; Rojas, L.; Zatarain-Barrón, Z.L.; Wills, B.; Reguart, N.; Carranza, H.; Vargas, C.; et al. EGFR Amplification and Sensitizing Mutations Correlate with Survival in Lung Adenocarcinoma Patients Treated with Erlotinib (MutP-CLICaP). Target. Oncol. 2018, 13, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Varghese, A.M.; Ou, S.H.I.; Kabraji, S.; Awad, M.M.; Katayama, R.; Pawlak, A.; Mino-Kenudson, M.; Yeap, B.Y.; Riely, G.J.; et al. ALK Rearrangements Are Mutually Exclusive with Mutations in EGFR or KRAS: An Analysis of 1,683 Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Timar, J.; Kashofer, K. Molecular Epidemiology and Diagnostics of KRAS Mutations in Human Cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Stiedl, A.C.; Wilbertz, T.; Petersen, K.; Scheble, V.; Menon, R.; Reischl, M.; Mikut, R.; Rubin, M.A.; Fend, F.; et al. Frequency and Clinicopathologic Correlates of KRAS Amplification in Non-Small Cell Lung Carcinoma. Lung Cancer 2011, 74, 118–123. [Google Scholar] [CrossRef]
- Goh, K.Y.; Lim, W.T. Cyclin D1 Expression in KRAS Mutant Non-Small Cell Lung Cancer-Old Wine into New Skins. Transl. Lung Cancer Res. 2020, 9, 2302–2304. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Jänne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated with Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef]
- Tong, J.H.; Yeung, S.F.; Chan, A.W.H.; Chung, L.Y.; Chau, S.L.; Lung, R.W.M.; Tong, C.Y.; Chow, C.; Tin, E.K.Y.; Yu, Y.H.; et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis. Clin. Cancer Res. 2016, 22, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.; Li, Y.; Rodrigues, F.M.; Sankararaman, S.; Kadara, H.; Goparaju, C.; Lanc, I.; Pepin, K.; Waqar, S.N.; Morgensztern, D.; et al. Genomic Profiling of Lung Adenocarcinoma in Never-Smokers. J. Clin. Oncol. 2021, 39, 3747–3758. [Google Scholar] [CrossRef]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-Institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar] [CrossRef]
- Löfling, L.; Karimi, A.; Sandin, F.; Bahmanyar, S.; Kieler, H.; Lambe, M.; Lamberg, K.; Wagenius, G. Clinical Characteristics and Survival in Non-Small Cell Lung Cancer Patients by Smoking History: A Population-Based Cohort Study. Acta Oncol. 2019, 58, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Bylicki, O.; Barazzutti, H.; Paleiron, N.; Margery, J.; Assié, J.B.; Chouaïd, C. First-Line Treatment of Non-Small-Cell Lung Cancer (NSCLC) with Immune Checkpoint Inhibitors. BioDrugs 2019, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Proto, C.; Ferrara, R.; Signorelli, D.; lo Russo, G.; Galli, G.; Imbimbo, M.; Prelaj, A.; Zilembo, N.; Ganzinelli, M.; Pallavicini, L.M.; et al. Choosing Wisely First Line Immunotherapy in Non-Small Cell Lung Cancer (NSCLC): What to Add and What to Leave Out. Cancer Treat. Rev. 2019, 75, 39–51. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Hong, D.S.; Ye, Y.; Cartwright, C.; Wheler, J.J.; Falchook, G.S.; Naing, A.; Fu, S.; Piha-Paul, S.; Janku, F.; et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study. JCO Precis. Oncol. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Pakkala, S.; Ramalingam, S.S. Personalized Therapy for Lung Cancer: Striking a Moving Target. JCI Insight 2018, 3, e120858. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.; Escriu, C.; Khan, S.; Hudson, E.; Mansy, T.; Conn, A.; Chan, S.; Powell, C.; Brock, J.; Conibear, J.; et al. Retrospective Analysis of Real-World Treatment Patterns and Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer Starting First-Line Systemic Therapy in the United Kingdom. BMC Cancer 2021, 21, 515. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, A.; Al-Hashami, Z.; Moore, S.; Pender, A.; Wong, S.K.; Wang, Y.; Leung, B.; Wu, J.; Ho, C. Effect of Targeted Therapy and Immunotherapy on Advanced Nonsmall-Cell Lung Cancer Outcomes in the Real World. Cancer Med. 2022, 11, 86–93. [Google Scholar] [CrossRef] [PubMed]

| Variable | |
|---|---|
| Age, mean ± SD | 63.2 ± 0.6 |
| Sex, n (%) | |
| Male | 217 (62.0) |
| Female | 133 (38.0) |
| Smoking history, n (%) | |
| Never | 65 (18.6) |
| Former smoker | 127 (36.3) |
| Current smoker | 158 (45.1) |
| Smoking load (former and current smokers), median (IQR) | 36 (23–50) |
| Years since quitting smoking (former smokers), median (IQR) | 12 (5–20) |
| Histology, n (%) | |
| Adenocarcinoma | 288 (82.3) |
| Large-cell carcinoma | 9 (2.6) |
| Squamous | 14 (4.0) |
| Sarcomatoid carcinoma | 11 (3.1) |
| Adenosquamous carcinoma | 3 (0.9) |
| Large-cell neuroendocrine carcinoma | 8 (2.3) |
| NOS | 17 (4.9) |
| Stage, n (%) | |
| IA | 38 (10.9) |
| IB | 24 (6.9) |
| IIA | 2 (0.6) |
| IIB | 17 (4.9) |
| IIIA | 28 (8.0) |
| IIIB | 19 (5.4) |
| IIIC | 9 (2.6) |
| IV | 200 (57.1) |
| Unknown | 13 (3.7) |
| Molecular Alteration | n | Drug |
|---|---|---|
| EGFR: p.(Leu858Arg) | 4 | Osimertinib |
| EGFR: p.(Glu746_Ala750del) | 3 | Osimertinib |
| EGFR: p.(Leu858Arg) + EGFR Amplification | 1 | Osimertinib |
| EGFR: p.(Gly719Ala) + p.(Ser768Ile) | 1 | Osimertinib |
| EGFR: p.(Leu861Gln) | 1 | Osimertinib |
| EGFR: p.(Glu709_Thr710delinsAsp) | 1 | Osimertinib |
| EGFR: p.(Ala767_Val769dup) | 1 | Amivantamab |
| EML4(13)-ALK(20) | 1 | Alectinib |
| KIF5B(17)-ALK(20) | 1 | Brigatinib |
| ALK Fusion (Unknown Partner) | 1 | Alectinib |
| MET(13)—MET(15) | 1 | Capmatinib |
| KRAS: p.(Gly12Cys) | 1 | Sotorasib |
| BRAF: p.(Val600Glu) | 1 | Dabrafenib + trametinib |
| SLC34A2(13)-ROS1(32) | 1 | Crizotinib |
| KIF5B(15)-RET(12) + IDH1: p.(Arg132His) + MYC Amplification | 1 | Selpercatinib |
| Variable | |
|---|---|
| Age, mean ± SD | 63.1 ± 12.1 |
| Sex, n (%) | |
| Male | 78 (39.0) |
| Female | 122 (61.0) |
| Smoking history, n (%) | |
| Never | 46 (23.0) |
| Former smoker | 63 (31.5) |
| Current smoker | 91 (45.5) |
| Sex and smoking history, n (%) | |
| Never-smoker female | 31 (15.5) |
| Former smoker female | 16 (8.0) |
| Current smoker female | 31 (15.5) |
| Never-smoker male | 15 (7.5) |
| Former smoker male | 47 (23.5) |
| Current smoker male | 60 (30.0) |
| Histology, n (%) | |
| Adenocarcinoma | 162 (81.0) |
| Large-cell carcinoma | 5 (2.5) |
| Squamous | 7 (3.5) |
| Sarcomatoid carcinoma | 5 (2.5) |
| Adenosquamous carcinoma | 2 (1.0) |
| Large-cell neuroendocrine carcinoma | 5 (2.5) |
| NOS | 14 (7.0) |
| Systemic treatment, n (%) | |
| No | 37 (19.0) |
| Yes | 158 (81.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simarro, J.; Pérez-Simó, G.; Mancheño, N.; Ansotegui, E.; Muñoz-Núñez, C.F.; Gómez-Codina, J.; Juan, Ó.; Palanca, S. Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital. Cancers 2023, 15, 1705. https://doi.org/10.3390/cancers15061705
Simarro J, Pérez-Simó G, Mancheño N, Ansotegui E, Muñoz-Núñez CF, Gómez-Codina J, Juan Ó, Palanca S. Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital. Cancers. 2023; 15(6):1705. https://doi.org/10.3390/cancers15061705
Chicago/Turabian StyleSimarro, Javier, Gema Pérez-Simó, Nuria Mancheño, Emilio Ansotegui, Carlos Francisco Muñoz-Núñez, José Gómez-Codina, Óscar Juan, and Sarai Palanca. 2023. "Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital" Cancers 15, no. 6: 1705. https://doi.org/10.3390/cancers15061705
APA StyleSimarro, J., Pérez-Simó, G., Mancheño, N., Ansotegui, E., Muñoz-Núñez, C. F., Gómez-Codina, J., Juan, Ó., & Palanca, S. (2023). Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital. Cancers, 15(6), 1705. https://doi.org/10.3390/cancers15061705








