Current Landscape of Immunotherapy for Advanced Sarcoma
Abstract
Simple Summary
Abstract
1. Introduction
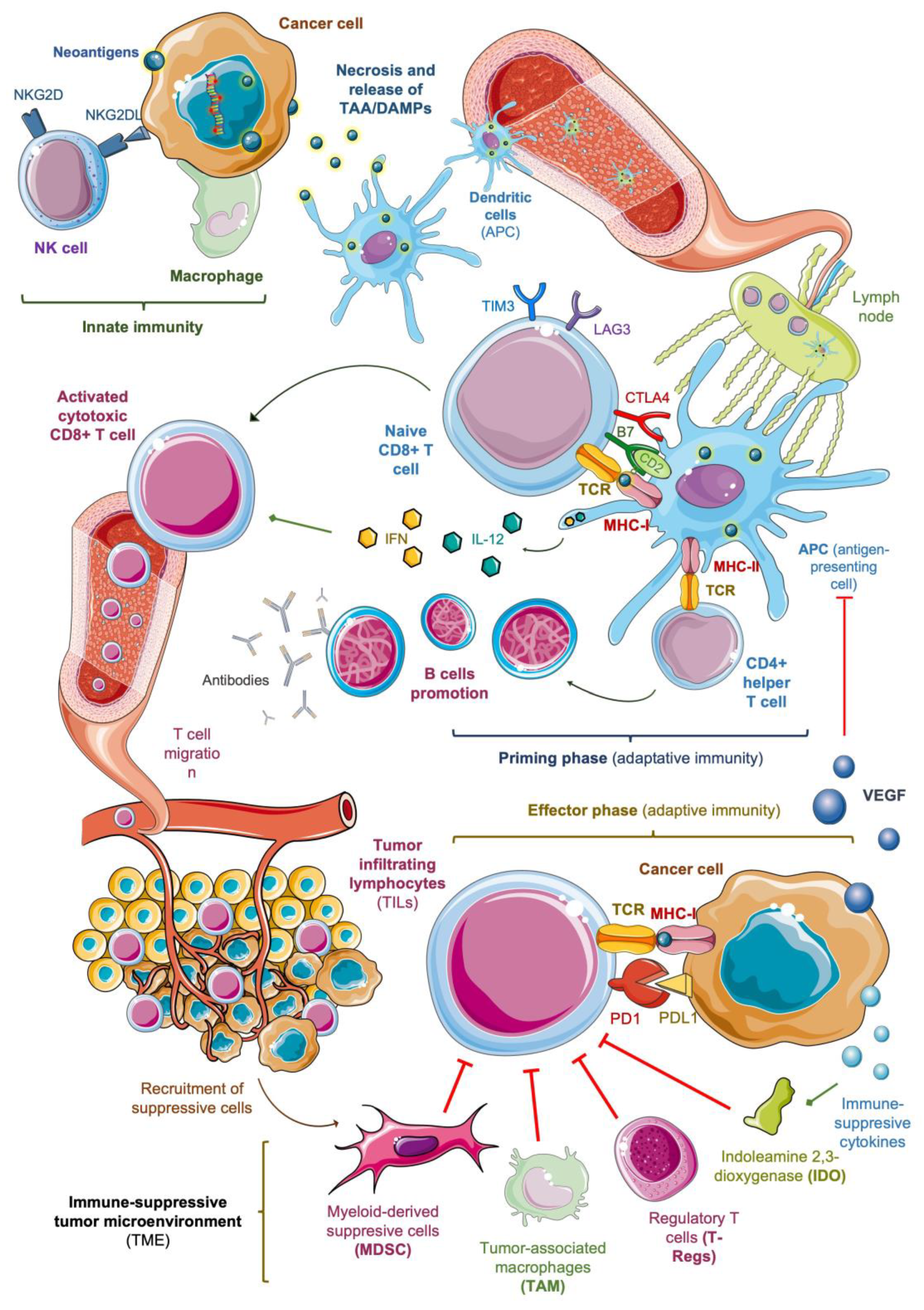
2. Immunogenicity of Sarcoma: Anti-Tumor Response and Biomarkers
2.1. Innate Immunity and Release of Neoantigens
2.2. Antigenic Presentation and Activation of T Cells
2.3. Tumor Infiltration of Activated Lymphocytes
2.4. Immune-Suppressive Tumor Microenvironment
3. Immunotherapy for Sarcoma: Clinical Results
3.1. Immune Checkpoint Inhibitors (ICIs)
3.2. Combination of ICIs and Conventional Chemotherapy
3.3. Combination of ICIs and Tyrosine–Kinase Inhibitors
3.4. Combination of ICIs and Other Agents
3.5. Therapeutic Vaccines
3.6. Adoptive Cell Therapy
3.6.1. Engineered TCR
3.6.2. CAR T Cells
3.6.3. TIL Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann. Surg. 2014, 260, 416–421. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Araki, N.; Sugiura, H.; Ueda, T.; Yonemoto, T.; Takahashi, M.; Morioka, H.; Hiraga, H.; Hiruma, T.; Kunisada, T.; et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: A randomised, open-label, phase 2 study. Lancet Oncol. 2015, 16, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- García-Del-Muro, X.; López-Pousa, A.L.; Maurel, J.; Martín, J.; Martínez-Trufero, J.; Casado, A.; Gómez-España, A.; Fra, J.; Cruz, J.; Poveda, A.; et al. Randomized Phase II Study Comparing Gemcitabine Plus Dacarbazine Versus Dacarbazine Alone in Patients With Previously Treated Soft Tissue Sarcoma: A Spanish Group for Research on Sarcomas Study. J. Clin. Oncol. 2011, 29, 2528–2533. [Google Scholar] [CrossRef]
- Van Der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; Cesne, A.L.; et al. Faculty Opinions recommendation of Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Lond. E 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Butrynski, J.E.; D’Adamo, D.R.; Hornick, J.L.; Cin, P.D.; Antonescu, C.R.; Jhanwar, S.C.; Ladanyi, M.; Capelletti, M.; Rodig, S.J.; Ramaiya, N.; et al. Crizotinib in ALK-Rearranged Inflammatory Myofibroblastic Tumor. N. Engl. J. Med. 2010, 363, 1727–1733. [Google Scholar] [CrossRef]
- Judson, I.; Morden, J.P.; Kilburn, L.; Leahy, M.; Benson, C.; Bhadri, V.; Campbell-Hewson, Q.; Cubedo, R.; Dangoor, A.; Fox, L.; et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): A double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Torricelli, E.; Cascinu, S.; Pierini, M.; De Paolis, M.; Donati, D.; Ferrari, S. Is there a role for chemotherapy after local relapse in high-grade osteosarcoma? Pediatr. Blood Cancer 2019, 66, e27792. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef]
- Felix, A.; Berlanga, P.; Toulmonde, M.; Landman-Parker, J.; Dumont, S.; Vassal, G.; Gaspar, N. Systematic review of phase-I/II trials enrolling refractory and recurrent Ewing sarcoma: Actual knowledge and future directions to optimize the research. Cancer Med. 2021, 10, 1589–1604. [Google Scholar] [CrossRef]
- Albarrán, V.; Villamayor, M.L.; Chamorro, J.; Rosero, D.I.; Pozas, J.; Román, M.S.; Calvo, J.C.; de Aguado, P.P.; Moreno, J.; Guerrero, P.; et al. Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 13784. [Google Scholar] [CrossRef]
- Ferrari, S.; Briccoli, A.; Mercuri, M.; Bertoni, F.; Picci, P.; Tienghi, A.; Del Prever, A.B.; Fagioli, F.; Comandone, A.; Bacci, G. Postrelapse Survival in Osteosarcoma of the Extremities: Prognostic Factors for Long-Term Survival. J. Clin. Oncol. 2003, 21, 710–715. [Google Scholar] [CrossRef]
- Cotterill, S.; Ahrens, S.; Paulussen, M.; Jürgens, H.; Voûte, P.; Gadner, H.; Craft, A. Prognostic Factors in Ewing’s Tumor of Bone: Analysis of 975 Patients From the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef]
- Coley, W.B. II Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Fyfe, G.A.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Long-term response data for 255 patients with met-astatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. Oncol. 1996, 14, 2410–2411. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, W.; Klass, V.; Benesch, M.; Lackner, H.; Dornbusch, H.J.; Sovinz, P.; Moser, A.; Schwantzer, G.; Urban, C. Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann. Oncol. 2005, 16, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Baluna, R.; Vitetta, E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Saerens, M.; Brusselaers, N.; Rottey, S.; Decruyenaere, A.; Creytens, D.; Lapeire, L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: A systematic review and meta-analysis. Eur. J. Cancer 2021, 152, 165–182. [Google Scholar] [CrossRef]
- Dajsakdipon, T.; Siripoon, T.; Ngamphaiboon, N.; Ativitavas, T.; Dejthevaporn, T. Immunotherapy and Biomarkers in Sarcoma. Curr. Treat. Options Oncol. 2022, 23, 415–438. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Rosin, D.L.; Okusa, M.D. Dangers within: DAMP responses to damage and cell death in kidney disease. J. Am. Soc. Nephrol. JASN 2011, 22, 416–425. [Google Scholar] [CrossRef]
- Fumet, J.-D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Bocchia, M.; Wentworth, P.A.; Southwood, S.; Sidney, J.; McGraw, K.; Scheinberg, D.A.; Sette, A. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood 1995, 85, 2680–2684. [Google Scholar] [CrossRef]
- Worley, B.S.; Broeke, L.T.V.D.; Goletz, T.J.; Pendleton, C.D.; Daschbach, E.M.; Thomas, E.K.; Marincola, F.M.; Helman, L.J.; Berzofsky, J.A. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001, 61, 6868–6875. [Google Scholar] [PubMed]
- Kakimoto, T.; Matsumine, A.; Kageyama, S.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Sudo, A. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol. Lett. 2019, 17, 3937–3943. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lafleur, E.A.; Koshkina, N.V.; Worth, L.L.; Lester, M.S.; Kleinerman, E.S. Interleukin-12 Up-Regulates Fas Expression in Human Osteosarcoma and Ewing’s Sarcoma Cells by Enhancing Its Promoter Activity. Mol. Cancer Res. 2005, 3, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Marchisone, C.; Benelli, R.; Albini, A.; Santi, L.; Noonan, D.M. Inhibition of Angiogenesis by Type I Interferons in Models of Kaposi’S Sarcoma. Int. J. Biol. Markers 1999, 14, 257–262. [Google Scholar] [CrossRef]
- Taylor, K.L.; Oates, R.K.; Grane, R.; Leaman, D.W.; Borden, E.C.; Lindner, D.J. IFN-alpha1,8 inhibits tumor-induced angiogenesis in murine angiosarcomas. J. Interferon Cytokine Res. 2006, 26, 353–361. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Dancsok, A.R.; Setsu, N.; Gao, D.; Blay, J.-Y.; Thomas, D.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod. Pathol. 2019, 32, 1772–1785. [Google Scholar] [CrossRef]
- Slaney, C.Y.; Kershaw, M.H.; Darcy, P.K. Trafficking of T Cells into Tumors. Cancer Res. 2014, 74, 7168–7174. [Google Scholar] [CrossRef]
- Kornepati, A.V.R.; Vadlamudi, R.K.; Curiel, T.J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 2022, 22, 174–189. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.-X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2014, 46, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; You, W.; Wan, P.; Jiang, X.; Chen, J.; Zheng, Y.; Li, W.; Tan, J.; Zhang, S. Clinicopathological and prognostic significance of PD-L1 expression in sarcoma: A systematic review and meta-analysis. Medicine 2018, 97, e11004. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xiao, W.; Guan, Y.-X.; Liang, Y.; Yan, S.-M.; Chen, H.-Y.; Li, Q.-Q.; Xu, B.-S.; Zhou, Z.-W.; Zhang, X. PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis. J. Cancer 2017, 8, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, R.M.; Busund, L.-T.; Kilvær, T.L.; Andersen, S.; Richardsen, E.; Paulsen, E.E.; Hald, S.; Khanehkenari, M.R.; Cooper, W.A.; Kao, S.C.; et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, S.; Donia, M.; Straten, P.T. Effector CD4 and CD8 T Cells and Their Role in the Tumor Microenvironment. Cancer Microenviron. 2012, 6, 123–133. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Liu, C.; Xiang, X.; Wang, S.; Wu, A.; Shen, Y.; Li, G. Prognostic value of the common tumour-infiltrating lymphocyte subtypes for patients with non-small cell lung cancer: A meta-analysis. PLoS ONE 2020, 15, e0242173. [Google Scholar] [CrossRef]
- Bi, Q.; Liu, Y.; Yuan, T.; Wang, H.; Li, B.; Jiang, Y.; Mo, X.; Lei, Y.; Xiao, Y.; Dong, S.; et al. Predicted CD4+ T cell infiltration levels could indicate better overall survival in sarcoma patients. J. Int. Med. Res. 2021, 49, 300060520981539. [Google Scholar] [CrossRef]
- Fujii, H.; Arakawa, A.; Utsumi, D.; Sumiyoshi, S.; Yamamoto, Y.; Kitoh, A.; Ono, M.; Matsumura, Y.; Kato, M.; Konishi, K.; et al. CD8+tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int. J. Cancer 2013, 134, 2393–2402. [Google Scholar] [CrossRef]
- Fridman, W.H.; Sibéril, S.; Pupier, G.; Soussan, S.; Sautès-Fridman, C. Activation of B cells in Tertiary Lymphoid Structures in cancer: Anti-tumor or anti-self? Semin. Immun. 2023, 65, 101703. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.-T. Prognostic Impact of Lymphocytes in Soft Tissue Sarcomas. PLoS ONE 2011, 6, e14611. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Shimizu, K.; Iyoda, T.; Okada, M.; Yamasaki, S.; Fujii, S. Immune suppression and reversal of the suppressive tumor microenvi-ronment. Int. Immunol. 2018, 30, 445–455. [Google Scholar] [CrossRef]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef]
- Mahnke, K.; Ring, S.; Johnson, T.S.; Schallenberg, S.; Schönfeld, K.; Storn, V.; Bedke, T.; Enk, A.H. Induction of immunosuppressive functions of dendritic cellsin vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur. J. Immunol. 2007, 37, 2117–2126. [Google Scholar] [CrossRef]
- Smolle, M.A.; Herbsthofer, L.; Granegger, B.; Goda, M.; Brcic, I.; Bergovec, M.; Scheipl, S.; Prietl, B.; Pichler, M.; Gerger, A.; et al. T-regulatory cells predict clinical outcome in soft tissue sarcoma patients: A clinico-pathological study. Br. J. Cancer 2021, 125, 717–724. [Google Scholar] [CrossRef]
- Brinkrolf, P.; Landmeier, S.; Altvater, B.; Chen, C.; Pscherer, S.; Rosemann, A.; Ranft, A.; Dirksen, U.; Juergens, H.; Rossig, C. A high proportion of bone marrow T cells with regulatory phenotype (CD4+CD25hiFoxP3+) in Ewing sarcoma patients is associated with metastatic disease. Int. J. Cancer 2009, 125, 879–886. [Google Scholar] [CrossRef]
- Camarillo, D.; Leslie, K.; Unemori, P.; Yashi, A.K.; McCune-Smith, K.; Maurer, T. Regulatory T cells are present in Kaposi’s sarcoma and increasingly frequent in advanced disease. Infect. Agents Cancer 2009, 4, P12–1. [Google Scholar] [CrossRef]
- Colomiere, M.; Ward, A.C.; Riley, C.; Trenerry, M.K.; Cameron-Smith, D.; Findlay, J.; Ackland, L.; Ahmed, N. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epi-thelial-mesenchymal transition in ovarian carcinomas. Br. J. Cancer 2009, 100, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Tartour, E.; Pere, H.; Maillere, B.; Terme, M.; Merillon, N.; Taieb, J.; Sandoval, F.; Quintin-Colonna, F.; Lacerda, K.; Karadimou, A.; et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011, 30, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Han, X.; Shi, H.; Sun, Y.; Shang, C.; Luan, T.; Wang, D.; Ba, X.; Zeng, X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions con-tributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef]
- Jung, K.Y.; Cho, S.W.; Kim, Y.; Kim, D.; Oh, B.-C.; Park, D.J.; Park, Y.J. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015, 49, 318–324. [Google Scholar] [CrossRef]
- Lee, C.-H.; Espinosa, I.; Vrijaldenhoven, S.; Subramanian, S.; Montgomery, K.D.; Zhu, S.; Marinelli, R.J.; Peterse, J.L.; Poulin, N.; Nielsen, T.O.; et al. Prognostic Significance of Macrophage Infiltration in Leiomyosarcomas. Clin. Cancer Res. 2008, 14, 1423–1430. [Google Scholar] [CrossRef]
- Nabeshima, A.; Matsumoto, Y.; Fukushi, J.; Iura, K.; Matsunobu, T.; Endo, M.; Fujiwara, T.; Iida, K.; Hatano, M.; Yokoyama, N.; et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br. J. Cancer 2015, 112, 547–555. [Google Scholar] [CrossRef]
- Oike, N.; Kawashima, H.; Ogose, A.; Hotta, T.; Hatano, H.; Ariizumi, T.; Sasaki, T.; Yamagishi, T.; Umezu, H.; Endo, N. Prognostic impact of the tumor immune microenvironment in synovial sarcoma. Cancer Sci. 2018, 109, 3043–3054. [Google Scholar] [CrossRef]
- Komohara, Y.; Takeya, H.; Wakigami, N.; Kusada, N.; Bekki, H.; Ishihara, S.; Takeya, M.; Nakashima, Y.; Oda, Y. Positive correlation between the density of macrophages and T-cells in undif-ferentiated sarcoma. Med. Mol. Morphol. 2019, 52, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Healey, J.; Ogura, K.; Yoshida, A.; Kondo, H.; Hata, T.; Kure, M.; Tazawa, H.; Nakata, E.; Kunisada, T.; et al. Role of Tumor-Associated Macrophages in Sarcomas. Cancers 2021, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-Infiltrating Macrophages Are Associated with Metastasis Suppression in High-Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immuno-histochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. OncoImmunology 2017, 6, e1331193. [Google Scholar]
- Handl, M.; Hermanova, M.; Hotarkova, S.; Jarkovsky, J.; Mudry, P.; Shatokhina, T.; Vesela, M.; Sterba, J.; Zambo, I. Clinicopathological correlation of tumor-associated macrophages in Ewing sarcoma. Biomed. Pap. 2018, 162, 54–60. [Google Scholar] [CrossRef]
- Lussier, D.M.; O’Neill, L.; Nieves, L.M.; McAfee, M.S.; Holechek, S.A.; Collins, A.W.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J.N. Enhanced T-Cell Immunity to Osteosarcoma Through Antibody Blockade of PD-1/PD-L1 Interactions. J. Immun. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef]
- Nafia, I.; Toulmonde, M.; Bortolotto, D.; Chaibi, A.; Bodet, D.; Rey, C.; Velasco, V.; Larmonier, C.B.; Cerf, L.; Adam, J.; et al. IDO Targeting in Sarcoma: Biological and Clinical Implications. Front. Immunol. 2020, 11, 274. [Google Scholar] [CrossRef]
- Mimura, K.; Kono, K.; Takahashi, A.; Kawaguchi, Y.; Fujii, H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 2006, 56, 761–770. [Google Scholar] [CrossRef]
- Oelkrug, C.; Ramage, J.M. Enhancement of T cell recruitment and infiltration into tumours. Clin. Exp. Immunol. 2014, 178, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Dubois, S.; Demetri, G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer 2007, 109, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.G.; Jungbluth, A.A.; Gnjatic, S.; Schwartz, G.K.; D’Adamo, D.R.; Keohan, M.L.; Wagner, M.J.; Scheu, K.; Chiu, R.; Ritter, E.; et al. A Pilot Study of Anti-CTLA4 Antibody Ipilimumab in Patients with Synovial Sarcoma. Sarcoma 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study: Nivolumab for Uterine Leiomyosarcoma. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Penel, N.; Ray-Coquard, I.L.; Cousin, S.; Bertucci, F.; Bompas, E.; Eymard, J.C.; Saada-Bouzid, E.; Soulie, P.; Boudou-Rouquette, P.; et al. High clinical activity of pembrolizumab in chordoma, alveolar soft part sarcoma (ASPS) and other rare sarcoma histotypes: The French AcSé pembrolizumab study from Unicancer. J. Clin. Oncol. 2021, 39 (Suppl. 15), 11520. [Google Scholar] [CrossRef]
- Delyon, J.; Resche-Rigon, M.; Renaud, M.; Le Goff, J.; Dalle, S.; Heidelberger, V.; Da Meda, L.; Allain, V.; Toullec, L.; Carcelain, G.; et al. 1077MO PD1 blockade with pembrolizumab in classic and endemic Kaposi sarcoma: A multicenter phase II study. Ann. Oncol. 2020, 31, S732. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Soto, L.S.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S.; et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol. 2022, 23, 1156–1166. [Google Scholar] [CrossRef]
- Livingston, M.B.; Jagosky, M.H.; Robinson, M.M.; Ahrens, W.A.; Benbow, J.H.; Farhangfar, C.J.; Foureau, D.M.; Maxwell, D.M.; Baldrige, E.A.; Begic, X.; et al. Phase II Study of Pembrolizumab in Combination with Doxorubicin in Metastatic and Unresectable Soft-Tissue Sarcoma. Clin. Cancer Res. 2021, 27, 6424–6431. [Google Scholar] [CrossRef]
- Pollack, S.M.; Redman, M.W.; Baker, K.K.; Wagner, M.J.; Schroeder, B.A.; Loggers, E.T.; Trieselmann, K.; Copeland, V.C.; Zhang, S.; Black, G.; et al. Assessment of Doxorubicin and Pembrolizumab in Patients With Advanced Anthracycline-Naive Sarcoma: A Phase 1/2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1778. [Google Scholar] [CrossRef]
- Gordon, E.M.; Chawla, S.P.; Tellez, W.A.; Younesi, E.; Thomas, S.; Chua-Alcala, V.S.; Chomoyan, H.; Valencia, C.; Brigham, D.A.; Moradkhani, A.; et al. SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma. Cancers 2023, 15, 906. [Google Scholar] [CrossRef]
- Pink, D.; Andreou, D.; Flörcken, A.; Golf, A.; Richter, S.; Kessler, T.; Kortüm, M.; Schmidt, C.A.; Kasper, B.; Wardelmann, E.; et al. Efficacy and safety of nivolumab and trabectedin in pretreated patients with advanced soft tissue sarcomas (STS): Preliminary results of a phase II trial of the German Interdisciplinary Sarcoma Group (GISG-15, NitraSarc) for the non-L sarcoma cohort. J. Clin. Oncol. 2021, 39, 11545. [Google Scholar] [CrossRef]
- Smrke, A.; Ostler, A.; Napolitano, A.; Vergnano, M.; Asare, B.; Fotiadis, N.; Thway, K.; Zaidi, S.; Miah, A.; van der Graaf, W.; et al. 1526MO GEMMK: A phase I study of gemcitabine (gem) and pembrolizumab (pem) in patients (pts) with leiomyosarcoma (LMS) and undifferentiated pleomorphic sarcoma UPS). Ann. Oncol. 2021, 32, S1114. [Google Scholar] [CrossRef]
- Nathenson, M.; Choy, E.; Carr, N.D.; Hibbard, H.D.; Mazzola, E.; Catalano, P.J.; Thornton, K.A.; Morgan, J.A.; Cote, G.M.; Merriam, P.; et al. Phase II study of eribulin and pembrolizumab in patients (pts) with metastatic soft tissue sarcomas (STS): Report of LMS cohort. J. Clin. Oncol. 2020, 38, 11559. [Google Scholar] [CrossRef]
- Wagner, M.J.; Zhang, Y.; Cranmer, L.D.; Loggers, E.T.; Black, G.; McDonnell, S.; Maxwell, S.; Johnson, R.; Moore, R.; Hermida de Viveiros, P.; et al. A Phase 1/2 Trial Combining Avelumab and Trabectedin for Advanced Lipo-sarcoma and Leiomyosarcoma. Clin. Cancer Res. 2022, 28, 2306–2312. [Google Scholar] [CrossRef]
- Toulmonde, M.; Brahmi, M.; Giraud, A.; Chakiba, C.; Bessede, A.; Kind, M.; Toulza, E.; Pulido, M.; Albert, S.; Guégan, J.-P.; et al. Trabectedin plus Durvalumab in Patients with Advanced Pretreated Soft Tissue Sarcoma and Ovarian Carcinoma (TRAMUNE): An Open-Label, Multicenter Phase Ib Study. Clin. Cancer Res. 2021, 28, 1765–1772. [Google Scholar] [CrossRef]
- Palmerini, E.; Lopez-Pousa, A.; Grignani, G.; Redondo, A.; Hindi, N.; Stacchiotti, S.; Sebio, A.; Lopez-Martin, J.A.; Morales, C.M.V.; Martinez-Trufero, J.; et al. IMMUNOSARC: A collaborative Spanish (GEIS) and Italian (ISG) sarcoma groups phase I/II trial of sunitinib and nivolumab in advanced soft tissue and bone sarcoma: Results from the phase II part, bone sarcoma cohort. J. Clin. Oncol. 2020, 38, 11522. [Google Scholar] [CrossRef]
- Broto, J.M.; Hindi, N.; Grignani, G.; Trufero, J.M.; Redondo, A.; Valverde, C.; Pousa, A.L.; Stacchiotti, S.; Palmerini, E.; De Alava, E.; et al. IMMUNOSARC: A collaborative Spanish (GEIS) and Italian (ISG) sarcoma groups phase I/II trial of sunitinib plus nivolumab in advanced soft tissue and bone sarcomas: Results of the phase II- soft-tissue sarcoma cohort. Ann. Oncol. 2019, 30, v684. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Xie, L.; Xu, J.; Sun, X.; Guo, W.; Gu, J.; Liu, K.; Zheng, B.; Ren, T.; Huang, Y.; Tang, X.; et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: A single-arm, open-label, phase 2 trial. J. Immunother. Cancer 2019, 8, e000798. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cho, H.J.; Yun, K.-H.; Lee, Y.H.; Kim, S.H.; Baek, W.; Jeon, M.K. Durvalumab and pazopanib in patients with advanced soft tissue sarcoma: A single-center, single-arm, phase 2 trial. J. Clin. Oncol. 2021, 39, 11551. [Google Scholar] [CrossRef]
- Cousin, S.; Bellera, C.; Guegan, J.-P.; Valentin, T.; Bahleda, R.; Metges, J.-P.; Cassier, P.; Cantarel, C.; Ceruso, M.S.; Kind, M.; et al. 1494P Regomune-a phase II study of regorafenib + avelumab in solid tumors: Results of the soft tissue sarcoma (STS) cohort. Ann. Oncol. 2022, 33, S1230. [Google Scholar] [CrossRef]
- Kelly, C.M.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Qin, L.-X.; Adamson, T.; Condy, M.M.; Biniakewitz, M.; Phelan, H.; et al. A phase II study of epacadostat and pembrolizumab in patients with advanced sarcoma. J. Clin. Oncol. 2019, 37, 11049. [Google Scholar] [CrossRef]
- Schöffski, P.; Bahleda, R.; Wagner, A.; Burgess, M.; Junker, N.; Chisamore, M.; Peterson, P.; Ceccarelli, M.; William, T. 154P Results of an open-label, phase Ia/Ib study of olaratumab plus pembrolizumab in patients with unresectable, locally advanced or metastatic soft tissue sarcoma. Ann. Oncol. 2021, 32, S1447. [Google Scholar] [CrossRef]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 402. [Google Scholar] [CrossRef]
- Chawla, N.S.; Kim, T.; Sherman, T.; Dang, J.; Chua, V.S.; Moradkhani, A.; Bhuiyan, I.; Krkyan, N.; Fernando, M.; Colletti, E.; et al. A phase 2 study of talimogene laherparepvec, nivolumab, and trabectedin (TNT) in advanced sarcoma. J. Clin. Oncol. 2021, 39, 11567. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Richards, A.L.; Conley, A.P.; Woo, H.J.; Dickson, M.A.; Gounder, M.; Kelly, C.; Keohan, M.L.; Movva, S.; Thornton, K.; et al. Pilot study of bempegaldesleukin in combination with nivolumab in patients with metastatic sarcoma. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Somaiah, N.; Block, M.S.; Kim, J.W.; Shapiro, G.I.; Do, K.T.; Hwu, P.; Eder, J.P.; Jones, R.L.; Lu, H.; ter Meulen, J.H.; et al. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019, 25, 5808–5817. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Movva, S.; Kelly, C.M.; Dickson, M.A.; Keohan, M.L.; Gounder, M.M.; Thornton, K.A.; Chi, P.; Chan, J.E.; Nacev, B.; et al. A phase 1b study of avelumab plus DCC-3014, a potent and selective inhibitor of colony stimulating factor 1 receptor (CSF1R), in patients with advanced high-grade sarcoma. J. Clin. Oncol. 2021, 39, 11549. [Google Scholar] [CrossRef]
- Chawla, S.P.; Chua-Alcala, V.S.; Gordon, E.M.; Kim, T.T.; Feske, W.; Gibson, B.L.; Chang, P.Y.; Robinson, D.; Song, P.Y. Interim analysis of a phase I study of SNK01 (Autologous Nongenetically Modified Natural Killer Cells with Enhanced Cytotoxicity) and avelumab in advanced refractory sarcoma. J. Clin. Oncol. 2022, 40, 11517. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Wada, T.; Ida, K.; Sato, Y.; Nagoya, S.; Tsukahara, T.; Kimura, S.; Sahara, H.; Ikeda, H.; Shimozawa, K.; et al. Phase I vaccination trial of SYT-SSX junction peptide in patients with disseminated synovial sarcoma. J. Transl. Med. 2005, 3, 1. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishibashi, Y.; Hiraoka, K.; Matsueda, S.; Kawano, K.; Kawahara, A.; Kage, M.; Ohshima, K.; Yamanaka, R.; Shichijo, S.; et al. Phase II study of personalized peptide vaccination for refractory bone and soft tissue sarcoma patients. Cancer Sci. 2013, 104, 1285–1294. [Google Scholar] [CrossRef]
- Pipia, N.; Baldueva, I.; Nekhaeva, T.; Novik, A.; Danilova, A.; Avdonkina, N.; Protsenko, S.; Komarov, Y.; Girdyuk, D.; Oganesyan, A.; et al. Autologous dendritic-cell vaccine based on cancer-testis antigens “CaTeVac” in the treatment of soft tissue sarcoma. Ann. Oncol. 2018, 29, viii410–viii411. [Google Scholar] [CrossRef]
- Chawla, S.P.; Van Tine, B.A.; Pollack, S.M.; Ganjoo, K.N.; Elias, A.D.; Riedel, R.F.; Attia, S.; Choy, E.; Okuno, S.H.; Agulnik, M.; et al. Phase II Randomized Study of CMB305 and Atezolizumab Compared With Atezolizumab Alone in Soft-Tissue Sarcomas Expressing NY-ESO-1. J. Clin. Oncol. 2022, 40, 1291–1300. [Google Scholar] [CrossRef]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.N.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A Pilot Trial Using Lymphocytes Genetically Engineered with an NY-ESO-1–Reactive T-cell Receptor: Long-term Follow-up and Correlates with Response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Demetri, G.; Tine, B.V.; Druta, M.; Glod, J.; Chow, W.; Araujo, D. 298 Final analysis of the phase 1 trial of NY-ESO-1–specific T-cell receptor (TCR) T-cell therapy (letetresgene autoleucel; GSK3377794) in patients with advanced synovial sarcoma (SS) [Internet]. In Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2020; p. A182–3. Available online: https://jitc.bmj.com/lookup/doi/10.1136/jitc-2020-SITC2020.0298 (accessed on 13 April 2023).
- Van Tine, B.A.; Butler, M.O.; Araujo, D.; Johnson, M.L.; Clarke, J.; Liebner, D.; Odunsi, K.; Olszanski, A.J.; Basu, S.; Brophy, F.; et al. ADP-A2M4 (MAGE-A4) in patients with synovial sarcoma. Ann. Oncol. 2019, 30, v684–5. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Van Tine, B.A.; Attia, S.; Blay, J.-Y.; Strauss, S.J.; Morales, C.M.V.; Razak, A.R.A.; Van Winkle, E.; Trivedi, T.; Biswas, S.; et al. SPEARHEAD-1: A phase 2 trial of afamitresgene autoleucel (Formerly ADP-A2M4) in patients with advanced synovial sarcoma or myxoid/round cell liposarcoma. J. Clin. Oncol. 2021, 39, 11504. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Navai, S.A.; Derenzo, C.; Joseph, S.; Sanber, K.; Byrd, T.; Zhang, H.; Mata, M.; Gerken, C.; Shree, A.; Mathew, P.R.; et al. Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 2019, 79 (Suppl. 13), LB-147. [Google Scholar] [CrossRef]
- Burgess, M.A.; Bolejack, V.; Schuetze, S.; Van Tine, B.A.; Attia, S.; Riedel, R.F.; Hu, J.S.; Davis, L.E.; Okuno, S.H.; Priebat, D.A.; et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J. Clin. Oncol. 2019, 37, 11015. [Google Scholar] [CrossRef]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Tawbi, H.A. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sar-coma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Z.; Bai, C.; Li, S.; Xue, R.; Gao, T.; Zhang, L.; Tan, Z.; Fang, Z. Real-world experience with pembrolizumab in patients with advanced soft tissue sarcoma. Ann. Transl. Med. 2021, 9, 339. [Google Scholar] [CrossRef]
- Saif, A.; Verbus, E.A.; Sarvestani, A.L.; Teke, M.E.; Lambdin, J.; Hernandez, J.M.; Kirsch, D.G. A Randomized Trial of Pembrolizumab & Radiotherapy Versus Radiotherapy in High-Risk Soft Tissue Sarcoma of the Extremity (SU2C-SARC032). Ann. Surg. Oncol. 2022, 30, 683–685. [Google Scholar] [CrossRef]
- le Guevelou, J.; Debaigt, C.; Saada-Bouzid, E.; Viotti, J.; Khalladi, N.; Thibouw, D.; Thariat, J. Phase II study of concomitant radiotherapy with atezolizumab in oli-gometastatic soft tissue sarcomas: STEREOSARC trial protocol. BMJ Open 2020, 10, e038391. [Google Scholar] [CrossRef]
- Keung, E.Z.; Tsai, J.-W.; Ali, A.M.; Cormier, J.N.; Bishop, A.J.; Guadagnolo, B.A.; Roland, C.L. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: Rationale for combination neoadjuvant immune checkpoint inhibition and radio-therapy. OncoImmunology 2018, 7, e1385689. [Google Scholar] [CrossRef]
- Takada, K.; Sugita, S.; Murase, K.; Kikuchi, T.; Oomori, G.; Ito, R.; Hayasaka, N.; Miyanishi, K.; Iyama, S.; Ikeda, H.; et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac. Cancer 2019, 10, 2312–2315. [Google Scholar] [CrossRef]
- Anžič, N.; Krasniqi, F.; Eberhardt, A.-L.; Tzankov, A.; Haslbauer, J.D. Ipilimumab and Pembrolizumab Mixed Response in a 41-Year-Old Patient with SMARCA4-Deficient Thoracic Sarcoma: An Interdisciplinary Case Study. Case Rep. Oncol. 2021, 14, 706–715. [Google Scholar] [CrossRef]
- Henon, C.; Blay, J.-Y.; Massard, C.; Mir, O.; Bahleda, R.; Dumont, S.; Postel-Vinay, S.; Adam, J.; Soria, J.-C.; Le Cesne, A. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann. Oncol. 2019, 30, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Sakakibara, R.; Ishizuka, M.; Honda, T.; Shirai, T.; Okamoto, T.; Tateishi, T.; Sakashita, H.; Tamaoka, M.; Takemoto, A.; et al. Notable response to nivolumab during the treatment of SMARCA4-deficient thoracic sarcoma: A case report. Immunotherapy 2020, 12, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, H.; Kunimasa, K.; Kukita, Y.; Nakamura, H.; Honma, K.; Kawamura, T.; Inoue, T.; Tamiya, M.; Kuhara, H.; Nishino, K.; et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy 2021, 13, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Okami, J.; Takenaka, S.; Honma, K.; Kukita, Y.; Nagata, S.; Kawamura, T.; Inoue, T.; Tamiya, M.; Kuhara, H.; et al. Conversion Surgery for Advanced Thoracic SMARCA4-Deficient Undifferentiated Tumor With Atezolizumab in Combination With Bevacizumab, Paclitaxel, and Carboplatin Treatment: A Case Report. JTO Clin. Res. Rep. 2021, 2, 100235. [Google Scholar] [CrossRef]
- Marcrom, S.; De Los Santos, J.F.; Conry, R.M. Complete response of mediastinal clear cell sarcoma to pembrolizumab with ra-diotherapy. Clin. Sarcoma Res. 2017, 7, 14. [Google Scholar] [CrossRef]
- Yu, X.-H.; Huang, J.; Ge, N.-J.; Yang, Y.-F.; Zhao, J.-Y. Recurrent undifferentiated embryonal sarcoma of the liver in adult patient treated by pembrolizumab: A case report. World J. Clin. Cases 2021, 9, 2281–2288. [Google Scholar] [CrossRef]
- Song, H.-N.; Kang, M.G.; Park, J.R.; Hwang, J.-Y.; Kang, J.H.; Lee, W.S.; Lee, G.-W. Pembrolizumab for Refractory Metastatic Myxofibrosarcoma: A Case Report. Cancer Res. Treat. 2018, 50, 1458–1461. [Google Scholar] [CrossRef]
- Lambden, J.P.; Kelsten, M.F.; Schulte, B.C.; Pa, S.A.; Hayes, J.P.; Villaflor, V.; Agulnik, M. Metastatic Myxofibrosarcoma with Durable Response to Temozolomide Followed by Atezolizumab: A Case Report. Oncologist 2021, 26, 549–553. [Google Scholar] [CrossRef]
- Tabata, M.M.; Novoa, R.A.; Bui, N.Q.; Zaba, L.C. Successful treatment of HIV-negative Kaposi sarcoma with ipilimumab and nivolumab and concurrent management of baseline psoriasis and bullous pemphigoid. JAAD Case Rep. 2020, 6, 447–449. [Google Scholar] [CrossRef]
- Zhou, M.; Bui, N.; Lohman, M.; van de Rjin, M.; Hwang, G.; Ganjoo, K. Long-Term Remission with Ipilimumab/Nivolumab in Two Patients with Different Soft Tissue Sarcoma Subtypes and No PD-L1 Expression. Case Rep. Oncol. 2021, 14, 459–465. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, J.; Liang, D.; Zhao, M.; Yu, W.; Chen, P. Nivolumab plus ipilimumab versus nivolumab in individuals with treatment-naive programmed death-ligand 1 positive metastatic soft tissue sarcomas: A multicentre retrospective study. BMC Cancer 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bui, N.; Bolleddu, S.; Lohman, M.; Becker, H.-C.; Ganjoo, K. Nivolumab plus ipilimumab for soft tissue sarcoma: A single institution retrospective review. Immunotherapy 2020, 12, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Zer, A.; Icht, O.; Joseph, L.; Avram, D.; Jacobi, O.; Fenig, E.; Shamai, S.; Frommer, R.S.; Bernstine, H.; Weitzen, R.; et al. A phase II single-arm study of nivolumab and ipilimumab (Nivo/Ipi) in previously treated Classic Kaposi sarcoma (CKS). J. Clin. Oncol. 2019, 37, 11064. [Google Scholar] [CrossRef]
- Lewin, J.; Davidson, S.; Anderson, N.D.; Lau, B.Y.; Kelly, J.; Tabori, U.; Salah, S.; Butler, M.O.; Aung, K.L.; Shlien, A.; et al. Response to Immune Checkpoint Inhibition in Two Patients with Alveolar Soft-Part Sarcoma. Cancer Immunol. Res. 2018, 6, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, V.; Bauer, S.; Hermes, B.; Ivanyi, P.; Lindner, L.H.; Pink, D.; Reichardt, P.; Richter, S.; Tuchscherer, A. A randomized phase II study of durvalumab and tremelimumab compared to dox-orubicin in patients with advanced or metastatic soft tissue sarcoma (MEDISARC, AIO-STS 0415). J. Clin. Oncol. 2019, 37 (Suppl. 15), TPS11075. [Google Scholar] [CrossRef]
- Bishop, M.W.; Kaste, S.C.; Sykes, A.; Pan, H.; Cruz, F.S.D.; Whittle, S.; Mascarenhas, L.; Thomas, P.G.; Youngblood, B.; Harman, J.L.; et al. OSTPDL1: A phase II study of avelumab, a monoclonal antibody targeting programmed death-ligand 1 (PD-L1) in adolescent and young adult patients with recurrent or progressive osteosarcoma. J. Clin. Oncol. 2020, 38, 10521. [Google Scholar] [CrossRef]
- Uboha, N.V.; Milhem, M.M.; Kovacs, C.; Amin, A.; Magley, A.; Das Purkayastha, D.; Piha-Paul, S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019, 37, 2553. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Long, G.V. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Mela-noma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Rivera Vargas, T.; Apetoh, L. Danger signals: Chemotherapy enhancers? Immunol. Rev. 2017, 280, 175–193. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, Y.; Yang, J.; Zhang, P.; Zhang, F.; Du, X.; Li, C.; Wang, J. Safety and Efficacy of PD-1 Inhibitors Plus Chemotherapy in Advanced Soft Tissue Sarcomas: A Retrospective Study. Cancer Manag. Res. 2020, 12, 1339–1346. [Google Scholar] [CrossRef]
- Italiano, A.; Bessede, A.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Bertucci, F.; Toulmonde, M.; Bellera, C.A.; Guegan, J.-P.; et al. PD1 inhibition in soft-tissue sarcomas with tertiary lymphoid structures: A multicenter phase II trial. J. Clin. Oncol. 2021, 39, 11507. [Google Scholar] [CrossRef]
- Spalato-Ceruso, M.; Bouteiller, F.; Guegan, J.-P.; Toulmonde, M.; Bessede, A.; Kind, M.; Cousin, S.; Buy, X.; Palussiere, J.; Le Loarer, F.; et al. Pembrolizumab combined with low-dose cyclophosphamide and in-tra-tumoral injection of the toll-like receptor 4 agonist G100 in patients with advanced pretreated soft tissue sarcoma: Results from the PEMBROSARC basket study. J. Hematol. Oncol. J. Hematol. Oncol. 2022, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Sankhala, K.K.; Ravicz, J.; Kang, G.; Liu, S.; Stumpf, N.; Leong, B.; Kim, S.; Arasheben, S.; Tseng, W.W.; et al. Clinical experience with combination chemo-/immunotherapy using trabectedin and nivolumab for advanced soft tissue sarcoma. J. Clin. Oncol. 2018, 36 (Suppl. 15), e23568. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Terry, R.L.; Meyran, D.; Omer, N.; Trapani, J.A.; Haber, M.; Neeson, P.J.; Ekert, P.G. Enhancing the Potential of Immunotherapy in Paediatric Sarcomas: Breaking the Immunosuppressive Barrier with Receptor Tyrosine Kinase Inhibitors. Biomedicines 2021, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Dorman, K.; Burkhard-Meier, A.; Di Gioia, D.; Kunz, W.G.; Knösel, T.; Holch, J.W.; Lindner, L.H. Treatment of metastatic alveolar soft part sarcoma with axitinib and pembrolizumab in an 80-year-old patient with a history of autoimmune disorders. Anti-Cancer Drugs 2022, 34, 311–316. [Google Scholar] [CrossRef]
- Li, H.-L.; Li, Y.; Hu, H.-T.; Shao, S.-S.; Chen, C.-S.; Guo, C.-Y.; Zhao, Y.; Yao, Q.-J. Clinical observation of local intervention combined with camrelizumab and apatinib in the treatment of metastatic soft-tissue sarcoma. J. Cancer Res. Ther. 2021, 17, 1718. [Google Scholar] [CrossRef]
- Paoluzzi, L.; Cacavio, A.; Ghesani, M.; Karambelkar, A.; Rapkiewicz, A.; Weber, J.; Rosen, G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin. Sarcoma Res. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Arora, S.; Rastogi, S.; Shamim, S.A.; Barwad, A.; Sethi, M. Good and sustained response to pembrolizumab and pazopanib in advanced undifferentiated pleomorphic sarcoma: A case report. Clin. Sarcoma Res. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Monga, V.; Miller, B.J.; Tanas, M.; Boukhar, S.; Allen, B.; Anderson, C.; Stephens, L.; Hartwig, S.; Varga, S.; Houtman, J.; et al. Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: Phase IB/II trial. J. Immunother. Cancer 2021, 9, e003119. [Google Scholar] [CrossRef]
- Diab, A.; Tannir, N.M.; Bentebibel, S.-E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Hanna, A.; Metge, B.J.; Bailey, S.K.; Chen, D.; Chandrashekar, D.S.; Varambally, S.; Samant, R.S.; Shevde, L.A. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology 2019, 8, 1548241. [Google Scholar] [CrossRef] [PubMed]
- Grund-Gröschke, S.; Ortner, D.; Szenes-Nagy, A.B.; Zaborsky, N.; Weiss, R.; Neureiter, D.; Wipplinger, M.; Risch, A.; Hammerl, P.; Greil, R. Epidermal activation of Hedgehog signaling establishes an immuno-suppressive microenvironment in basal cell carcinoma by modulating skin immunity. Mol. Oncol. 2020, 14, 1930–1946. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yao, F.; Davis, P.F.; Tan, S.T.; Hall, S.R.R. CD73, Tumor Plasticity and Immune Evasion in Solid Cancers. Cancers 2021, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, N.; Livingston, J.A.A.; Ravi, V.; Lin, H.Y.; Amini, B.; Solis, L.M.; Conley, A.P.; Zarzour, M.A.; Ludwig, J.A.; Ratan, R.; et al. A phase II multi-arm study to test the efficacy of oleclumab and durvalumab in specific sarcoma subtypes. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS11594. [Google Scholar] [CrossRef]
- Italiano, A.; Isambert, N.; Metges, J.-P.; Toulmonde, M.; Cousin, S.; Pernot, S.; Spalato, M.; Grellety, T.; Auzanneau, C.; Lortal, B.; et al. CAIRE: A basket multicenter open-label phase 2 study evaluating the EZH2 inhibitor tazemetostat in combination with durvalumab in patients with advanced solid tumors. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS2703. [Google Scholar] [CrossRef]
- Lin, C.-C. Clinical Development of Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors. J. Immunother. Precis. Oncol. 2021, 4, 105–114. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Sato, Y.; Nabeta, Y.; Tsukahara, T.; Hirohashi, Y.; Syunsui, R.; Maeda, A.; Sahara, H.; Ikeda, H.; Torigoe, T.; Ichimiya, S.; et al. Detection and Induction of CTLs Specific for SYT-SSX-Derived Peptides in HLA-A24+ Patients with Synovial Sarcoma. J. Immunol. 2002, 169, 1611–1618. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Tsukahara, T.; Ida, K.; Kimura, S.; Murase, M.; Kano, M.; Emori, M.; Nagoya, S.; Kaya, M.; Torigoe, T.; et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: A study from the Japanese Musculoskeletal Oncology Group. Cancer Sci. 2012, 103, 1625–1630. [Google Scholar] [CrossRef]
- Krishnadas, D.K.; Shusterman, S.; Bai, F.; Diller, L.; Sullivan, J.E.; Cheerva, A.C.; George, R.E.; Lucas, K.G. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015, 64, 1251–1260. [Google Scholar] [CrossRef]
- Dillman, R.O.; Wiemann, M.; Nayak, S.K.; DeLeon, C.; Hood, K.; DePriest, C. Interferon-gamma or granulocyte-macrophage col-ony-stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short-term cell line cultures: A randomized phase 2 trial of the cancer biotherapy research group. J. Immun. Hagerstown Md. 2003, 26, 367–373. [Google Scholar]
- Kershaw, M.; Westwood, J.A.; Darcy, P. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 2013, 13, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Gyurdieva, A.; Zajic, S.; Chang, Y.-F.; Houseman, E.A.; Zhong, S.; Kim, J.; Nathenson, M.; Faitg, T.; Woessner, M.; Turner, D.C.; et al. Biomarker correlates with response to NY-ESO-1 TCR T cells in patients with synovial sarcoma. Nat. Commun. 2022, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Druta, M.; Liebner, D.A.; Schuetze, S.; Somaiah, N.; Van Tine, B.A.; Tap, W.D.; Pulham, T.; Chagin, K.; Norry, E.; et al. Pilot study of NY-ESO-1c259 T cells in advanced myxoid/round cell liposarcoma. J. Clin. Oncol. 2018, 36, 3005. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Attia, S.; Blay, J.-Y.; Strauss, S.J.; Morales, C.M.V.; Razak, A.R.A.; Van Tine, B.A. Identification of response stratification factors from pooled efficacy analyses of afami-tresgene autoleucel (“Afami-cel” [Formerly ADP-A2M4]) in metastatic synovial sarcoma and myxoid/round cell liposarcoma phase 1 and phase 2 trials. J. Clin. Oncol. 2022, 40 (Suppl. 16), 11562. [Google Scholar]
- Moreno Tellez, C.; Leyfman, Y.; D’Angelo, S.P.; Wilky, B.A.; Dufresne, A. Immunotherapy in Sarcoma: Where Do Things Stand? Surg. Oncol. Clin. N. Am. 2022, 31, 381–397. [Google Scholar] [CrossRef]
- Huang, X.; Park, H.; Greene, J.; Pao, J.; Mulvey, E.; Zhou, S.X.; Albert, C.M.; Moy, F.; Sachdev, D.; Yee, D.; et al. IGF1R-and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PLoS ONE 2015, 10, e0133152. [Google Scholar] [CrossRef]
- Leuci, V.; Casucci, G.M.; Grignani, G.; Rotolo, R.; Rossotti, U.; Vigna, E.; Gammaitoni, L.; Mesiano, G.; Fiorino, E.; Donini, C.; et al. CD44v6 as innovative sarcoma target for CAR-redirected CIK cells. Oncoimmunology 2018, 7, e1423167. [Google Scholar] [CrossRef]
- Lehner, M.; Götz, G.; Proff, J.; Schaft, N.; Dörrie, J.; Full, F.; Ensser, A.; Muller, Y.; Cerwenka, A.; Abken, H.; et al. Redirecting T Cells to Ewing’s Sarcoma Family of Tumors by a Chimeric NKG2D Receptor Expressed by Lentiviral Transduction or mRNA Transfection. PLoS ONE 2012, 7, e31210. [Google Scholar] [CrossRef]
- Fernández, L.; Metais, J.-Y.; Escudero, A.; Vela, M.; Valentín, J.; Vallcorba, I.; Leivas, A.; Torres, J.; Valeri, A.; Patiño-García, A.; et al. Memory T Cells Expressing an NKG2D-CAR Efficiently Target Osteosarcoma Cells. Clin. Cancer Res. 2017, 23, 5824–5835. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef] [PubMed]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, Ö.; Kessels, R.; Foppen, M.H.G.; Granhøj, J.S.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Nie, C.-P.; Liu, X.-F.; Song, B.; Yue, J.-H.; Xu, J.-X.; He, J.; Li, K.; Feng, Y.-L.; Wan, T.; et al. Phase I study of adjuvant immunotherapy with autologous tumor-infiltrating lymphocytes in locally advanced cervical cancer. J. Clin. Investig. 2022, 132, e157726. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus–associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Jazaeri, A.A.; Zsiros, E.; Amaria, R.N.; Artz, A.S.; Edwards, R.P.; Wenham, R.M.; Slomovitz, B.M.; Walther, A.; Thomas, S.S.; Chesney, J.A.; et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J. Clin. Oncol. 2019, 37, 2538. [Google Scholar] [CrossRef]
- Creelan, B.; Wang, C.; Teer, J.; Toloza, E.; Yao, J.; Kim, S.; Landin, A.; Mullinax, J.; Saller, J.; Saltos, A.; et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: A phase 1 trial. Immune ACCESS 2021, 27, 1410–1418. [Google Scholar] [CrossRef]
- Pedersen, M.; Westergaard, M.; Milne, K.; Nielsen, M.; Borch, T.H.; Poulsen, L.G.; Hendel, H.W.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: A pilot study. Oncoimmunology 2018, 7, e1502905. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Feldman, S.A. Immune recognition of somatic mutations leading to complete durable re-gression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epi-thelial Cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Mullinax, J.E.; Hall, M.; Beatty, M.; Weber, A.M.; Sannasardo, Z.; Svrdlin, T.; Hensel, J.; Bui, M.; Richards, A.; Gonzalez, R.J.; et al. Expanded Tumor-infiltrating Lymphocytes From Soft Tissue Sarcoma Have Tu-mor-specific Function. J. Immunother Hagerstown Md. 2021, 44, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Coward, V.S.; Gokgoz, N.; Dickson, B.C.; Tsoi, K.; Wunder, J.S.; Andrulis, I.L. Investigating the Potential of Isolating and Expanding Tumour-Infiltrating Lymphocytes from Adult Sarcoma. Cancers 2022, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, J.; Duan, C.; Liu, Y. Retrospective Analysis of Adoptive TIL Therapy plus Anti-PD1 Therapy in Patients with Chemotherapy-Resistant Metastatic Osteosarcoma. J. Immunol. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial | Agent | Tumor | N | Age Range | Outcomes | Reported G3/G4 AEs |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors (ICI) | ||||||
| Maki et al. (phase I/II) [85] | Ipilimumab | Synovial sarcoma | 6 | 23–57 | ORR 0% mPFS 1.5 m mOS 8.8 m | Nausea (50%), diarrhea (33.3%), lymphopenia (33.3%), hyperbilirubinemia (16.7%), thrombopenia (16.7%) |
| Merchant et al. (phase I) [86] | Ipilimumab | Pediatric sarcoma | 17 | 2–17 | ORR 0%; DCR 17.6% (3 SD) mPFS/mOS: NA | Diarrhea (9%), AST/ALT increase (6%), endocrinopathies (3%), other irAEs (9%) |
| Ben-Ami et al. (phase II) [87] | Nivolumab | Uterine leiomyo-sarcoma | 12 | 29–73 | ORR 0% mPFS 1.8 m; mOS NA | Lipase/amylase increase (8.3%), fatigue (8.3%), abdominal pain (8.3%) |
| Tawbi et al. (phase II) (SARC028) [54] | Pembrolizumab | BS cohort (22 OST, 13 ES, 5 CS) | 40 | 16–70 | mPFS 8 w; mOS 52 w OST: ORR 5%; DCR 32% (1 PR, 6 SD) ES: ORR 0%; DCR 15% (2 SD) CS: ORR 20%; DCR 40% (1 PR, 1 SD) | Interstitial nephritis (2%), infectious pneumonia (2%), bone pain (2%), pleural effusion (2%), hypoxia (2%) |
| STS cohort (10 LMS, 10 LPS, 10 SS, 10 UPS) | 40 | 18–81 | mPFS 18 w; mOS 49 w LMS: ORR 0%; DCR 60% (6 SD) / LPS: ORR 20%; DCR 60% (2 PR, 4 SD)/SS: ORR 10%; DCR 30% (1 PR, 2 SD)/UPS: ORR 40%; DCR 70% (1 CR, 3 PR, 3 SD) | Pulmonary embolism (2%), adrenal insufficiency (2%), pneumonitis (2%) | ||
| Blay et al. (phase II) (AcSé) [88] | Pembro-lizumab | Advanced rare sarcoma | 98 | >18 (NA) | ORR 15.3%; DCR 49% (1 CR, 14 PR, 33 SD); mDR 8.2 m; mPFS 2.8 m; mOS 19.7 m | NA |
| Delyon et al. (phase II) [89] | Pembrolizumab | Classic/ endemic KS | 17 | NA | ORR 70.1%; DCR 88% (1 CR, 10 PR, 4 SD) | Reversible acute cardiac decompensation (6%) |
| D’Angelo et al. (phase II) (Alliance A091401) [90] | Nivo/ipi vs. ipi | Advanced sarcoma (BS and STS) | 85 | 21–81 | ORR 16% vs. 5%; mDR 6.2 mmPFS 4.1 m vs. 1.7 m mOS 14.3 m vs. 10.7 m | Pain (7% vs. 5%), thrombopenia (0% vs. 2%), pulmonary edema (2% vs. 0%), respiratory failure (5% vs. 5%), skin infection (2% vs. 0%), intestinal obstruction (2% vs. 2%), spinal fracture (0% vs. 2%), thrombo-embolic event (2% vs. 2%), urinary tract infection (7% vs. 2%), urinary obstruction (0% vs. 5%), fistula (2% vs. 0%), vomiting (0% vs. 2%) |
| Somaiah et al. (phase II) [91] | Durva-lumab + tremeli-mumab | Advanced sarcoma (BS and STS) | 57 | 35–59 | mPFS 2.8 m; mOS: 21.6 m; PFS at 12 m (all): 28%; PFS at 12 m (ASPS): 80% ORR (irRECIST) (all): 12%; ORR (irRECIST) (ASPS): 40% | Lipase increase (7%), pneumonitis (6%), colitis (6%), myocarditis (4%), autoimmune disorders (4%), endocrine disorders (2%), diarrhea (2%), gastrointestinal disorders (2%), lung infection (2%), ALP increase (2%), amylase increase (2%), myositis (2%) |
| Immune checkpoint inhibitors (ICI) + conventional CT | ||||||
| Livingston et al. (phase II) [92] | Pembro + doxorubicin | Anthracy-cline naïve STS | 30 | NA | ORR 36.7%; DCR 80% (1 CR, 10 PR, 13 SD) mPFS 5.7 m; mOS 17 m PFS at 6 m: 44% PFS at 12 m: 62% | Neutropenia (36.7%), anemia (26.7%), febrile neutropenia (16.7%), arthralgia (13.3%), lymphopenia (13.3%), nausea (13.3%), fatigue (10.0%), hyponatremia (10.0%), vomiting (10.0%), lung infection (10.0%), muscle weakness (10.0%) |
| Pollack et al. (phase I/II) [93] | Pembro + doxorubicin | Anthracy-cline naïve STS | 37 | 25–80 | ORR 19%; DCR 78% (7 PR, 22 SD); mPFS 8.1 m; mOS 27.6 m PFS at 12 m: 27% | Neutropenia (27.0%), oral mucositis (8.1%), anemia (5.4%), febrile neutropenia (5.4%), lymphopenia (5.4%), ejection fraction decrease (5.4%), anorexia (5.4%), diarrhea (2.7%), hypothyroidism (2.7%), nausea (2.7%), weight loss (2.7%) |
| Toulmonde et al. (phase II) [79] | Pembro + mCP | Advanced STS | 50 | 18–84 | ORR 2%; DCR 34% (1 PR, 16 SD); PFS at 6 m: 0% (LMS, UPS), 11.1% (GIST), 14.3% (others) | Anemia (7.0%), fatigue (3.5%), lymphopenia (3.5%), oral mucositis (3.5%) |
| Gordon et al. (phase I/II) (SAINT) [94] | Ipi/nivo + trabectedin (trab) | Advanced STS | 79 | NA | ORR 25.3%; DCR 87.3% (6 CR *, 14 PR, 49 SD) mPFS 6.7 m; mOS 24.6 m * One surgical CR | ALT increase (25%), fatigue (8.7%), AST increase (8.7%), decreased neutrophil count (5.4%), anemia (4.6%) |
| Pink et al. (phase II) (NITRA-SARC) [95] | Nivo + trab | Advanced STS | 25 | NA | ORR 8%; DCR 48% (2 PR, 10 SD); mPFS 4 m | Leukopenia (47.2%), neutropenia (41.7%), thrombopenia (33.3%), increased ALT (30.6%), anemia (27.8%) |
| Smrke et al. (phase I) [96] | Pembro + gemcitabine | LMS, UPS | 13 | 40–67 | LMS (11): DCR 73% (8 SD) UPS (2): DCR 100% (2 PR) | NA |
| Nathenson et al. (phase II) [97] | Pembro + eribulin | LMS cohort | 19 | 48–80 | ORR 5.3%; DCR 26.3% (1 PR, 5 SD); mPFS 11 w | Most commonly, neutropenia, anemia, weight loss, diarrhea, lipase/ALP increase |
| Wagner et al. (phase I/II) [98] | Avelumab + trab | LMS, LPS | 23 | NA | ORR 13%; DCR 56% (3 PR, 10 SD); mPFS 8.3 m | NA |
| Toulmonde et al. (phase Ib) [99] | Durva + trab | Advanced STS cohort | 16 | NA | ORR 7%; PFS at 6 m: 28.6% | NA |
| Immune checkpoint inhibitors (ICI) + tyrosine–kinase inhibitors/antiangiogenic drugs | ||||||
| Martin-Broto et al. (phase I/II) (IMMU-NOSARC) | Nivo + suni-tinib | BS cohort (17 OST, 14 CS, 8 ES, 1 UPS) [100] | 40 | 21–74 | ORR 5%; DCR 60% (1 CR, 1 PR, 22 SD) mPFS 3.7 m; mOS 14.2 m | Neutropenia (10%), anemia (10%), AST/ALT increase (7.5%), fatigue (5%), oral mucositis (5%), hemorrhage (2.5%), dysphagia (2.5%), thrombopenia (2.5%), malaise (2.5%), thromboembolism (2.5%), pneumonitis (2.5%) |
| STS cohort [101] | 43 | 19–77 | ORR 9.3%; DCR 69.3% (1 CR, 3 PR, 26 SD); mPFS 5.9 m; mOS not reached (follow up 6.1 m) | AST increase (11.8%), ALT increase (9.8%), neutropenia (9.8%), fatigue (5.9%), thrombopenia (3.9%), diarrhea (3.9%), renal failure (3.9%) | ||
| Wilky et al. (phase II) [102] | Pembro + axitinib | 12 ASPS, 6 LMS, 5 UPS, 2 DDLPS, 8 others (2 BS) | 33 | 27–62 | ORR 25%; DCR 53.1% (8 PR, 9 SD); mPFS (all): 4.7 m; mOS (all): 18.7 m; mPFS (ASPS): 12.4 m; mPFS (others): 3.0 m. | Hypertension (15%), autoimmune toxic effects (15%), nausea (6%), ALT/AST increase (3%), oral mucositis (3%), diarrhea (3%), abdominal pain (3%), hemoptysis (3%), hyperlipidemia (3%) |
| Xie et al. (phase II) [103] | Camre-lizumab + apatinib | CT-refractory OST | 43 | 11–43 | ORR 20.1% (9 PR) mDR 6.2 m mPFS 6.2 m; mOS 11.3 m | Wound dehiscence (14%), ALP increase (9.3%), AST/ALT increase (9.3%), blood bilirubin increase (9.3%), hypertriglyceridemia (7.0%), anorexia (7.0%), weight loss (7.0%), pneumothorax (7.0%), platelet count decrease (4.7%), diarrhea (4.7%), PPS (4.7%), limb pain (4.7%), leukopenia (4.7%), rash (4.7%), oral mucositis (4.7%), hypertension (4.7%), toothache (4.7%), nausea (4.7%), non-cardiac chest pain (4.7%), hypothyroidism (2.3%), LDH increase (2.3%), proteinuria (2.3%), cough (2.3%), hemorrhage (2.3%), fatigue (2.3%), peripheral neuroinflammation (2.3%) |
| Kim et al. (phase II) [104] | Durva + pazopanib | Advanced STS | 47 | NA | ORR 28.3% (1 CR, 12 PR) mPFS 8.6 m | NA |
| Cousin et al. (phase II) [105] | Avelumab + regorafenib | Advanced STS | 43 | NA | ORR 9.3%; DCR 48.8% (4 PR, 17 SD); mDR 7.8 m; mPFS 1.8 m; mOS 15.1 m | PPS (12.2%), fatigue (10.2%), diarrhea (10.2%) |
| Kelly et al. (phase II) [106] | Pembro + epacadostat | Advanced STS | 29 | 24–78 | ORR 3%; DCR 48% (1 PR, 13 SD); mPFS 8 w; PFS at 24 w 27.9%; mOS NA | AST increase (10%), ALT increase (3%), anemia (3%), hypophosphatemia (3%), lipase increase (3%) |
| Schöffski et al. (phase Ib) [107] | Pembro + olaratumab | Advanced STS | 28 | NA | ORR 21.4%; DCR 53.5%; mDR 16.2 m; mPFS 2.7 m; mOS 14.8 m | NA |
| Immune checkpoint inhibitors (ICI) + other agents | ||||||
| Kelly et al. (phase II) [108] | Pembro + T-VEC | Advanced STS | 20 | 24–90 | ORR 35%; DCR 70% (7 PR, 7 SD); mPFS 17.1 w | Pneumonitis (5%), fever (5%), anemia (5%) |
| Chawla et al. (phase II) [109] | Trabectedin + nivo + T-VEC | Advanced sarcoma | 36 | NA | ORR 8.3%; DCR 86.1% (3 PR, 27 SD); mPFS 5.5 m; mOS 9.0 m; OS at 6 m 73% | Anemia (33.3%), ALT increase (22.2%), fatigue (11.1%), thrombopenia (11.1%), neutropenia (11.1%) |
| D’Angelo et al. (phase I) [110] | Nivo + bempegal-desleukin | Advanced STS | 84 | 13–80 | ORR 10.4% (PR: 3/8 in AS, 1/4 in ASPS, 2/10 in UPS, 1/10 in LMS, 1/10 in CS); mDR 9.3 m | Anemia (10%), lipase increase (10%), amylase increase (7%), hypertension (7%), pain (8%), thromboembolic events (5%) |
| Somaiah et al. (phase I) [111] | LV305 | STS (13 SS, 6 MRCL) | 24 | 25–72 | ORR 4.2%; DCR 62.5% (1 PR, 14 SD); mPFS 4.6 m; mOS 33 m | No G3/G4 adverse events |
| Rosenbaum et al. (phase I) [112] | Avelumab + DCC-3014 | Advanced STS (7 LMS) | 13 | 32–71 | DCR 23% (3 SD); decreased circulating MDSCs in 5/7 patients (median 26.9%) | ALT/AST increase (31%), CK increase (23%), amylase/lipase increase (16%), anemia (8%), hypertension (8%) |
| Chawla et al. (phase I) [113] | Avelumab + SNK01 | Advanced sarcoma | 15 | 20–75 | ORR 13.3%; DCR 33.3% (2 PR, 3 SD); mPFS 11.1 w | No G3/G3 adverse events related to SNK01 |
| Therapeutic vaccines | ||||||
| Kawaguchi et al. (phase I) [114] | SYT-SSX vaccine | SS | 21 | 21–69 | DCR 50% (6 SD out of 12 assessable patients) | NA |
| Takahashi et al. (phase II) [115] | Peptide vaccine | Advanced sarcoma | 20 | 23–75 | DCR 30% (6 SD); mOS 9.6 m | NA |
| Pipia et al. (phase I/II) [116] | DC vaccine | Advanced STS | 74 | NA | Cohort 1 (adj/maint): mOS 24.4 m; cohort 2 (mono): mOS 14.2 m | NA |
| Chawla et al. (phase II) [117] | Atezo +/− CMB305 | SS, MLS | 89 | NA | mPFS 2.6 m vs. 1.6 m mOS 18 m in both groups | 4 G3/G4 events in each group (not specified) |
| Adoptive cell therapy | ||||||
| Robbins et al. (phase II) [118] | NY-ESO1 TCR | SS | 18 | 19–65 | ORR 61% (1 CR, 10 PR); PFS 3–47 m; estimated 3-y OS: 38% | No toxicities attributed to the transferred cells * |
| D’Angelo et al. (phase II) [119] | NY-ESO1 TCR | SS | 45 | NA | ORR 33% (1 CR, 14 PR); mPFS 8.6–22.4 w | No toxicities attributed to the transferred cells * |
| Van Tine et al. (phase I) [120] | MAGE-A4 TCR | SS | 8 | NA | ORR 50%; DCR 87.5% (4 PR, 3 SD) | No toxicities attributed to the transferred cells * |
| D’Angelo et al. (phase II) [121] | MAGE-A4 TCR | STS (23 SS,2 MLS) | 25 | 24–73 | ORR 40%; DCR 84% (2 CR, 8 PR, 11 SD) | CRS (5%) * |
| Ahmed et al. (phase I/II) [122] | Her2-CAR T cells | Advanced sarcoma (16 OST, 3 others) | 19 | 7–29 | DCR 23.5% (4 SD); mOS 10.3 m | Anemia (5.3%), muscle weakness (5.3%), back pain (5.3%) |
| Navai et al. (phase I) [123] | Her2-CAR T cells | Advanced sarcoma (5 OST, 5 others) | 10 | 4–54 | ORR 20%; DCR 50% (2 CR, 3 SD) (CR in 1 OST and 1 RMS) | No toxicities attributed to the transferred cells * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarrán, V.; Villamayor, M.L.; Pozas, J.; Chamorro, J.; Rosero, D.I.; San Román, M.; Guerrero, P.; Pérez de Aguado, P.; Calvo, J.C.; García de Quevedo, C.; et al. Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers 2023, 15, 2287. https://doi.org/10.3390/cancers15082287
Albarrán V, Villamayor ML, Pozas J, Chamorro J, Rosero DI, San Román M, Guerrero P, Pérez de Aguado P, Calvo JC, García de Quevedo C, et al. Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers. 2023; 15(8):2287. https://doi.org/10.3390/cancers15082287
Chicago/Turabian StyleAlbarrán, Víctor, María Luisa Villamayor, Javier Pozas, Jesús Chamorro, Diana Isabel Rosero, María San Román, Patricia Guerrero, Patricia Pérez de Aguado, Juan Carlos Calvo, Coral García de Quevedo, and et al. 2023. "Current Landscape of Immunotherapy for Advanced Sarcoma" Cancers 15, no. 8: 2287. https://doi.org/10.3390/cancers15082287
APA StyleAlbarrán, V., Villamayor, M. L., Pozas, J., Chamorro, J., Rosero, D. I., San Román, M., Guerrero, P., Pérez de Aguado, P., Calvo, J. C., García de Quevedo, C., González, C., & Vaz, M. Á. (2023). Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers, 15(8), 2287. https://doi.org/10.3390/cancers15082287






