Simple Summary
Liquid biopsy has recently been introduced as a novel method in cancer diagnostics. It is less invasive for patients than conventional tissue biopsies, as the assay material is drawn from peripheral blood. The detected DNA fragments, as well as the cells and extracellular vesicles, can be used as biomarkers for cancer. Not all biomarkers are equally reliable in cancer diagnostics. In this review, we give an overview of the lung cancer biomarkers identified in liquid biopsy assays and discuss the differences, current applications, and future perspectives of liquid biopsies in lung cancer.
Abstract
As lung cancer has the highest cancer-specific mortality rates worldwide, there is an urgent need for new therapeutic and diagnostic approaches to detect early-stage tumors and to monitor their response to the therapy. In addition to the well-established tissue biopsy analysis, liquid-biopsy-based assays may evolve as an important diagnostic tool. The analysis of circulating tumor DNA (ctDNA) is the most established method, followed by other methods such as the analysis of circulating tumor cells (CTCs), microRNAs (miRNAs), and extracellular vesicles (EVs). Both PCR- and NGS-based assays are used for the mutational assessment of lung cancer, including the most frequent driver mutations. However, ctDNA analysis might also play a role in monitoring the efficacy of immunotherapy and its recent accomplishments in the landscape of state-of-the-art lung cancer therapy. Despite the promising aspects of liquid-biopsy-based assays, there are some limitations regarding their sensitivity (risk of false-negative results) and specificity (interpretation of false-positive results). Hence, further studies are needed to evaluate the usefulness of liquid biopsies for lung cancer. Liquid-biopsy-based assays might be integrated into the diagnostic guidelines for lung cancer as a tool to complement conventional tissue sampling.
1. Introduction
In recent years, lung cancer has become the second most frequent, newly diagnosed malignant disease worldwide, and is the main cause of cancer-specific mortality [1]. The timely detection of these tumors is difficult, as early symptoms, such as cough or dyspnea, are unspecific or absent [2], and because current screening methods, such as low dose computed tomography (LD-CT), albeit effective, are not available on a large scale. Managing the tumor patient and adapting the therapy can be challenging due to the fact that lung cancer is a very heterogeneous disease, even in a single patient. For instance, PD-L1 expression can differ between the primary tumor, lymph node, and metastatic site tissue [3]. Currently, the standard sampling of suspected tumor tissue is resource consuming, painful, and potentially dangerous for the patient. In addition, the obtained material only represents the tissue at the specific biopsy site, and thus can entirely overlook the aspect of tumor heterogeneity, which is essential for effective therapy. This is especially true in patients undergoing targeted treatment of driver mutations as the treatment-induced selection of drug resistant cells [4] with the subsequent progression of such being a major prognostic issue. Progressive lesions in the CT scan might call for a therapeutic switch or treatment beyond disease progression (TBP), that also might be beneficial in immunotherapeutic or targeted therapy settings [5,6]. However, such decisions would be hard to make without definitive data on the actual makeup of the tumor.
In this setting, the liquid-biopsy-based assay, or simply the liquid biopsy, a method for detecting, identifying, and quantifying DNA and cell fragments in body fluids, can be an important addition to the palette of methods for the diagnosis and management of lung cancer patients. The method itself has a long history—scientists first detected cell-free DNA (cfDNA) in blood in 1948 [7]. Others were able to identify specific mutations of cfDNA in 1994, but it wasn’t until 2016 that the FDA approved the first liquid biopsy test for detecting EGFR gene mutations in lung cancer. The foremost value of a liquid biopsy is that one can obtain a synopsis of the relevant tumor information, not only of the known tumors, but also of any yet undetected metastatic tumors in the body, by analyzing the sample for circulating tumor DNA (ctDNA).
In brief, samples of peripheral blood are analyzed for cfDNA (150–200 base pairs [bp]) and ctDNA (90–150 bp), as well as for microRNA (miRNA), which directly originate from apoptotic or necrotic cells or are released from tumor-associated macrophages (TAMs). Circulating tumor cells (CTCs) may also be found in the blood samples, but the information from the circulating DNA fragments is more revealing than that from single tumor cells, since it represents the heterogeneity of all shed cells, including NSCLC cells at a specific time point.
As mentioned previously, cfDNA consists of small DNA fragments that are either passively released from apoptotic or necrotic cells or released by phagocytes due to digestion [8]. ctDNA is a subentity of cfDNA that is shed by malignant cells [9]. Apoptosis promotes protein-bound cfDNA and ctDNA in peripheral blood, also known as oligo- or mono-nucleosomes. However, ctDNA can also be found in membrane-bearing EVs that are actively released by cells [10].
In the current guidelines for the management of patients with non-curatively treated lung cancer, the liquid biopsy has found its way into the routine diagnostics for EGFR-mutated tumors, once the disease has progressed under a non-T790 M-addressing EGFR tyrosine kinase inhibitor [11]. Due to the fact that the concentration of fragmented DNA in the blood plasma of cancer patients is orders of magnitude higher than that in non-cancer controls (5–1500 ng/mL vs. 1–5 ng/mL), it can be used as a diagnostic tool [12]. A liquid biopsy offers an altogether minimally invasive method for monitoring treatment responses (i.e., minimal residual disease [MRD] monitoring), and helps us to understand genomic tumor evolution and to identify resistance mutations earlier than is possible with radiological findings.
2. Methodology (Figure 1)
The materials and methods currently used in liquid and tissue biopsies for diagnostic assessments of lung cancer can be found in Figure 1.
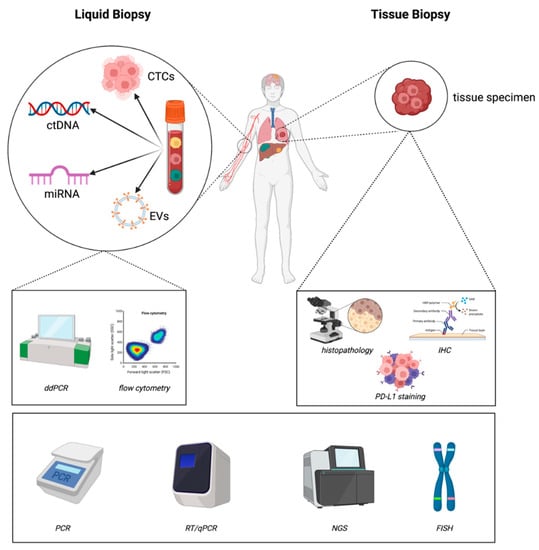
Figure 1.
Materials and methods currently used in liquid and tissue biopsies for diagnostic assessments of lung cancer (created with https://biorender.com/ accessed on 22 February 2023).
2.1. Cell Free DNA (cfDNA) and Circulating Tumor DNA (ctDNA)
2.1.1. Sample Collection and Preparation Timeline
Although the cfDNA content is higher in serum than in plasma [13], plasma is the preferred source for extraction, since there is less DNA contamination due to clotting, cell lysis, and the release of cfDNA from white blood cells [14]. A standard volume for a sample collection has not yet been defined, but 20 mL blood samples are usually employed for a cfDNA analysis in most isolation protocols [14].
The detection and identification of ctDNA is the preferred method of the liquid biopsy for lung cancer. As the levels of ctDNA in the plasma of NSCLC patients are very low (<0.5% of total cfDNA) [15], and it has only a short half-life < 3 h [16], time-sensitive isolation protocols should be implemented. The procedural steps in isolating ctDNA are: (1) drawing blood, (2) centrifugation, (3) DNA extraction, and (4) DNA analysis.
2.1.2. Collecting Tubes
Blood samples can be collected in either standard EDTA tubes or in specialized tubes containing a cfDNA preservative (see Table 1). EDTA tubes are widely available and considerably less inexpensive than the specialized tubes with the preservative. Disadvantageously, the samples from EDTA tubes have to be processed within 1–2 h to avoid their contamination with cfDNA from leukocyte lysis [17], but the samples from the tubes containing the cfDNA preservative can be stored at room temperature for up to 14 days [18]. However, for cfDNA preservative tubes, the contamination with leucocyte DNA can commence earlier so that processing should begin within three days [14]. The disadvantage with these tubes is that they require special processing and must often be sent to external laboratories, incurring delays and further costs.
Even in immediately processed samples, tumor-specific somatic mutations often represent < 1% of the total cfDNA for the region of interest, and any increase in the contamination with “diluting” cfDNA from in vitro lysis may result in false-negative results [19]. Consequently, a two-step centrifugation of the EDTA samples is recommended to reduce the contamination from leukocyte DNA, prior to their freezing and storage [19].

Table 1.
Comparison of blood collection tubes.
Table 1.
Comparison of blood collection tubes.
| EDTA Tubes | Preservative Tubes | |
|---|---|---|
| Advantages | Inexpensive and easily available Broad experience with use Better than heparin or citrate for anticoagulation [20] Transport to external laboratory not required | PAXgene® Blood DNA tubes (Qiagen) or Cell-free DNA BCT® tubes (Streck) cfDNA stable up to 14 days and CTC up to 7 days [18] Can be stored at room temperature [21] |
| Disadvantages | Must be processed within 1–2 h to prevent contamination with DNA from white blood cells | Expensive Limited availability and experience Strict adherence to product guidelines necessary Transportation to external laboratory required |
2.1.3. Methods for DNA Analysis
In total, two established methods are available for analyzing nucleic acids (e.g., DNA and RNA): targeted polymerase chain reaction (PCR)-based, and next generation sequencing (NGS)-based assays. Table 2 highlights the differences between the methods.

Table 2.
Comparison of targeted PCR-based assays and NGS for DNA analysis (adopted from [9,12,22]).
2.1.4. Real-Time (quantitative) PCR (qPCR) Assay
A qPCR is a widely used method for DNA analysis, which also allows for the semi-quantification of samples. The cost of the method has decreased markedly, and the results are easy to interpret. There are several commercially available FDA- and EMA-approved tests for detecting, activating, or resistance-causing EGFR mutations in NSCLC. Because only a single gene or an already known genetic alteration can be examined, the sensitivity is only 70–80% (detection limit 1–5%) [23,24], and using a qPCR alone carries the risk of false-negative results.
2.1.5. Digital Droplet PCR (ddPCR) Assay
A ddPCR is more sensitive than a qPCR (detection limit 0.1–1%) [23]. In ddPCR, a sample oil emulsion is fractionated into many thousands of droplets, upon which a PCR is subsequently performed in the individual compartments of a microtiter plate. This method allows for the detection of even rare events and quantification at the level of a single molecule. BEAMing (beads, emulsions, amplifications, and magnetics) has further improved the ddPCR technique using DNA templates that are bound to magnetic beads [25].
2.1.6. Next Generation Sequencing (NGS) Assay
NGS is a high-throughput method for sequencing DNA and RNA that allows for the detection of single nucleotide polymorphisms (SNPs), and small (insertions and deletions) as well as large (insertions, deletions, amplifications, inversions, and translocations) genetic alterations. The method is very sensitive (detection limit 0.001–2%) and can detect even unknown genetic alterations in small DNA fragments [22]. Whole genome sequencing (WGS) and whole exome sequencing (WES) have not been established, as the amount of ctDNA obtained with liquid biopsies might be too low. So far, only hybrid capture-based and amplicon-based (PCR capture) NGS approaches are regularly used in routine testing for cancer diagnostics.
Hybrid Capture-Based NGS
The hybrid capture-based NGS approach is not based on primary amplification, and thus allows for a more reliable quantification of copy numbers than amplicon-based NGS. Targeted DNA sequences are hybridized (“captured”) to biotinylated probes, which are bound to magnetic beads. The beads are captured by magnets, and the non-hybridized DNA is washed off. Since the ctDNA plasma concentration is very low, and sequencing does not rely on prior amplification, this method is at a greater risk of sequencing errors, including false-positive results. This explains its low specificity (approx. 60%) [12].
Amplicon-Based NGS (PCR Capture)
The amplicon-based NGS approach is based on the primary PCR amplification of specific genomic regions of interest, especially hotspot genes. Before sequencing, the amplified DNA sequences (“amplicons”) are multiplexed and marked with a distinct molecular barcode for identification. As this approach uses primary PCR amplification, it is valuable for liquid biopsies with their low amounts of ctDNA [26]. The risk of false-positive results is effectively reduced using molecular barcodes. While amplification biases the quantification of allele frequencies and copy number variations (CNVs), this method is useful for the detection of SNPs, indels, or known gene fusions [12].
2.1.7. Comparison of PCR-Based and NGS-Based Methods
PCR-based methods (qPCR, ddPCR) are well established, require short turnaround times of 2–3 days, and are fairly inexpensive. However, they allow for the detection of only a limited number of genetic alterations at a time (e.g., no multiplexing across different genes) and detect neither previously unknown alterations, nor gene fusions. NGS-based methods, on the other hand, have a longer turnaround time of about 1–2 weeks [27], but are able to detect known and unknown genetic variants, including CNVs, SNPs, and gene fusions, which might promote the use of diagnostic algorithms for cancer diagnostics and treatment monitoring. Lately, the NGS of ALK-positive NSCLC has offered a more detailed characterization of fusion partners than standard pathological techniques like IHC or FISH [28]. For quantification analysis, NGS and ddPCR are more suitable than the semi-quantitative qPCR. Despite the high sensitivity of NGS-based methods, a single false-positive read (with an error rate of approximately 10−3) can impact the result [29]. Hence, error-proofing techniques and algorithms need to be implemented.
2.2. Circulating Tumor Cells (CTCs)
Circulating tumor cells are rare in peripheral blood (1 CTC per 106−7 leukocytes), where they occur as single tumor cells in cell clusters, as so-called circulating tumor microemboli [30], or attached to stromal cells originating from the primary tumor [31]. Their plasma half-life (1–2.4 h) is short, so that they must be promptly separated from other blood cells [32]. CTCs can be isolated by using antibodies to identify the expression of intracellular (DNA) or transmembrane molecules (cytokeratins, epithelial adhesion molecules [EGDR and HER3], and CD45) [33]. They can also be identified according to their density or size [12]. Circulating epithelial cells are present in only 46% of blood samples from stage IV NSCLC patients, and circulating cells with an epithelial phenotype can also be found in 7% of healthy controls [34]. Thus, the presence of CTCs in the bloodstream alone is an inadequate marker for the tumor burden. However, some studies have shown that the baseline CTC count is a prognostic marker for tumors other than lung cancer [35,36,37].
2.2.1. Antigen-Based CTC Isolation
In the CellSearch® CTC Test (Menarini Silicon Biosystems Inc., Castel Maggiore, Italy) the CTCs are separated magnetically from other blood cells using anti-EpCAM antibodies conjugated with magnetic nanoparticles. The separated cells are stained for nuclear DNA with 4′,6-diamidino-2-phenylindol (DAPI), and with fluorescently labeled antibodies for cytokeratins (CK) and CD45. They are then analyzed with automated fluorescence microscopy. CTCs are defined as DAPI+, CK+, and CD45−, while leukocytes are DAPI+ and CD45+ [33]. While this widely used assay is considered the gold standard for CTC detection, it has, so far, only been approved by the FDA for routine use in metastatic breast, prostate, and colorectal cancer, but not yet in lung cancer.
A different commercially available test (AdnaTest® (Qiagen GmbH, Hilden, Germany)) uses a combination of different antibodies (e.g., anti-EpCAM, anti-MUC1, anti-HER2, and anti-EGFR) conjugated with magnetic beads for cell separation, which increases the sensitivity of the CTC detection when only the antibodies against EpCAM are used [38]. However, this test has not yet been approved by the FDA for routine use in cancer.
2.2.2. CTC Isolation Based on Biological and Physical Characteristics
Antigen-based isolation methods have a poor sensitivity and specificity [31] due to the cells’ tendency for epithelial-to-mesenchymal transition (EMT) [39], with a subsequent loss of epithelial surface markers [40]. Focusing on the physical and biological characteristics of the CTCs, instead of the epithelial cell surface antigens, would bypass this problem.
CTCs can also be isolated by using the physical properties of the cells, i.e., size and density. One method employs a porous membrane (pore diameter 8 μm) (e.g., ISET® system,(Rarecells Diagnostics SAS, Paris, France)) to isolate the CTCs. Here, leukocytes are not totally eliminated. Thus, a subsequent further cell characterization with a cytomorphological or immunocytochemical analysis is required [41,42]. Another method is to centrifuge the plasma in a density gradient, e.g., a Ficoll-Paque® solution, to separate the mononuclear cells (including the CTCs) from other blood cells (e.g., OncoQuick® system, (Greiner BioOne International GmbH, Kremsmuenster, Austria)). The method is inexpensive but has a high rate of contamination with leukocytes [43,44].
Microfluidic technologies have recently evolved as a very appealing approach to CTC isolation [45]. Exemplarily, one such “CTC-chip” uses anti-EpCAM-coated microposts under precisely controlled laminar flow conditions to capture the CTCs [46], while the vortex technology uses microscale vortices to isolate the CTCs based on their physical characteristics, such as size or compressibility [12,47,48]. These approaches are antigen-independent, resulting in a high sensitivity (a low risk of false-negative results due to EMT) and high purity of the CTC isolates [12].
2.2.3. Interpretation of CTCs
Apart from using the common morphological and biological features of malignant cells (e.g., nucleus size and chromatin structure) to discriminate the CTCs from other blood cells, immunocytochemistry can be used for CTC identification. Moreover, genomic analyses, such as DNA analysis, as well as RNA analysis (qPCR or RNA sequencing), can be performed on CTCs, and may reflect the tumor heterogeneity [33]. So far, proteomics using mass spectrometry and immunoblotting have not been implemented in CTC analysis.
2.3. miRNA
MicroRNAs (miRNAs) are noncoding RNAs involved in the posttranscriptional regulation of gene expression that can act as tumor suppressors or oncogenes. Cell-free miRNA can be detected in various body fluids. It is passively released during cell lysis (e.g., necrosis and apoptosis) and actively secreted by cells for intercellular communication [49]. miRNAs are usually packed into EVs (see below), or coupled with Argonaute2 (Ago2) protein [50] or high-density lipoprotein (HDL) [51], and dispersed into the extracellular environment. A genomic analysis can be performed using the same methods as those for cfDNA (see previous section).
2.4. Extracellular Vesicles (EVs)
EVs are small membrane particles that are shed by all living cells and mediate intercellular communication by carrying proteins, lipids, and nucleic acids (e.g., DNA, RNA) from the secreting to the surrounding cells. A total of two distinct EV populations have so far been described: small EVs (sEVs, also called exosomes) with a diameter between 50–150 nm, and larger microvesicles (intermediate size vesicles, IEVs) with a diameter between 100–1000 nm [52]. sEVs are formed inside the cell through the inward budding of endosomal membranes, while IEVs bud directly from the plasma membrane [53]. Numerous studies have demonstrated that tumor-derived EVs are crucial in establishing a favorable tumor microenvironment, and thus pave the way for metastatic spread [54]. The large amount of EVs shed by tumor cells into the blood and other body fluids has opened up a new perspective on using EVs as cancer biomarkers in liquid biopsies, particularly in lung cancer. Due to their small size, the isolation and analysis of sEVs is time-consuming, and thus not suitable for clinical routine. In contrast, the extraction and analysis of IEVs with flow cytometry is less demanding and time-consuming [52]. Although EVs can be isolated from body fluids [55,56], an internationally standardized isolation method, which would be necessary for their clinical use, is lacking. Inherent to every method is the risk of co-isolating the other subtypes of EVs or lipoparticles, and the separation of different EV subtypes remains difficult. Further details are highlighted in the review “Extracellular vesicles in liquid biopsies” in this Special Issue.
3. Clinical Applications
Liquid biopsies have a great potential for initial tumor diagnosis, as well as for monitoring the response to therapy. Here, we elucidate the current applications and future perspectives of blood-plasma-based assays in lung cancer. An overview of studies, partly using liquid biopsy in mutational diagnostics, cited in this review can be found in the Supplementary Materials (Table S1).
3.1. ctDNA
The most robust data on all methods exist for the use and assessment of ctDNA, which has been integrated into the diagnostic work-up of NSCLC during the past decade. Current studies have shown the amount of ctDNA to correlate with the tumor stage (TNM), tumor burden, and metabolism [57,58,59]. Moreover, ctDNA assessment is suitable for monitoring therapeutic responses, as well as for detecting minimal residual disease (MRD). This would allow for the timely adjustments of individualized treatment, if needed.
However, some key preanalytical considerations should be made ahead of ctDNA testing to improve the test performance. Most important is the identification of the correct patient cohort to be tested. As there is a variety of different tests available, each of them should be used carefully in the right clinical setting. The test requirements for a screening test of healthy individuals highly differ from the requirements for those of metastatic cancer to monitor the treatment response. In locally advanced and metastatic lung cancer, for example, state-of-the-art ctDNA tests focus on the evaluation of specific mutation sites, such as EGFR, ALK, and BRAF. However, upfront WGS might enable the detection of rare alterations and allow for an individualized treatment approach. A recent study evaluated the cost-effectiveness of a standard-of-care diagnostic approach vs. an upfront WGS for lung cancer patients [60]. If reimbursed and established, a liquid biopsy might allow for an easy and all-encompassing approach to evaluate for actionable mutations via ctDNA WGS. Another relevant state-of-the-art diagnostic focus in metastatic lung cancer is ctDNA testing for resistance genotyping under (targeted) therapy. Depending on the test method (e.g., targeted PCR-based assays vs. NGS), not only on-target resistance mutations, but also off-target resistance mutations can be detected (Table 2), thus implicating the importance of preanalytical considerations ahead of testing. Apart from that, a number of factors (e.g., disease status, tumor stage, histology, molecular pathology, and type of therapy, etc.) have an impact on the specific test performance, and should be considered carefully before choosing the right ctDNA test method.
3.1.1. Mutational Assessment
Due to the rising number of targetable oncogenic drivers, the current NSCLC guidelines include molecular testing for already known driver mutations in regular diagnostic work-ups [11]. The diagnostic assessment should be performed at initial diagnosis and in cases of relapse or progression. Tissue analysis was formerly the accepted standard, but biopsies are often scarce, difficult to obtain, and may not represent the heterogeneity of the tumor. The use of liquid biopsies in the assessment of NSCLC is increasing, as they are easy to obtain and can possibly overcome interspatial/intratumoral variations. However, liquid biopsies should be obtained prior to the beginning of cytotoxic or targeted therapies, since effective therapy reduces DNA shedding and thus lowers the amount of ctDNA below a detectable threshold within 1–2 weeks after the therapy induction [61]. A recent analysis of the German CRISP register revealed that only 75% of all patients were tested for EGFR mutations. Most unsettling was that 12.4% of patients were not tested for any marker by any method [62,63], mostly because they were unable to obtain tissue biopsies and were unaware of the liquid biopsy. Here, by analyzing both tissue and liquid biopsy DNA samples, targetable oncogenic driver mutations were detected in 30–60% of patients [64,65]. Multiple meta-analyses have revealed the feasibility of assessing the mutational status via a liquid biopsy [66,67]. Moreover, the subsequent prospective trial validation led to the recommendation of liquid biopsies within current guidelines in Europe and the US, especially in cases with insufficient or unattainable tissue biopsies [68,69]. Recently, the International Association for the Study of Lung Cancer (IASLC) underlined the outstanding potential of liquid biopsies for the evaluation of oncogenic drivers and proposed that ctDNA-based mutational analysis be used preferentially. Especially in patients with progressive disease, they facilitate the choice of a specific therapy (“plasma first”) [23]. Moreover, the IASLC recommended their concurrent use in cases of questionable adequacy of the accessible specimens. The currently ongoing LIQUIK Trial (NCT04703153) aims to prove the non-inferiority of liquid biopsies compared to tissue biopsies in detecting the guideline-recommended molecular biomarkers, and might further foster the role of liquid biopsies in NSCLC diagnostics.
3.1.2. EGFR Mutations
ctDNA analyses initially entered the diagnostic field with the detection of EGFR mutations and the subsequent management of NSCLC treatment with tyrosine kinase inhibitors (TKI). In total, two PCR-based tests have been approved by the FDA and EMA, respectively, for detecting EGFR mutations. The cobas EGFR mutation test ® detects 42 defined EGFR mutations with a high sensitivity [70], and allows a treatment selection from the companion drugs gefitinib and erlotinib or osimertinib. The therascreen® EGFR kit [12] permits the initiation of treatment with afatinib, gefitinib, and dacomitinib in cases of finding an EGFR mutation. Comparative molecular analyses of liquid and tissue biopsies showed concordance in some trials, but often resulted in contradictory findings [70]. However, PCR-based methods can only detect a limited number of known EGFR mutations, which must be predefined before testing (c.f., comparison of PCR-based and NGS-based methods). Thus, both tests are unable to discover new EGFR mutations. Furthermore, PCR-based methods showed a high specificity (97% in a meta-analysis of nine studies) in EGFR detection, albeit with a low sensitivity of 51% [71]. In addition, high rates of false-negative results when detecting T790M mutations, a harbored resistance to first-generation TKIs, and only a 51% agreement to the matched tissue samples were seen with the cobas® test in the AURA3 trial [72]. Hence, guidelines (e.g., ESMO practical guideline [73]) require a subsequent tissue biopsy in the case of a negative result from PCR-based liquid biopsies. Nevertheless, the less expensive PCR-based EGFR testing methods remain highly relevant as an initial step, especially for patients at risk of EGFR mutations. Still, unaddressed mutations can result in false-negative EGFR mutation test results, and a negative PCR-based test does not prove the absence of an EGFR mutation.
NGS-based approaches might overcome the latter disadvantage, as they allow for the simultaneous detection of all actionable targets, facilitate the detection of actionable variants, and thus permit rapid genotype-matched therapeutic decision making. Furthermore, NGS-based methods achieve a higher sensitivity and specificity [71,74], are therefore currently preferred over PCR-based methods, and are the first choice for liquid biopsy testing in the current ESMO guidelines for metastatic NSCLC. Thus, they might supersede PCR-based methods in the upcoming years. A total of two NGS-based methods were approved by the FDA in 2020: Guardant360® CDx (Guardant Health, Palo Alto, CA, USA) and Foundation One® Liquid Cdx (Roche, Basel, Switzerland). The Guardant360® CDx-test, which allows for the use of osimertinib as a companion drug in the case of an EGFR mutation, showed a significantly increased number of molecular assessments compared to tissue-based standard diagnostics in a shorter time-period with a high (>98%) concordance between plasma and tissue [27]. The Foundation One® Liquid Cdx test can assess over 300 gene alterations, allows the companion use of first- and second-generation TKI after the detection of sensitizing EGFR mutations, and is also approved for other cancers (e.g., breast and colon cancer).
A liquid biopsy also allows for the early detection of disease progression, which is eventually seen in most patients undergoing TKI therapy as an expression of an acquired (secondary) resistance to EGFR TKI. There is an urgent need to evaluate the on- and off-target mechanisms of resistance (MOR), in order to better understand the treatment-induced tumor evolution and to adjust further therapies. The most common MOR is the EGFR T790M mutation, which can be effectively targeted with the third generation TKI osimertinib. The assessment of T790M with liquid biopsies is very well examined, and its detection in plasma could obviate the need for tissue biopsies, as it predicts an excellent response to osimertinib, similar to that observed in tumor genotyping [75,76]. Moreover, a subsequent ctDNA analysis in patients undergoing TKI treatment detected the T790M mutation in a median time of two months prior to the clinical progression, in 45% of all the patients monitored [77,78], allowing for the treatment to be adjusted before the clinical tumor progressed and the detriment of patient performance statuses were evident. The results from the FLAURA-trial pointed out that osimertinib significantly improved the survival of treatment-naive patients compared to the comparator-TKI, and the application of first-line osimertinib led to a lower frequency of T790M mutations [79]. Nonetheless, a resistance to osimertinib is emerging, and an evaluation of the distinct on- and off-target MOR to EGFR TKI via NGS-based liquid biopsies is urgently needed to effectively treat osimertinib-resistant NSCLC. This is the aim of the APPLE trial [80]. Beyond this, the MELROSE study (NCT03865511) addresses the question of whether there is an association between ctDNA levels and the occurrence of resistance to osimertinib or therapeutic escape, in order to choose the ideal treatment for the individual patient [81]. For instance, the promising results of the use of the c-MET inhibitor crizotinib in NSCLC cell lines harboring MET amplification, a known MOR to osimertinib, has shown a potential to overcome the acquired resistance [82,83].
In patients with T790M-negative plasma samples, a tumor biopsy is still warranted due to the possibility of false-negative results and a limited sensitivity of the liquid biopsy. In addition, other acquired EGFR mutations besides T790M should be examined, as some of them can be targeted by switching to first- and second-generation TKIs [84]. Interestingly, even when no other mutations are found and no tumor material can be assessed, a small cohort of patients might nonetheless profit from treatment with osimertinib. In the AURA1-trial, patients with tumor progression after TKI treatment, in whom neither the original EGFR-sensitizing mutation nor a T790M mutation could be detected with ctDNA, showed an improved outcome under osimertinib compared to those patients with proof of their sensitizing mutation (progression-free survival [PFS] of 15.2 months vs. 4.4 months). The negative test results for the T790M mutation were thus misleading in these cases. The lack of any detectable mutation in the plasma test might be explained by low DNA shedding, lower aggressiveness, and/or a lower disease burden in those patients.
3.1.3. ALK Rearrangement
ALK rearrangement preferentially occurs in young patients, as well as in never-smokers. Fortunately, the treatment inhibition of downstream signaling via TKI is effective, irrespective of the, so far, 19 known fusion partners [85,86]. Where ALK fusions have not been effectively detected by PCR-based methods, an NGS-based liquid biopsy attains a high specificity (100%) and sensitivity (79.2%) [87]. In 2011, the multi-kinase-inhibiting drug crizotinib was the first FDA-approved substance for ALK-rearranged NSCLC, achieving response rates of 60% and disease control rates of 90% [88,89]. However, most patients ultimately progress while on crizotinib treatment [14]. Consequently, the current ESMO guideline [11] endorses the first-line treatment of ALK-driven NSCLC with a second-generation TKI, such as alectinib or its comparators brigatinib, ceritinib, or ensartinib [90,91,92]. Likewise, each of these substances is able to induce a renewed therapeutic response in up to 55% of patients with progression [93]. Other than these, lorlatinib is a potent third-generation ALK-TKI that has shown efficacy in patients without a response to one or more second-generation TKIs, and was recently EMA-approved, even as a first-line treatment [94]. However, the various TKIs have individual binding affinities and therefore a sensitivity to the ALK resistance mechanisms. Thus, even if the exact characterization of the mutations under treatment with ALK-TKI is not required for a therapeutic switch, this characterization via NGS panels, covering a variety of mutations, might help with finding the optimum choice of TKI for the patient. Most recently, the FDA approved alectinib as a companion drug for ALK rearrangement detected with the Foundation One® Liquid Cdx test, since the first results of the ongoing BFAST trial not only underlined the suitability of NGS-based assays in detecting ALK-fusions, but also demonstrated that a subsequent treatment with alectinib yielded high overall response rates (ORR) [95]. NGS-based longitudinal monitoring also has prognostic value, as the detection of ctDNA (based on matched targeted NGS and shallow WGS) at the time of the initial diagnosis indicates a shorter time until progression, and the ctDNA levels might be highly elevated in some patients throughout TKI therapy until death [96,97].
3.1.4. ROS1 Gene Fusions
Oncogenic ROS1 gene fusions that induce the relevant activation of the ROS-1 receptor tyrosine kinase can be detected with NGS-based liquid biopsies and subsequently targeted by crizotinib (with ORR of 73–93%) and entrectinib (ORR 41%, some cases of durable responses) in a first-line setting [98,99]. According to preliminary results, the consecutive ctDNA monitoring of a ROS1 rearrangement may help to predict the response to therapy [100], and allows for the early detection of resistance-mediating mutations in ROS1 genes [101] that would ultimately require an adjustment of therapy.
3.1.5. MET Rearrangements
The MET gene encodes for a receptor tyrosine kinase that, i.a., activates the signaling pathways involved in crucial cellular processes such as cell proliferation, survival, and growth [102]. Most prominently in NSCLC, MET exon 14 (METex14) skipping mutations are characterized by the fusion of exons 13 and 15, which ultimately impairs receptor degradation, thus overactivating the MET-mediated signaling [102]. METex14 alterations are usually observed in the absence of other oncogenic driver mutations [102]. NGS-based Guardant360® CDx and Foundation One® Liquid Cdx tests are FDA-approved for the assessment of METex14 skipping mutations in NSCLC. Encouraging results from the phase II GEOMETRY mono-1 trial led to the FDA approval of the MET-inhibiting oral agent capmatinib; patients with qPCR tissue-confirmed METex14 skipping NSCLC, that was retrospectively assessed by Foundation One® Liquid Cdx, achieved an ORR of 68% in first-line and 41% in second- or third-line treatment with capmatinib. More so, the study resulted in a 92% intracranial disease control and a 31% complete remission in patients with brain metastases [103]. Capmatinib was approved by the EMA in 2022. Most recently, the second-line use of tepotinib, another oral MET kinase inhibitor, was approved by the FDA and EMA, based on the results of the phase II VISION trial [104]. Here, METex14 skipping was either determined by Guardant360® CDx in plasma or with RNA from tissue specimens. Treatment with tepotinib induced an ORR rate of 43% over all therapeutic lines, with a median duration of response (mDOR) of approximately 11 months, and likewise demonstrated intracranial activity [105]. Furthermore, a high concordance of clinical response and decreasing ctDNA levels during treatment were observed [104,106]. Preliminary results of a second-line phase II trial (NCT02897479) with savolitinib indicated a mORR of 46%, while the mDOR was not reached in the interim analysis at 6.9 months [107].
3.1.6. RET Rearrangements
RET rearrangements represent oncogenic drivers in young NSCLC patients with a non-smoking history. These patients are especially prone to brain metastases [108]. Several studies have shown liquid biopsies to be a reliable tool in identifying RET rearrangements [64,109,110]. The results of the LIBRETTO trial highlighted a high ORR after a RET-inhibiting selpercatinib administration in second- and first-line treatment (64% and 84%, respectively). While in both treatment lines, cases of durable response and intracranial activity [111] were observed, the approval was restricted to second-line treatment after platinum-based chemotherapy. With praseltinib being a highly effective treatment (an ORR of 70%, an mDOR not reached after 6 months, and the presence of CR in some cases), the ARROW trial [112] led to the approval of praseltinib in the first-line setting of RET-rearranged NSCLC. In line with other TKIs, the use of RET inhibitors is periodically limited, e.g., with acquired RET V480M gatekeeper resistance mutations [113] that are identifiable by a liquid biopsy [114]. With even greater impact, an analysis of over 32,000 samples showed that the finding of non-KIF5B-RET fusions contributed to anti-EGFR therapy resistance in cases with co-mutations [115].
3.1.7. BRAF Mutations
BRAF mutations, and predominantly the V600E mutation, which accounts for over 50% of all cases and can reliably be detected in liquid biopsies [116], are associated with a dismal response to cytotoxic treatment [117]. In melanoma, in which BRAF mutations are more frequent, the BRAF inhibitors vemurafenib or dabrafenib have shown high response rates and have improved the survival of patients dramatically [118,119]. In parallel, the application of vemurafenib or dabrafenib alone, or with additional downstream MEK inhibition, has shown promising antitumor activity (an ORR of 33% for dabrafenib, 42% for vemurafenib, and 67% for dabrafenib/trametinib) in phase II trials for NSCLC [120]. Consequently, the EMA and FDA approved the combination therapy of dabrafenib and trametinib for BRAF-mutated, advanced NSCLC.
3.1.8. ERBB-2/Her-2 Alterations:
ERBB-2/Her-2 alterations (overexpression, amplification, or point mutations) are common in breast cancer patients, where the monitoring of ctDNA under treatment has shown a high predictive value regarding the response to therapy [121]. ERBB2/Her-2-overexpressing NSCLC implies an inferior outcome [122], as ERBB2/Her-2 amplification is known to be one of the acquired EGFR TKI resistance mechanisms [122] that can be identified by NGS-based liquid biopsies [123]. Recently, the Her-2/neu-targeting antibody-drug conjugate trastuzumab deruxtecan has shown an impressive ORR of 54% in heavily pretreated NSCLC patients [124].
3.1.9. KRAS Mutations
Activating mutations in the KRAS gene are the most prevalent mutations in NSCLC, with the KRAS p.G12C variant being most frequent. The phase II CodeBreaK100 trial recently showed promising results for the use of sotorasib, a small molecule that irreversibly inhibits KRAS G12C, in pretreated KRAS p.G12C-mutated NSCLC [125]. Here, sotorasib exhibited an ORR of 37.1%, a disease control of 80.6%, and an mDOR of 11.1 months. However, the median PFS (6.8 months) and median overall survival (OS, 12.5 months) were still low in a cohort of pretreated, advanced NSCLC patients. Most recently, the first results of CodeBreaK200 phase III trial demonstrated that treatment with sotorasib significantly improved the disease control rate, as well as the PFS, and had a more favorable safety profile in a head-to-head comparison with docetaxel (NCT04303780), while OS data have not been provided yet [126]. The ongoing CodeBreaK101 trial (NCT04185883) is currently investigating the role of sotorasib in various combination therapies, including first-line settings.
The feasibility of the NGS-based KRAS mutation assessment of liquid biopsies from NSCLC patients has already been proven, especially in cases with insufficient or unavailable tissue samples [127]. Other than NGS, the Idylla qPCR platform, that has a 100% concordance with NGS and a high sensitivity, may expedite cost-effective tools to assess the KRAS p.G12C mutation [128]. So far, the FDA has only approved the NGS-based Guardant360® liquid biopsy CDx for tumor mutation profiling to identify KRAS p.G12C mutated patients with locally advanced or metastatic NSCLC, who may benefit from sotorasib.
3.1.10. NTRK Fusions
Rare NTRK fusions can effectively be targeted by two FDA- and EMA-approved drugs (larotrectinib and entrectinib). Although the FDA approved the NGS-based FoundationOne® CDx tissue test as a companion diagnostic to identify the fusions of NTRK genes in solid tumors, including NSCLC, so far, no NGS-based test for liquid biopsies has been approved.
3.1.11. Immunotherapy and Tumor Mutational Burden (TMB)
Immune checkpoint inhibitors (ICI), e.g., antibodies against PD-1 and PD-L1, have dramatically improved the outcome of advanced stage NSCLC patients. Independent of PD-L1 expression in tumor tissue, a combination of immunotherapy with platinum-based chemotherapy positively impacts the OS, more so than standard platinum-based chemotherapy alone, both in non-squamous [129] and squamous cell lung cancer [130]. However, NSCLC patients with a high tumor PD-L1 expression (i.e., their tumor proportion score [TPS]) and/or a high PD-L1 expression in tumor-infiltrating immune cells (i.e., their combined positive score [CPS]) benefit more from a single-agent immunotherapy compared to the previous standard-of-care platinum-based chemotherapy [131,132,133]. Unlike targeted therapies, ICIs reactivate cytotoxic T cells to overcome the tumor immune escape [134]. Although the tissue PD-L1 expression positively correlates with the treatment response, not all patients respond to immunotherapy, and to date, there is no alternative reliable cell-surface biomarker to predict the efficacy of ICI.
The tumor mutational burden, which is usually assessed by whole exome sequencing (WES) or targeted NGS (the sequencing of cancer gene panels (CGP)) of tissue specimens (tTMB), refers to the total number of somatic coding mutations, base substitutions, short insertions, and deletions per tumor genome. A high TMB is positively correlated with an environment of a high tumor neoantigen load, which contributes to a lower PFS and OS [135]. Multiple retrospective trials have shown that a high tTMB (determined with a cut-off >20 mut/mb in the CHECKMATE-026 trial) predicts a greater benefit of the PD-1 inhibitors nivolumab or pembrolizumab, in both treatment-naive and pretreated NSCLC patients [136,137,138]. Furthermore, tTMB is non-overlapping with PD-L1 expression, as it constitutes another aspect of the immune phenotype, and thus enables the selection of ICI-treatable patients. Despite conflicting results regarding the relationship of the tTMB and ICI response in clinical trials [139,140], the determination of tTMB and the subsequent selection of patients for pembrolizumab treatment in advanced non-resectable or metastasized cancers, including NSCLC, was approved by the FDA in 2020 [141]. However, tTMB diagnosis is still limited to a minor proportion of NSCLC patients (34–59%), due to the mentioned difficulties in obtaining tissue specimens [142]. With established ctDNA panels or optimized gene panel algorithms for NGS-based blood TMB (bTMB) determination, a liquid biopsy may offer a valuable substitute for tissue. Here, bTMB has been demonstrated to have a positive concordance with tTMB [143,144], and was validated in several trials, confirming that a high bTMB predicts beneficial PFS and ORR when treated with immunotherapy [32,145]. bTMB can be measured precisely using targeted gene panels, but the accuracy is compromised when the bait size is less than 0.5 MB [139]. The FDA-approved tests, Guardant OMNI ® (500 genes, 2.1 MB) and Foundation Medicine Liquid Cdx® bTMB (394 genes, 1.14 MB), have been demonstrated to test for sufficiently large baits and to correlate with tissue [146]. In the MYSTIC phase III trial, ‘high’ bTMB was defined as a threshold of at least 20 mut/mb, assessed by the Guardant OMNI® test [147]. High bTMB similarly predicted a clinical benefit of ICI therapy, with durvalumab and CTLA-4-inhibiting tremelimumab, over chemotherapy (a median OS of 21.9 months vs. 10 months, respectively). In addition, a cut-off concentration of ≥ 16 mut/mb measured with the Foundation Medicine® bTMB test [145] identified ‘high’ bTMB in patients particularly prone to ICI [131,148]. This cut-off was defined in the POPLAR study and validated by the OAK trial [143,149]. It was likewise confirmed in the first randomized, prospective trial using bTMB (B-F1RST) as a predictor for the response to treatment with the PD-L1 inhibitor atezolizumab, showing a beneficial PFS and OS beyond the cut-off of ≥16 mut/mb [150].
Nonetheless, there is currently a high variability among techniques and their interpretations, and a lack of a unified threshold for “high TMB”. The preliminary findings of the TMB Harmonization Project [151], an initiative for standardization, recently demonstrated the feasibility of reference control lines to further align the estimation of TMB and identify targeted NGS assays, which must now be validated in prospective trials. Synoptically, the incorporation of both TMB and PD-L1 expression into multivariate predictive models [152] might be helpful in forecasting ICI response.
3.1.12. Prognostic Value of DNA Methylation in cfDNA and CTCs
One of the major epigenetic modifications is DNA methylation. In tumorigenesis especially, the aberrant methylation of gene promoters might enhance or silence the transcription of RNA; hence, this directs a certain biological behavior of the malignant cell. When focusing on methylation analysis, one has to consider the different approaches of the obtained tissue and sample: while the liquid biopsy and analysis of cfDNA resulted in relatively high levels of APCme and SLFN11me sites in lung cancer patients, e.g., its corresponding CTCs exhibited a significantly lower frequency of promoter methylation [153]. In comparison to solid tissue, cfDNA and CTCs had fewer methylation signatures than tumor tissue itself, but cfDNA-detected alterations might derive from tumor adjacent tissue (non-ctDNA) [153]. Thus, before implementing the liquid biopsy analysis with regards to the methylation analysis, a predefined standardization and normalization of the tissue observed (e.g., cfDNA and CTCs in a liquid biopsy versus tumor tissue and adjacent tissue from tissue samples) has to be implemented and validly evaluated.
However, some early studies of cfDNA that evaluated DNA methylation analyses in cancer diagnostics allow a little glance at future perspectives. Here, Constâncio et al. detected a sensitivity of 64% and a specificity of 70% for a ‘PanCancer’ cfDNA analysis compiled of FOXA1me, RARβ2me, and RASSF1Ame in male early lung and prostate cancer patients. While positive results were also detected in 30% of the cfDNA healthy controls [154], its value as a potentially harmless method for cancer screening has to be verified by larger prospective trials.
With a focus on lung cancer patient diagnostics and staging, a liquid biopsy gathered APCme and RASSF1Ame cfDNA methylation panel predicted the disease-specific mortality of lung cancer patients: compared to unmethylated sites, APCme plus RASSF1Ame resulted in a Hazards Ratio of 3.9 (1.9–7.9) in lung cancer patients [154]. Such data could help to predict the cancer course and be useful for treatment decisions in the future.
Of current clinical relevance, TKI resistance mechanisms can be driven by DNA methylation. Here, a liquid biopsy cfDNA methylation analysis of prespecified sites (e.g., RASSF1A, RASSF10, APC, WIF-1, BRMS1, SLFN11, RARβ, SHISA3, and FOXA1) indicates the development of EGFR-TKI resistance in osimertinib-treated patients [155]. Likewise, a specific DNA methylation (i.e., 5-mC score) score was used to monitor the treatment efficacy and predict the disease progression in TKI-treated ALK-driven NSCLC patients with a liquid biopsy cfDNA analysis [156].
In conclusion, epigenetic changes such as DNA methylation might frequently be found in the liquid biopsies of cfDNA and CTCs. Still, adjacent tissue, tumor, and cfDNA analysis have to be normalized, and their deductive consequences must be well defined.
3.2. CTCs
CTCs provide parallel information on the mutational profile, CNVs, genomic rearrangements, and gene expression of a tumor. As they are released from the primary tumor site, as well as from metastases, the analysis of the CTCs might overcome the intratumor heterogeneity. Nevertheless, the CTC count does not correlate with the tumor burden, as only a fraction of NSCLCs shed CTCs [33], and the current data regarding the counting of CTCs and their correlation with the tumor stage are conflicting [157,158,159]. However, there is strong evidence that a high baseline CTC count predicts a poorer outcome in NSCLC patients, and is thus an independent prognostic factor in lung cancer [160]. Interestingly, monitoring the CTC count during therapy allows for an assessment of the disease development in real-time [33]. The maintenance of high CTC counts under therapy and during aftercare indicates a poorer prognosis, and identifies patients at risk of progression [161].
3.2.1. Mutational Assessment
Even if mutational assessment is more established in the field of ctDNA, a molecular analysis of CTCs can provide additional information about the underlying driver mutations. Allele-specific PCR amplification in CTCs has been shown to confirm tissue EGFR-mutation in NSCLC in 11 of 12 patients (92%) [162]. Interestingly, in cases where no oncogenic drivers were found in the tissue-based examination, the assessment of CTCs was also able to identify EGFR mutations [163]. In addition to EGFR mutations, ALK rearrangements can be detected with a high sensitivity and high concordance with tissue [164] when analyzing CNVs, even though this is with a less broad range of mutations than with NGS-based methods in ctDNA [162,165,166]. The expression of MET in CTCs has been shown to correlate with their expression in the primary tumor tissues of NSCLC patients [167], expanding the number of molecular drivers that can be observed in CTCs. If a mutation is identified, CNVs can serve as a simple tool to monitor the genomic evolution of the tumor under TKI therapy, and predict the therapy resistance and clinical outcome [168].
3.2.2. Immunotherapy and PD-L1 Expression
PD-L1 is co-expressed with EpCAM on >80% of CTCs from patients with metastatic lung cancer [169], making CTCs a suitable tool for identifying patients that qualify for ICI treatment. Interestingly, PD-L1 expression on CTCs in NSCLC patients is more often positive than on that in tissue, thus highlighting the potential use of CTC PD-L1 expression as a biomarker under immunotherapy [170,171]. Generally, the presence of PD-L1-positive (>1%) CTCs prior to treatment is associated with a poor outcome [170,172,173] in most trials. The persistence of PD-L1-positive CTCs after three and six months of ICI treatment was proposed as a predictor of mortality [174]. While conclusive proof is lacking, it is believed that the expression of PD-L1 on CTCs mediates immune escape [175], as patients with PD-L1-positive CTCs are more often non-responders to nivolumab [170]. More importantly, Nicolazzo et al. found PD-L1-positive CTCs in all patients that developed a resistance under ICI therapy [174], supporting the latter hypothesis. Nonetheless, further studies are needed to prove that correlation.
Comparing PD-L1 expression on CTCs and in tumor tissue yielded widely divergent results: while Ilie et al. described a 93% concordance in their tissue-matched study [176], others saw no correlation [170], which might be explained by the different methods and/or antibodies used to analyze the PD-L1 expression in those trials [170,177,178]. This illustrates the need for standardizing the methods, and for future trials to validate the current knowledge and enlighten future perspectives on CTCs in NSCLC diagnostics.
3.3. miRNA
miRNA profiles can reliably distinguish healthy controls from lung cancer patients [179,180,181]. Some profiles are specific for particular NSCLC entities [182,183,184], e.g., the detection of the highly specific hsa-miR-205 in plasma identifies a squamous cell carcinoma of the lung, with a high sensitivity and specificity [185]. In addition, the levels of blood-based miRNAs correlate with the disease stage and poor prognosis [186,187].
The analysis of miRNAs can also be used in early tumor stages for genotyping, monitoring TKI therapy [188], and selecting the patients that benefit from immunotherapy [189]. High expression levels of miR-195 and miR-122 were found to be associated with EGFR-mutant tumors, and were, moreover, independent predictors of survival [190]. In addition, increasing miR-21 levels were observed until there was a clinical progression under first-line TKI in EGFR-mutated NSCLC, anticipating TKI resistance and probably serving as MOR [191]. As miRNAs play a role in antitumor immunity by influencing the mRNA levels, and thus modulating the T-/NK-cell response [192] or PD-L1 expression [193], circulating miRNAs also have the potential to predict the response to ICI. Even though miRNAs have great potential, there is an urgent need for future trials to evaluate their role in disease progression, the evaluation of the therapeutic response, the prediction of progression, and the outcome of NSCLC patients.
3.4. EVs
The analysis of EVs has emerged as a method that can potentially complement the current gold standard of ctDNA analysis in the field of liquid biopsies for NSCLC (c.f. review “Extracellular vesicles in liquid biopsies” in this Special Issue). They are released from the primary tumor into the blood already at early tumor stages, are not as scarce as CTCs, and have the ability to pass the blood–brain barrier, thus allowing early access to tumors and metastases and their molecular compositions [194]. In NSCLC, EVs incorporate miRNAs, which are solely expressed in cancer but not in healthy subjects [195], and of which levels rise during tumor progression [196].
3.4.1. sEVs
The role of sEVs and their potential usefulness in diagnostics, disease monitoring, and therapeutic stratification have been highly studied over the past years. Regarding their mutational assessment in NSCLC especially, the analysis of EV-derived miRNA has been proven to detect oncogenic drivers with a higher sensitivity than ctDNA [197], thus extending the therapeutic options of TKI therapy for more patients. Interestingly, the combined evaluation of resistance mechanisms in ctDNA and EV-derived miRNA showed a high sensitivity in lung cancer patients [198]. The miRNA analysis of sEVs can also offer prognostic information with regards to the response to osimertinib, and might thus be a screening tool regarding the individual choice of TKI [199,200].
Furthermore, PD-L1-positive EVs can inhibit the activation of CD8+ T cells. The finding of PD-L1+-EVs in plasma, and a rapid decline of EV levels under ICI treatment, seems to be highly prognostic with regards to an expectable response [201] and the outcome of NSCLC patients [202]. Furthermore, PD-L1 expression in sEVs is correlated with the progression, tumor burden, TNM stages, and metastatic capacity of NSCLC [203]. The results regarding the correlation between PD-L1 expression in EVs and the tissue remain controversial, ranging from strong [204] to no correlation [203]. EVs are also involved in immune escape mechanisms and the resistance to chemo- and immunotherapy, e.g., by distinct miRNA expression patterns or the overexpression of PD-L1 [55]. Thus, the assessment of sEVs might also help to predict the response to ICI or the failure of immunotherapy [201,205].
3.4.2. lEVs
lEVs have long been known for their involvement in tumor progression and metastasis formation [55]. lEV levels are elevated in lung cancer patients [206], tend to be increased in late stage cancers [55,206], and predict a poor outcome [207], which demonstrates their potential as diagnostic and prognostic biomarkers in liquid biopsies.
The combined analysis of EV-associated transcripts and ctDNA improved the sensitivity of EGFR mutational detection [208], with a high concordance with tissue biopsies [209]. As an alternative approach, an analysis of sEV mRNA levels to determine the oncogenic driver mutations of NSCLC has demonstrated a greater sensitivity than that of ctDNA assessment [197]. However, lEVs seem to reflect the mutational status even better than sEVs [210]. Furthermore, lEVs might be involved in the induction of resistance to both EGFR and ALK inhibitors via the trafficking of EGFR or ALK-mutated isoforms, and the subsequent activation of oncogenic pathways [211,212].
In synopsis, the monitoring of the presence of tumor-derived EVs and the investigation of the vesicle-specific content have dramatically emerged over the last years, and are a promising methodology for a rapid and accurate diagnosis, early tumor detection, and the guidance of therapy. At present, their clinical applications are hampered by a lack of standardization and multicenter studies, as well as a lack of efficient and cost-effective methods for the separation of EVs from non-EV lipid particles.
4. State-of-the-Art Approaches and Future Perspectives
4.1. Screening Programs
Since the clinical appearance of lung cancer is unspecific, the detection of tumors in early, potentially curable stages is challenging. With respect to liquid biopsies, little DNA is shed from small tumor masses and ctDNA concentrations are very low, even prior to treatment [23]. Thus, the low sensitivity of detecting ctDNA in early-stage lung cancer is a major challenge. The sensitivity has been increased by the use of NGS-panels [213] or targeted error sequencing (TEC-Seq) [214]. Several programs have fused ctDNA diagnostics with other parameters, and by integrating distinct molecular features, the lung cancer likelihood in the plasma (Lung-CLIP) algorithm was able to robustly discriminate cancer patients from the risk-matched controls [213]. The multi-analytic blood test CancerSEEK combines ctDNA detection with known protein biomarkers of early cancer stages [215,216]. As early cancer detection ultimately reduces the number of cancer deaths, these multi-analytic blood tests (such as CancerSEEK) have shown promising results for cancer screening. However, ongoing trials aim to increase the clinical sensitivity of those tests. Similar to the implementation of LD-CT scans as a screening method for lung cancer, the use of such multi-analytic blood tests should be limited to patients at risk for cancer, in order to increase the sensitivity. In lung cancer, factors like the patient’s smoking status, their history of exposure to carcinogens, and their age should be considered when selecting the most suitable group of patients for blood screening. Additionally, the prevalence of cancer should be taken into account in order to increase sensitivity [217]. However, CancerSEEK has been proven to be highly specific, e.g., only 7 of 812 healthy individuals tested positive [215]. Thus, it might even be possible in future to screen not only patients at risk, but also healthy individuals for cancer.
Prospective trials, such as the DETECT-A trial [218], the SUMMIT trial (NCT03934866), or the ASCEND trial (NCT04213326) will provide new insights into a risk-stratified approach based on the results of the liquid biopsies (Table S2).
Moreover, with few exceptions, such as Crohn disease [219], endometriosis [220], or pregnancy [221], CTCs might enable an early tumor detection in lung cancer with a high specificity. The detection of CTCs in patients with CT-confirmed suspicious pulmonary nodules reliably discriminates malignant from benign lesions [222]. More importantly, “sentinel” CTC detection in COPD patients identified those patients who developed cancer in the follow-up period [223]. The ongoing multi-center cohort AIR-trial [224] aims to prove whether the image-based monitoring of “sentinel” CTC-positive COPD patients allows for the early diagnosis of lung cancer and improves prognosis (Table S2).
4.2. Monitoring MRD after Curative Intended Therapy and Surgery
ctDNA and CTCs in liquid biopsies are also being investigated for their use as biomarkers to determine the MRD levels and monitor the follow-up after curative intended therapy and surgery.
In a cohort of 41 NSCLC patients, 91.7% (22) of the pre-op-identified 24 plasma ctDNA mutations had a decrease in their mutation frequency within only two days after surgical tumor resection. Moreover, the presence of ctDNA had a higher positive predictive value of residual disease than that of six tumor biomarkers [225]. The ongoing randomized and controlled phase III SUPE_R trial (NCT03740126) is currently investigating the efficacy of ctDNA analysis using serial liquid biopsies every three months in stage I-III NSCLC patients after curative intended treatment [226]. In addition, the observational ORACLE (NCT05059444) study aims at demonstrating the ability of a novel ctDNA assay to detect recurrence in individuals treated for early-stage solid tumors.
Newman et al. developed a method called CAncer Personalized Profiling by deep Sequencing (CAPP-Seq) to identify cancer-specific genetic aberrations in ctDNA analysis [227]. In 94% of patients with tumor recurrence after the curative intended treatment for stage I-III lung cancer, ctDNA detection using CAPP-Seq was positive in the first post-treatment blood sample, indicating the reliable identification of MRD [228]. Post-treatment ctDNA detection preceded radiographic progression in 72% of patients by a median of 5.2 months [228]. Likewise, in the ongoing TRACERx (NCT01888601) study, pre- and post-surgical plasma ctDNA profiling is performed, blinded to relapse statuses to assess the clonal tumor evolution and tumor heterogeneity, and the mutational changes under therapy. The ctDNA profiling used in TRACERx was able to identify 93% (n = 13) of those patients with a confirmed relapse, prior to or at clinical relapse. The median interval between the ctDNA detection and the NSCLC relapse confirmed by a CT scan was 70 days [229]. Interestingly, it was shown that maintenance with immunotherapy in MRD-positive patients after curative intended therapy provided better outcomes [230].
Although patients with detectable ctDNA after surgery have a significantly lower PFS and OS than those with undetectable ctDNA after surgery [228], there are still some patients with undetectable ctDNA that ultimately recur [231]. Thus, interpreting the absence of ctDNA remains difficult.
Although the preliminary data for using ctDNA after surgery to identify the patients at risk of relapse appear promising, further data, including the awaited MRD data from the ADAURA trial [232], are required to elucidate which patients would benefit from an adjuvant therapy such as osimertinib (EGFR-TKI).
4.3. Treatment Monitoring in Advanced Stage (Metastatic) NSCLC
ctDNA detection can also be used to monitor the treatment response in advanced (metastatic) tumor stages. As one knows that the mutation load is correlated with the survival rate [233], the ctDNA-based monitoring of EGFR mutations might allow for an early prediction of the resistance to EGFR-TKI in NSCLC patients [234]. The FASTACT-2 study found that the changes in the cfDNA EGFR mutation status might predict clinical outcomes, even prior to routine CT scans [235]. In patients with EGFR mut+ at baseline, the median PFS and median OS were shorter if the patients were still EGFR mut+ at cycle 3, than if they were EGFR mut− at that point (7.2 vs. 12.0 months and 18.2 vs. 31.9 months, respectively.) [24].
The prognostic value of (i) the detection of the EGFR mutation in the plasma at baseline, as well as of (ii) the clearance of the EGFR mutated ctDNA under systemic therapy, was confirmed by several other studies, including the FLAURA trial [235,236,237,238]. The ongoing phase II APPLE trial (NCT02856893) on EGFR mutant, advanced NSCLC patients, compares the sequential T790M test using a ctDNA analysis of liquid biopsies to conventional radiological procedures, with regards to the prediction tumor progression [80].
Confirming the therapeutic response to ICI with imaging methods is challenging due to the induction of inflammatory processes with leukocyte infiltration that can be misinterpreted (pseudoprogression) in up to 6% of patients [239]. The subsequent monitoring of ctDNA levels via targeted NGS-based methods can predict the response to ICI [240]: After an initial peak due to the massive induction of apoptosis, a rapid decrease in the ctDNA levels under treatment, especially in the first four to eight weeks, correlates with a response and favorable outcome [241,242], while patients without a molecular response had a shorter PFS and OS compared to molecular responders [243]. There is also evidence from a small patient cohort that the increase in ctDNA might identify ICI non-responders prior to imaging [244]. The now-recruiting LIBERTYLUNG trial (NCT04790682) prospectively examines the suitability of ctDNA to predict the response to the PD-L1 inhibitor pembrolizumab (+/− chemotherapy) in treatment-naive metastatic NSCLC patients, with at least one detectable mutation. However, further studies are crucial to standardize the diagnostic thresholds and refine the methodology of liquid biopsies in this regard.
4.4. Limitations
Despite the great potential for the use of liquid biopsies in diagnostic and therapeutic decision making for patients with lung cancer, there are still several limitations of the current applications.
4.4.1. Sensitivity and Specificity
Tumor tissue genotyping is generally associated with a higher sensitivity than liquid-biopsy-based genotyping [14,23]. The sensitivity of ctDNA analysis mainly depends on the amount of ctDNA shed by the tumor; hence, its stage and type. The sensitivity is increased in patients with extra-thoracic metastases (especially bone and liver metastases), [245] as well as in patients with a high tumor burden [246]. However, 20% of stage IV NSCLC patients do not shed ctDNA [62]. Currently, highly sensitive assays can achieve sensitivity levels of up to 85% in advanced stage lung cancer [14], which have been further improved with the use of deep sequencing methods. Although a positive result in ctDNA analysis using a validated test is usually sufficient to initiate targeted therapy [14], a negative result should be further clarified, e.g., with a tissue biopsy, due to the high risk of false-negative results, especially in slow-growing or early-stage tumors [71,247].
Although the evolvement of deep sequencing methods such as NGS has increased sensitivity, it brings the inherent risk of false-positive results—variants with low allele frequencies especially can result in a single false-positive result and impact the data interpretation. However, some technical adaptations have been developed to further improve specificity (e.g., molecular barcoding or error-proofing algorithms).
False-positive results can also be caused by germline variants or the clonal hematopoiesis of indeterminate potentials (CHIP) that contribute to the bulk of cfDNA, and can interfere with the interpretation of ctDNA analysis results [247,248,249].
Some methods have been developed to avoid the false-positive detection of mutations caused by CHIP, e.g., a combined screening of matched white blood cells and ctDNA [250,251]. Moreover, a machine learning method, which can discriminate clonal hematopoiesis mutations from frequently recurring genetic alterations in NSCLC, has been validated [213,247]. It must be noted that conditions unrelated to cancer, such as infections, trauma, or inflammations, can increase the cfDNA levels in blood and might also lead to false-positive results [252].
4.4.2. Early Stages
Molecular testing in the early stages of NSCLC is not as widely reflected in current guidelines as it is for advanced stages. However, it has gained importance in the adjustment and individualization of therapy. The ADAURA trial demonstrated that adjuvant treatment with osimertinib in stage IB-IIIA EGFR-mutated NSCLC significantly improved the disease-free survival, thus leading to the approval of osimertinib in Ex19del or L858R EGFR-mutated NSCLC [232]. Consequently, testing for EGFR mutations, which were found in 30% of stage I-III NSCLCs in a meta-analysis [253], is now recommended in the current National Comprehensive Cancer Network (NCCN) guidelines, to determine whether a patient might benefit from adjuvant treatment with osimertinib [254]. CTCs can also be detected in the early NSCLC stages. Crosbie et al. outlined the large potential of CTCs for post-surgical risk stratification [255]. In their study, in 33 patients with NSCLC stage IA-IIIA, they found that the detection of tumor micro-emboli or ≥2 CTCs in the pulmonary vein draining the tumor was associated with an eight-fold increase in the risk of disease recurrence, and a seven-fold increased risk of death. Hence, the CTCs detected during surgery might identify the patients for whom an adjuvant therapy during early-stage disease would prevent the disease recurrence.
4.4.3. Financial Aspects/Accessibility
As more patients are given targeted therapies, the outcome of the patients suffering from NSCLC has improved. An analysis in Canada showed that therapeutic treatments yielded a gain of 168 life years, but at a cost of 14.7 million US dollars [256]. Even if the cost of an initial broad NGS-based mutational assessment (e.g., using liquid biopsies) is now comparable to that of sequential testing or hotspot panels [257], standard-of-care tissue testing is significantly less expensive [23]. In addition, a reimbursement from national health systems or health insurance is lacking in many countries, e.g., in Spain. NGS-based methods for analyzing liquid biopsies require the newest equipment that is generally only available in First World countries, which increases the global social gap and disparity, and also hinders their broad clinical introduction.
5. Conclusions
As a result of intensified research activity, the scope of the application of liquid biopsies is currently expanding. The new NGS-based techniques have enhanced the ability to analyze ctDNA, EVs, or CTCs, in order to assess an increasing number of accessible targets. Liquid biopsies, and ctDNA in particular, have already secured their position in clinical routine, where they are useful for the risk stratification of NSCLC patients, as well as for monitoring therapeutic efficacy, although further elucidation of the pathophysiological role and the implications of CTCs and EVs is required.
However, there are several obstacles limiting the role of this technique. The absence of standardized protocols for extracting and analyzing the DNA, and interpreting the data, together with the low amount of targeted biomarkers in plasma, are impeding the further integration of liquid biopsies into diagnostic routine. Additionally, most studies in this field are based on small sample sizes, which gives them an inferior level of evidence. Hence, further large-scale, prospective, randomized, and controlled trials are needed to clarify the role of liquid biopsies, to extend the current knowledge, and to strengthen the weight of liquid biopsies in diagnostics, risk stratification, the assessment of individualized therapy, disease monitoring, and, ultimately, in achieving better outcomes for NSCLC patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15051430/s1. Table S1: Overview of studies, partly using liquid biopsy in mutational diagnostics, cited in this review; Table S2: Use of liquid biopsies in lung cancer screening, post-treatment surveillance and therapy response prediction.
Author Contributions
M.K., C.K. and A.B.S. wrote the manuscript. K.M., G.L., G.E. and A.B. revised the manuscript. M.K., K.M. and A.B. acquired funding for the project. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the German Ministry of Education and Research (BMBF) e:Med project MyPathSem (031 L0024), the Else Kröner-Fresenius-Stiftung (project 2019_A162), the innovative medical research (IMF) of the Medical Faculty, University of Münster (project ME 12 19 14) and the Excellence Cluster “Cells in Motion”. The project was supported by the Volkswagen Foundation within the research project MTB-Report (ZN3424). Marcel Kemper received research funds from Novartis Pharma GmbH (InCa (Inflammation & Lung Cancer) Förderpreis 2021).
Acknowledgments
We would like to thank Thomas Crozier for revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung Cancer: Diagnosis and Management. Am. Fam. Phys. 2007, 75, 56–63. [Google Scholar]
- Shen, X.; Wang, Y.; Jin, Y.; Zheng, Q.; Shen, L.; Chen, Y.; Li, Y. PD-L1 Expression in Non-Small Cell Lung Cancer: Heterogeneity by Pathologic Types, Tissue Sampling and Metastasis. J. Thorac. Dis. 2021, 13, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hou, S.; Ji, H.; Han, X. Evolution from Genetics to Phenotype: Reinterpretation of NSCLC Plasticity, Heterogeneity, and Drug Resistance. Protein Cell 2017, 8, 178–190. [Google Scholar] [CrossRef]
- Gandara, D.R.; von Pawel, J.; Mazieres, J.; Sullivan, R.; Helland, Å.; Han, J.-Y.; Ponce Aix, S.; Rittmeyer, A.; Barlesi, F.; Kubo, T.; et al. Atezolizumab Treatment Beyond Progression in Advanced NSCLC: Results from the Randomized, Phase III OAK Study. J. Thorac. Oncol. 2018, 13, 1906–1918. [Google Scholar] [CrossRef]
- Schuler, M.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S.; et al. First-Line Afatinib vs Gefitinib for Patients with EGFR Mutation-Positive NSCLC (LUX-Lung 7): Impact of Afatinib Dose Adjustment and Analysis of Mode of Initial Progression for Patients Who Continued Treatment beyond Progression. J. Cancer Res. Clin. Oncol. 2019, 145, 1569–1579. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. Nuclear Acids in Human Blood Plasma. C R Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Gray, J.; Thompson, J.C.; Carpenter, E.L.; Elkhouly, E.; Aggarwal, C. Plasma Cell-Free DNA Genotyping: From an Emerging Concept to a Standard-of-Care Tool in Metastatic Non-Small Cell Lung Cancer. Oncology 2021, 26, e1812. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Pradines, A.; Favre, G.; Mazieres, J. Current and Future Applications of Liquid Biopsy in Nonsmall Cell Lung Cancer from Early to Advanced Stages. Eur. Respir. Rev. 2020, 29, 190052. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Burges, A.; Reich, O.; Spelsberg, F.W.; Stieber, P. DNA Integrity in Plasma and Serum of Patients with Malignant and Benign Diseases. Ann. N. Y. Acad. Sci. 2008, 1137, 162–170. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Pisapia, P.; Rocco, D.; Smeraglio, R.; di Spirito, M.; Bellevicine, C.; Troncone, G. Next Generation Sequencing Techniques in Liquid Biopsy: Focus on Non-Small Cell Lung Cancer Patients. Transl. Lung Cancer Res. 2016, 5, 505–510. [Google Scholar] [CrossRef]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and Comparison of in Vitro Degradation Kinetics of DNA in Serum, Urine and Saliva: A Qualitative Study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.L.; Corcoran, C.; Brown, H.; Sharpe, A.D.; Musilova, M.; Kohlmann, A. Optimised Pre-Analytical Methods Improve KRAS Mutation Detection in Circulating Tumour DNA (CtDNA) from Patients with Non-Small Cell Lung Cancer (NSCLC). PLoS ONE 2016, 11, e0150197. [Google Scholar] [CrossRef]
- Rothwell, D.G.; Smith, N.; Morris, D.; Leong, H.S.; Li, Y.; Hollebecque, A.; Ayub, M.; Carter, L.; Antonello, J.; Franklin, L.; et al. Genetic Profiling of Tumours Using Both Circulating Free DNA and Circulating Tumour Cells Isolated from the Same Preserved Whole Blood Sample. Mol. Oncol. 2016, 10, 566–574. [Google Scholar] [CrossRef]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Goethals, S.; Reclusa, P.; Van Casteren, K.; Augustus, E.; Lardon, F.; Roeyen, G.; et al. Circulating Cell-Free DNA and RNA Analysis as Liquid Biopsy: Optimal Centrifugation Protocol. Cancers 2019, 11, 458. [Google Scholar] [CrossRef]
- Lam, N.Y.L.; Rainer, T.H.; Chiu, R.W.K.; Lo, Y.M.D. EDTA Is a Better Anticoagulant than Heparin or Citrate for Delayed Blood Processing for Plasma DNA Analysis. Clin. Chem. 2004, 50, 256–257. [Google Scholar] [CrossRef]
- Medina Diaz, I.; Nocon, A.; Mehnert, D.H.; Fredebohm, J.; Diehl, F.; Holtrup, F. Performance of Streck CfDNA Blood Collection Tubes for Liquid Biopsy Testing. PLoS ONE 2016, 11, e0166354. [Google Scholar] [CrossRef] [PubMed]
- Pellini, B.; Szymanski, J.; Chin, R.-I.; Jones, P.A.; Chaudhuri, A.A. Liquid Biopsies Using Circulating Tumor DNA in Non-Small Cell Lung Cancer. Thorac. Surg. Clin. 2020, 30, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement from the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Wu, Y.-L.; Lee, J.S.; Yu, C.-J.; Sriuranpong, V.; Sandoval-Tan, J.; Ladrera, G.; Thongprasert, S.; Srimuninnimit, V.; Liao, M.; et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-Line Intercalated Erlotinib and Chemotherapy. Clin. Cancer Res. 2015, 21, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Hrebien, S.; Beaney, M.; Fribbens, C.; Garcia-Murillas, I.; Jiang, J.; Li, Y.; Huang Bartlett, C.; André, F.; Loibl, S.; et al. Comparison of BEAMing and Droplet Digital PCR for Circulating Tumor DNA Analysis. Clin. Chem. 2019, 65, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Hu, Y.; Feeney, N.; Kuang, Y.; Plagnol, V.; Jones, G.; Howarth, K.; Beeler, J.F.; Paweletz, C.P.; Oxnard, G.R. Amplicon-Based Next-Generation Sequencing of Plasma Cell-Free DNA for Detection of Driver and Resistance Mutations in Advanced Non-Small Cell Lung Cancer. Ann. Oncol. 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical Utility of Comprehensive Cell-Free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef]
- Batra, U.; Nathany, S.; Sharma, M.; Pasricha, S.; Bansal, A.; Jain, P.; Mehta, A. IHC versus FISH versus NGS to Detect ALK Gene Rearrangement in NSCLC: All Questions Answered? J. Clin. Pathol. 2021, 75, 405–409. [Google Scholar] [CrossRef]
- Shagin, D.A.; Shagina, I.A.; Zaretsky, A.R.; Barsova, E.V.; Kelmanson, I.V.; Lukyanov, S.; Chudakov, D.M.; Shugay, M. A High-Throughput Assay for Quantitative Measurement of PCR Errors. Sci. Rep. 2017, 7, 2718. [Google Scholar] [CrossRef]
- Hofman, V.; Ilie, M.; Long, E.; Guibert, N.; Selva, E.; Washetine, K.; Mograbi, B.; Mouroux, J.; Venissac, N.; Reverso-Meinietti, J.; et al. Detection of Circulating Tumor Cells from Lung Cancer Patients in the Era of Targeted Therapy: Promises, Drawbacks and Pitfalls. CMM 2014, 14, 440–456. [Google Scholar] [CrossRef]
- Duda, D.G.; Duyverman, A.M.M.J.; Kohno, M.; Snuderl, M.; Steller, E.J.A.; Fukumura, D.; Jain, R.K. Malignant Cells Facilitate Lung Metastasis by Bringing Their Own Soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef] [PubMed]
- Brozos-Vázquez, E.M.; Díaz-Peña, R.; García-González, J.; León-Mateos, L.; Mondelo-Macía, P.; Peña-Chilet, M.; López-López, R. Immunotherapy in Nonsmall-Cell Lung Cancer: Current Status and Future Prospects for Liquid Biopsy. Cancer Immunol. Immunother. 2021, 70, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Maly, V.; Maly, O.; Kolostova, K.; Bobek, V. Circulating Tumor Cells in Diagnosis and Treatment of Lung Cancer. Vivo 2019, 33, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Bosma, A.J.; van de Heuvel, M.M.; Heemsbergen, W.; Voest, E.E.; Schellens, J.H.M. Circulating Tumor Cell Detection in Advanced Non-Small Cell Lung Cancer Patients by Multi-Marker QPCR Analysis. Lung Cancer 2012, 75, 242–247. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.A.; Mitchell, E.; Miller, M.C.; et al. Prognostic Significance of Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2009, 20, 1223–1229. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating Tumor Cell Technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, Detection, and Clinical Implications of Circulating Tumor Cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Barrière, G.; Tartary, M.; Rigaud, M. Epithelial Mesenchymal Transition: A New Insight into the Detection of Circulating Tumor Cells. ISRN Oncol. 2012, 2012, 382010. [Google Scholar] [CrossRef]
- Hofman, V.; Long, E.; Ilie, M.; Bonnetaud, C.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Morphological Analysis of Circulating Tumour Cells in Patients Undergoing Surgery for Non-Small Cell Lung Carcinoma Using the Isolation by Size of Epithelial Tumour Cell (ISET) Method: Circulating Tumour Cells and Cytological Analysis. Cytopathology 2012, 23, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, G.; Tirino, V.; Camerlingo, R.; La Rocca, A.; Martucci, N.; Scognamiglio, G.; Franco, R.; Cantile, M.; Normanno, N.; Rocco, G. Prognostic Value of Cancer Stem Cells, Epithelial-Mesenchymal Transition and Circulating Tumor Cells in Lung Cancer. Oncol. Rep. 2013, 29, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, K.; Fan, Z.H. Circulating Tumor Cell Isolation and Analysis. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 75, pp. 1–31. ISBN 978-0-12-804688-3. [Google Scholar]
- Descamps, L.; Le Roy, D.; Deman, A.-L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. IJMS 2022, 23, 1981. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Sollier-Christen, E.; Renier, C.; Kaplan, T.; Kfir, E.; Crouse, S.C. VTX-1 Liquid Biopsy System for Fully-Automated and Label-Free Isolation of Circulating Tumor Cells with Automated Enumeration by BioView Platform. Cytometry 2018, 93, 1240–1245. [Google Scholar] [CrossRef]
- Khamenehfar, A.; Li, P. Microfluidic Devices for Circulating Tumor Cells Isolation and Subsequent Analysis. CPB 2016, 17, 810–821. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular MiRNAs: The Mystery of Their Origin and Function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating MicroRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs Are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and Characterization of Microvesicles from Peripheral Blood. JoVE 2017, 55057. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral Sphingomyelinases Control Extracellular Vesicles Budding from the Plasma Membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.-C. Extracellular Vesicles-Mediated Intercellular Communication: Roles in the Tumor Microenvironment and Anti-Cancer Drug Resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. IJMS 2020, 21, 5373. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Nygaard, A.D.; Holdgaard, P.C.; Spindler, K.-L.G.; Pallisgaard, N.; Jakobsen, A. The Correlation between Cell-Free DNA and Tumour Burden Was Estimated by PET/CT in Patients with Advanced NSCLC. Br. J. Cancer 2014, 110, 363–368. [Google Scholar] [CrossRef]
- Hyun, M.H.; Sung, J.S.; Kang, E.J.; Choi, Y.J.; Park, K.H.; Shin, S.W.; Lee, S.Y.; Kim, Y.H. Quantification of Circulating Cell-Free DNA to Predict Patient Survival in Non-Small-Cell Lung Cancer. Oncotarget 2017, 8, 94417–94430. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Demuth, C.; Fledelius, J.; Madsen, A.T.; Hjorthaug, K.; Meldgaard, P.; Sorensen, B.S. Correlation between Circulating Mutant DNA and Metabolic Tumour Burden in Advanced Non-Small Cell Lung Cancer Patients. Br. J. Cancer 2017, 117, 704–709. [Google Scholar] [CrossRef]
- Simons, M.J.H.G.; Uyl-de Groot, C.A.; Retèl, V.P.; Mankor, J.M.; Ramaekers, B.L.T.; Joore, M.A.; van Harten, W.H. Cost-Effectiveness and Budget Impact of Future Developments with Whole-Genome Sequencing for Patients with Lung Cancer. Value Health 2023, 26, 71–80. [Google Scholar] [CrossRef]
- Plagnol, V.; Woodhouse, S.; Howarth, K.; Lensing, S.; Smith, M.; Epstein, M.; Madi, M.; Smalley, S.; Leroy, C.; Hinton, J.; et al. Analytical Validation of a next Generation Sequencing Liquid Biopsy Assay for High Sensitivity Broad Molecular Profiling. PLoS ONE 2018, 13, e0193802. [Google Scholar] [CrossRef]
- Malapelle, U.; Tiseo, M.; Vivancos, A.; Kapp, J.; Serrano, M.J.; Tiemann, M. Liquid Biopsy for Biomarker Testing in Non-Small Cell Lung Cancer: A European Perspective. JMP 2021, 2, 255–273. [Google Scholar] [CrossRef]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.-O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. Biomarker Testing in Non-Small Cell Lung Cancer in Routine Care: Analysis of the First 3,717 Patients in the German Prospective, Observational, Nation-Wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184. [Google Scholar] [CrossRef]
- Mack, P.C.; Banks, K.C.; Espenschied, C.R.; Burich, R.A.; Zill, O.A.; Lee, C.E.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of Driver Mutations and Clinical Impact of Circulating Tumor DNA Analysis in Non–Small Cell Lung Cancer: Analysis of over 8000 Cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef]
- Sabari, J.K.; Offin, M.; Stephens, D.; Ni, A.; Lee, A.; Pavlakis, N.; Clarke, S.; Diakos, C.I.; Datta, S.; Tandon, N.; et al. A Prospective Study of Circulating Tumor DNA to Guide Matched Targeted Therapy in Lung Cancers. JNCI J. Natl. Cancer Inst. 2019, 111, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, L.; Zheng, D. Diagnostic Value of Circulating Free DNA for the Detection of EGFR Mutation Status in NSCLC: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 4, 6269. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, J.; Xu, Y.; Ding, X.; Li, M.; Jiang, F.; Xu, L.; Yin, R. Circulating Tumor DNA Is Effective for the Detection of EGFR Mutation in Non–Small Cell Lung Cancer: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2015, 24, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients with Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. JCO 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating Tumor DNA and Liquid Biopsy in Oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, F.; Zhang, M.; Sun, B.; Yin, W.; Deng, S.; Wan, Y.; Lu, W. The Diagnostic Accuracy of Liquid Biopsy in EGFR-Mutated NSCLC: A Systematic Review and Meta-Analysis of 40 Studies. SLAS Technol. 2021, 26, 42–54. [Google Scholar] [CrossRef]
- Wu, Y.L.; Jenkins, S.; Ramalingam, S.; Han, J.-Y.; Delmonte, A.; Hsia, T.-C.; Laskin, J.; Kim, S.-W.; He, Y.; Patel, S.; et al. MA08.03 Osimertinib vs Platinum-Pemetrexed for T790M-Mutation Positive Advanced NSCLC (AURA3): Plasma CtDNA Analysis. J. Thorac. Oncol. 2017, 12, S386. [Google Scholar] [CrossRef]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v1–v27. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Giovannetti, E.; Hong, D.S.; Bivona, T.; Raez, L.E.; Bronte, G.; Buffoni, L.; Reguart, N.; Santos, E.S.; Germonpre, P.; et al. Novel Therapeutic Strategies for Patients with NSCLC That Do Not Respond to Treatment with EGFR Inhibitors. Cancer Treat. Rev. 2014, 40, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Thress, K.S.; Alden, R.S.; Lawrance, R.; Paweletz, C.P.; Cantarini, M.; Yang, J.C.-H.; Barrett, J.C.; Jänne, P.A. Association Between Plasma Genotyping and Outcomes of Treatment with Osimertinib (AZD9291) in Advanced Non–Small-Cell Lung Cancer. JCO 2016, 34, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Sacher, A.G.; Paweletz, C.; Dahlberg, S.E.; Alden, R.S.; O’Connell, A.; Feeney, N.; Mach, S.L.; Jänne, P.A.; Oxnard, G.R. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol. 2016, 2, 1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Qing, X.; Xiumin, W.; Yali, B.; Chi, S.; Bak, S.H.; Lee, H.Y.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; et al. Longitudinal Monitoring of EGFR Mutations in Plasma Predicts Outcomes of NSCLC Patients Treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC-12-02). Oncotarget 2016, 7, 6984–6993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ye, X.; Zhang, M.Z.; Sun, Y.; Wang, J.Y.; Ni, J.; Zhang, H.P.; Zhang, L.; Luo, J.; Zhang, J.; et al. Plasma EGFR T790M CtDNA Status Is Associated with Clinical Outcome in Advanced NSCLC Patients with Acquired EGFR-TKI Resistance. Sci. Rep. 2016, 6, 20913. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Gray, J.E.; Ohe, Y.; Cho, B.C.; Vansteenkiste, J.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; Shah, R.; et al. Osimertinib vs Comparator EGFR-TKI as First-Line Treatment for EGFRm Advanced NSCLC (FLAURA): Final Overall Survival Analysis. Ann. Oncol. 2019, 30, v914–v915. [Google Scholar] [CrossRef]
- Remon, J.; Menis, J.; Hasan, B.; Peric, A.; De Maio, E.; Novello, S.; Reck, M.; Berghmans, T.; Wasag, B.; Besse, B.; et al. The APPLE Trial: Feasibility and Activity of AZD9291 (Osimertinib) Treatment on Positive PLasma T790M in EGFR-Mutant NSCLC Patients. EORTC 1613. Clin. Lung Cancer 2017, 18, 583–588. [Google Scholar] [CrossRef]
- Bennouna, J.; Girard, N.; Audigier-Valette, C.; le Thuaut, A.; Gervais, R.; Masson, P.; Marcq, M.; Molinier, O.; Cortot, A.; Debieuvre, D.; et al. Phase II Study Evaluating the Mechanisms of Resistance on Tumor Tissue and Liquid Biopsy in Patients With EGFR-Mutated Non-Pretreated Advanced Lung Cancer Receiving Osimertinib Until and Beyond Radiologic Progression: The MELROSE Trial. Clin. Lung Cancer 2020, 21, e10–e14. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Mignard, X.; Ruppert, A.-M.; Antoine, M.; Vasseur, J.; Girard, N.; Mazières, J.; Moro-Sibilot, D.; Fallet, V.; Rabbe, N.; Thivolet-Bejui, F.; et al. C-MET Overexpression as a Poor Predictor of MET Amplifications or Exon 14 Mutations in Lung Sarcomatoid Carcinomas. J. Thorac. Oncol. 2018, 13, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shao, Y.; Qin, H.-F.; Tai, Y.-H.; Gao, H.-J. ALK- Rearrangement in Non-Small-Cell Lung Cancer (NSCLC): ALK Rearrangement in Lung Cancer. Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The Anaplastic Lymphoma Kinase in the Pathogenesis of Cancer. Nat. Rev. Cancer 2008, 8, 11–23. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, P.-W.; Wang, W.-Y.; Wang, K.; Zhang, Z.; Chen, B.-J.; He, Y.-Q.; Li, L.; Liu, H.; Chuai, S.; et al. Noninvasive Genotyping and Monitoring of Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small Cell Lung Cancer by Capture-Based Next-Generation Sequencing. Oncotarget 2016, 7, 65208–65217. [Google Scholar] [CrossRef]
- Kwak, E.L.; Bang, Y.-J.; Camidge, D.R.; Shaw, A.T.; Solomon, B.; Maki, R.G.; Ou, S.-H.I.; Dezube, B.J.; Jänne, P.A.; Costa, D.B.; et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef]
- Camidge, D.R.; Doebele, R.C. Treating ALK-Positive Lung Cancer—Early Successes and Future Challenges. Nat. Rev. Clin. Oncol. 2012, 9, 268–277. [Google Scholar] [CrossRef]
- Gainor, J.F.; Tan, D.S.W.; De Pas, T.; Solomon, B.J.; Ahmad, A.; Lazzari, C.; de Marinis, F.; Spitaleri, G.; Schultz, K.; Friboulet, L.; et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin. Cancer Res. 2015, 21, 2745–2752. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Bazhenova, L.A.; Langer, C.J.; Salgia, R.; Gold, K.A.; Rosell, R.; Shaw, A.T.; Weiss, G.J.; Tugnait, M.; Narasimhan, N.I.; et al. Activity and Safety of Brigatinib in ALK -Rearranged Non-Small-Cell Lung Cancer and Other Malignancies: A Single-Arm, Open-Label, Phase 1/2 Trial. Lancet Oncol. 2016, 17, 1683–1696. [Google Scholar] [CrossRef]
- Kim, D.-W.; Tiseo, M.; Ahn, M.-J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.-W.; Huber, R.M.; West, H.L.; Groen, H.J.M.; Hochmair, M.J.; et al. Brigatinib in Patients with Crizotinib-Refractory Anaplastic Lymphoma Kinase–Positive Non–Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. JCO 2017, 35, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-H.I.; Ahn, J.S.; De Petris, L.; Govindan, R.; Yang, J.C.-H.; Hughes, B.; Lena, H.; Moro-Sibilot, D.; Bearz, A.; Ramirez, S.V.; et al. Alectinib in Crizotinib-Refractory ALK- Rearranged Non–Small-Cell Lung Cancer: A Phase II Global Study. JCO 2016, 34, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK -Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Mok, T.S.K.; Peters, S.; Alexander, J.A.A.; Leighl, N.B.; Sriuranpong, V.; Perol, M.; De Castro, G.; Nadal, E.; De Marinis, F.; et al. Phase II/III Blood First Assay Screening Trial (BFAST) in Patients (Pts) with Treatment-Naïve NSCLC: Initial Results from the ALK+ Cohort. Ann. Oncol. 2019, 30, v918. [Google Scholar] [CrossRef]
- Angeles, A.K.; Christopoulos, P.; Yuan, Z.; Bauer, S.; Janke, F.; Ogrodnik, S.J.; Reck, M.; Schlesner, M.; Meister, M.; Schneider, M.A.; et al. Early Identification of Disease Progression in ALK-Rearranged Lung Cancer Using Circulating Tumor DNA Analysis. NPJ Precis. Onc. 2021, 5, 100. [Google Scholar] [CrossRef]
- Kwon, M.; Ku, B.M.; Park, S.; Jung, H.A.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Longitudinal Monitoring by next Generation Sequencing of Plasma Cell-Free DNA in ALK-Rearranged Non-Small Cell Lung Cancer (NSCLC) Patients Treated with ALK Tyrosine Kinase Inhibitors. JCO 2020, 38, 9603. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1 -Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef]
- Swalduz, A.; Mezquita, L.; Ortiz-Cuaran, S.; Jovelet, C.; Avrillon, V.; Planchard, D.; Marteau, S.; Recondo, G.; Martinez, S.; Howarth, K.; et al. MA16.09 Feasibility, Clinical Relevance of ALK/ROS1 Fusion Variant Detection by Liquid Biopsy in Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, S413–S414. [Google Scholar] [CrossRef]
- Mezquita, L.; Swalduz, A.; Jovelet, C.; Ortiz-Cuaran, S.; Howarth, K.; Planchard, D.; Avrillon, V.; Recondo, G.; Marteau, S.; Benitez, J.C.; et al. Clinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients with Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2020, 272–282. [Google Scholar] [CrossRef]
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non–Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Viteri, S.; Mazieres, J.; Veillon, R.; Felip, E.; Le, X.; Garassino, M.C.; Stanton, T.S.; Morise, M.; Lee, J.-S.; Matsumoto, S.; et al. 1286P Activity of Tepotinib in Brain Metastases (BM): Preclinical Models and Clinical Data from Patients (Pts) with MET Exon 14 (METex14) Skipping NSCLC. Ann. Oncol. 2020, 31, S831. [Google Scholar] [CrossRef]
- Abdayem, P.; Planchard, D. Update on Molecular Pathology and Role of Liquid Biopsy in Nonsmall Cell Lung Cancer. Eur. Respir. Rev. 2021, 30, 200294. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fang, J.; Li, X.; Cao, L.; Zhou, J.; Guo, Q.; Liang, Z.; Cheng, Y.; Jiang, L.; Yang, N.; et al. Phase II Study of Savolitinib in Patients (Pts) with Pulmonary Sarcomatoid Carcinoma (PSC) and Other Types of Non-Small Cell Lung Cancer (NSCLC) Harboring MET Exon 14 Skipping Mutations (METex14+). JCO 2020, 38, 9519. [Google Scholar] [CrossRef]
- Drilon, A.; Lin, J.J.; Filleron, T.; Ni, A.; Milia, J.; Bergagnini, I.; Hatzoglou, V.; Velcheti, V.; Offin, M.; Li, B.; et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET–Rearranged Lung Cancers. J. Thorac. Oncol. 2018, 13, 1595–1601. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Vincent, J.J.; Mortimer, S.; Vowles, J.V.; Ulrich, B.C.; Banks, K.C.; Fairclough, S.R.; Zill, O.A.; Sikora, M.; Mokhtari, R.; et al. Validation of a Plasma-Based Comprehensive Cancer Genotyping Assay Utilizing Orthogonal Tissue- and Plasma-Based Methodologies. Clin. Cancer Res. 2018, 24, 3539–3549. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Patil, T.; Kirtane, K.; Rich, T.A.; Espenschied, C.R.; Weipert, C.M.; Raymond, V.M.; Santana-Davila, R.; Doebele, R.C.; Baik, C.S. Duration of Targeted Therapy in Patients with Advanced Non–Small-Cell Lung Cancer Identified by Circulating Tumor DNA Analysis. Clin. Lung Cancer 2020, 21, 545–552.e1. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET Fusion-Positive Non-Small-Cell Lung Cancer (ARROW): A Multi-Cohort, Open-Label, Phase 1/2 Study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Drusbosky, L.M.; Rodriguez, E.; Dawar, R.; Ikpeazu, C.V. Therapeutic Strategies in RET Gene Rearranged Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2021, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.A.; Reckamp, K.L.; Chae, Y.K.; Doebele, R.C.; Iams, W.T.; Oh, M.; Raymond, V.M.; Lanman, R.B.; Riess, J.W.; Stinchcombe, T.E.; et al. Analysis of Cell-Free DNA from 32,989 Advanced Cancers Reveals Novel Co-Occurring Activating RET Alterations and Oncogenic Signaling Pathway Aberrations. Clin. Cancer Res. 2019, 25, 5832–5842. [Google Scholar] [CrossRef]
- Iaccarino, A.; Pisapia, P.; Pepe, F.; Sgariglia, R.; Nacchio, M.; Russo, G.; Gragnano, G.; De Luca, C.; Troncone, G.; Malapelle, U. Liquid Biopsy for BRAF Mutations Testing in Non-Small Cell Lung Cancer: A Retrospective Study. J. Clin. Pathol. 2022, 75, 58–60. [Google Scholar] [CrossRef]
- Marchetti, A.; Felicioni, L.; Malatesta, S.; Grazia Sciarrotta, M.; Guetti, L.; Chella, A.; Viola, P.; Pullara, C.; Mucilli, F.; Buttitta, F. Clinical Features and Outcome of Patients with Non–Small-Cell Lung Cancer Harboring BRAF Mutations. JCO 2011, 29, 3574–3579. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-Mutated Metastatic Melanoma: A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib Combined with Vemurafenib in Advanced BRAFV600-Mutant Melanoma (CoBRIM): Updated Efficacy Results from a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- O’Leary, C.G.; Andelkovic, V.; Ladwa, R.; Pavlakis, N.; Zhou, C.; Hirsch, F.; Richard, D.; O’Byrne, K. Targeting BRAF Mutations in Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 1119–1124. [Google Scholar] [CrossRef]
- Guan, X.; Liu, B.; Niu, Y.; Dong, X.; Zhu, X.; Li, C.; Li, L.; Yi, Z.; Sun, X.; Chen, H.; et al. Longitudinal HER2 Amplification Tracked in Circulating Tumor DNA for Therapeutic Effect Monitoring and Prognostic Evaluation in Patients with Breast Cancer. Breast 2020, 49, 261–266. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, Y. Targeting HER2 Alterations in Non–Small-Cell Lung Cancer: A Comprehensive Review. JCO Precis. Oncol. 2020, 411–425. [Google Scholar] [CrossRef]
- Mayo de las Casas, C.; Garzón-Ibañez, M.; Jordana-Ariza, N.; Viteri-Ramírez, S.; Moya-Horno, I.; Karachaliou, N.; Yeste, Z.; Campos, R.; Villatoro, S.; Balada-Bel, A.; et al. Prospective Analysis of Liquid Biopsies of Advanced Non-Small Cell Lung Cancer Patients after Progression to Targeted Therapies Using GeneReader NGS Platform. Transl. Cancer Res. 2018, 8, S3–S15. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. LBA45 Primary Data from DESTINY-Lung01: A Phase II Trial of Trastuzumab Deruxtecan (T-DXd) in Patients (Pts) with HER2-Mutated (HER2m) Metastatic Non-Small Cell Lung Cancer (NSCLC). Ann. Oncol. 2021, 32, S1323–S1324. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.-M.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus Docetaxel for Previously Treated Non-Small-Cell Lung Cancer with KRASG12C Mutation: A Randomised, Open-Label, Phase 3 Trial. Lancet 2023, S0140673623002210. [Google Scholar] [CrossRef] [PubMed]
- Nacchio, M.; Sgariglia, R.; Gristina, V.; Pisapia, P.; Pepe, F.; De Luca, C.; Migliatico, I.; Clery, E.; Greco, L.; Vigliar, E.; et al. KRAS Mutations Testing in Non-Small Cell Lung Cancer: The Role of Liquid Biopsy in the Basal Setting. J. Thorac. Dis. 2020, 12, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Gelibter, A.; Bottillo, I.; Belardinilli, F.; Pisegna, S.; De Renzi, G.; Marinelli, D.; Grammatico, P.; Cortesi, E.; Giannini, G.; et al. Comparison of Two Blood-Based Genotyping Tests to Investigate the KRAS G12C Mutation in Patients with Non-Small-Cell Lung Cancer at Failure of First-Line Treatments. Diagnostics 2021, 11, 2196. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncology 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Davis, A.; Agte, S.; Pan, A.; Mohindra, N.; Villaflor, V.; Giles, F. OA 07.03 Circulating Tumor DNA Mutant Allele Frequency and Tumor Burden as Biomarkers for Response to Immune Checkpoint Blockade. J. Thorac. Oncol. 2017, 12, S1762. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Lopes, G.; Kowalski, D.M.; Nishio, M.; Wu, Y.-L.; de Castro Junior, G.; Baas, P.; Kim, D.-W.; Gubens, M.A.; Cristescu, R.; et al. Association between Tissue TMB (TTMB) and Clinical Outcomes with Pembrolizumab Monotherapy (Pembro) in PD-L1-Positive Advanced NSCLC in the KEYNOTE-010 and -042 Trials. Ann. Oncol. 2019, 30, v916–v917. [Google Scholar] [CrossRef]
- Fenizia, F.; Pasquale, R.; Roma, C.; Bergantino, F.; Iannaccone, A.; Normanno, N. Measuring Tumor Mutation Burden in Non-Small Cell Lung Cancer: Tissue versus Liquid Biopsy. Transl. Lung Cancer Res. 2018, 7, 668–677. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden–High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Davis, A.A.; Chae, Y.K.; Agte, S.; Pan, A.; Simon, N.I.; Taxter, T.J.; Behdad, A.; Carneiro, B.A.; Cristofanilli, M.; Giles, F.J. Comparison of Tumor Mutational Burden (TMB) across Tumor Tissue and Circulating Tumor DNA (CtDNA). JCO 2017, 35, e23028. [Google Scholar] [CrossRef]
- Fabrizio, D.; Lieber, D.; Malboeuf, C.; Silterra, J.; White, E.; Coyne, M.; Brennan, T.; Ma, J.; Kennedy, M.; Schleifman, E.; et al. Abstract 5706: A Blood-Based Next-Generation Sequencing Assay to Determine Tumor Mutational Burden (BTMB) Is Associated with Benefit to an Anti-PD-L1 Inhibitor, Atezolizumab. Cancer Res. 2018, 78, 5706. [Google Scholar] [CrossRef]
- Koeppel, F.; Blanchard, S.; Jovelet, C.; Genin, B.; Marcaillou, C.; Martin, E.; Rouleau, E.; Solary, E.; Soria, J.-C.; André, F.; et al. Whole Exome Sequencing for Determination of Tumor Mutation Load in Liquid Biopsy from Advanced Cancer Patients. PLoS ONE 2017, 12, e0188174. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-Based Tumor Mutational Burden as a Predictor of Clinical Benefit in Non-Small-Cell Lung Cancer Patients Treated with Atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Poehlein, C.H.; Marton, M.J.; Laterza, O.F.; Levitan, D. Measuring Tumor Mutational Burden (TMB) in Plasma from MCRPC Patients Using Two Commercial NGS Assays. Sci. Rep. 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or Without Tremelimumab vs Standard Chemotherapy in First-Line Treatment of Metastatic Non–Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Cai, S.; Han, M.; Dong, H.; Zhao, J.; Zhu, B.; Wang, S.; Zhuo, M.; Sun, J.; et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients with Non–Small Cell Lung Cancer with Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol. 2019, 5, 696. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Socinski, M.; Velcheti, V.; Mekhail, T.; Chae, Y.K.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; Dakhil, C.S.; Stella, P.; Shen, V.; et al. Final Efficacy Results from B-F1RST, a Prospective Phase II Trial Evaluating Blood-Based Tumour Mutational Burden (BTMB) as a Predictive Biomarker for Atezolizumab (Atezo) in 1L Non-Small Cell Lung Cancer (NSCLC). Ann. Oncol. 2019, 30, v919–v920. [Google Scholar] [CrossRef]
- Merino, D.M.; McShane, L.; Butler, M.; Funari, V.A.; Hellmann, M.D.; Chaudhary, R.; Chen, S.-J.; Chen, W.S.; Conroy, J.M.; Fabrizio, D.; et al. TMB Standardization by Alignment to Reference Standards: Phase II of the Friends of Cancer Research TMB Harmonization Project. JCO 2019, 37, 2624. [Google Scholar] [CrossRef]
- Bravaccini, S.; Bronte, G.; Ulivi, P. TMB in NSCLC: A Broken Dream? IJMS 2021, 22, 6536. [Google Scholar] [CrossRef]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef]
- Constâncio, V.; Nunes, S.P.; Henrique, R.; Jerónimo, C. DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types. Cells 2020, 9, 624. [Google Scholar] [CrossRef]
- Ntzifa, A.; Londra, D.; Rampias, T.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. DNA Methylation Analysis in Plasma Cell-Free DNA and Paired CTCs of NSCLC Patients before and after Osimertinib Treatment. Cancers 2021, 13, 5974. [Google Scholar] [CrossRef]
- Janke, F.; Angeles, A.K.; Riediger, A.L.; Bauer, S.; Reck, M.; Stenzinger, A.; Schneider, M.A.; Muley, T.; Thomas, M.; Christopoulos, P.; et al. Longitudinal Monitoring of Cell-Free DNA Methylation in ALK-Positive Non-Small Cell Lung Cancer Patients. Clin. Epigenet. 2022, 14, 163. [Google Scholar] [CrossRef]
- Lou, J.; Ben, S.; Yang, G.; Liang, X.; Wang, X.; Ni, S.; Han, B. Quantification of Rare Circulating Tumor Cells in Non-Small Cell Lung Cancer by Ligand-Targeted PCR. PLoS ONE 2013, 8, e80458. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhu, D.; Yang, Y.; Yuan, G.; Xie, H.; Shen, R. The Application of Nano-Enrichment in CTC Detection and the Clinical Significance of CTCs in Non-Small Cell Lung Cancer (NSCLC) Treatment. PLoS ONE 2019, 14, e0219129. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-R.; Zhu, L.-Y.; Qian, K.; Feng, Y.-G.; Zhou, J.-H.; Wang, R.-W.; Bai, L.; Deng, B.; Liang, N.; Tan, Q.-Y. Circulating Tumor Cells in Early Stage Lung Adenocarcinoma: A Case Series Report and Literature Review. Oncotarget 2017, 8, 23130–23141. [Google Scholar] [CrossRef] [PubMed]
- Kapeleris, J.; Kulasinghe, A.; Warkiani, M.E.; Vela, I.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front. Oncol. 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Xu, Y.; Zhao, J.; Chen, M.; Xing, J.; Zhong, W.; Wang, M. Prognostic Significance of Circulating Tumor Cells in Non-Small Cell Lung Cancer Patients Undergoing Chemotherapy. Oncotarget 2017, 8, 86615–86624. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, S.; Sequist, L.V.; Nagrath, S.; Ulkus, L.; Brannigan, B.; Collura, C.V.; Inserra, E.; Diederichs, S.; Iafrate, A.J.; Bell, D.W.; et al. Detection of Mutations in EGFR in Circulating Lung-Cancer Cells. N. Engl. J. Med. 2008, 359, 366–377. [Google Scholar] [CrossRef]
- Yanagita, M.; Redig, A.J.; Paweletz, C.P.; Dahlberg, S.E.; O’Connell, A.; Feeney, N.; Taibi, M.; Boucher, D.; Oxnard, G.R.; Johnson, B.E.; et al. A Prospective Evaluation of Circulating Tumor Cells and Cell-Free DNA in EGFR -Mutant Non–Small Cell Lung Cancer Patients Treated with Erlotinib on a Phase II Trial. Clin. Cancer Res. 2016, 22, 6010–6020. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Lim, T.H.; Lim, T.K.; Tan, D.S.-W.; Chua, Y.W.; Ang, M.K.; Pang, B.; Lim, C.T.; Takano, A.; Lim, A.S.-T.; et al. Concordance of Anaplastic Lymphoma Kinase (ALK) Gene Rearrangements between Circulating Tumor Cells and Tumor in Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 23251–23262. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.C.-H.; Wang, J.; Wang, Y.; Behren, A.; Cebon, J.; Trau, M. Characterising the Phenotypic Evolution of Circulating Tumour Cells during Treatment. Nat. Commun. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Adam, J.; Barthélémy, A.; Oulhen, M.; Auger, N.; Valent, A.; Borget, I.; Planchard, D.; Taylor, M.; André, F.; et al. Detection of Circulating Tumor Cells Harboring a Unique ALK Rearrangement in ALK-Positive Non–Small-Cell Lung Cancer. JCO 2013, 31, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Szafer-Glusman, E.; Hofman, V.; Long-Mira, E.; Suttmann, R.; Darbonne, W.; Butori, C.; Lalvée, S.; Fayada, J.; Selva, E.; et al. Expression of MET in Circulating Tumor Cells Correlates with Expression in Tumor Tissue from Advanced-Stage Lung Cancer Patients. Oncotarget 2017, 8, 26112–26121. [Google Scholar] [CrossRef]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular Analysis of Circulating Tumor Cells Identifies Distinct Copy-Number Profiles in Patients with Chemosensitive and Chemorefractory Small-Cell Lung Cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef]
- Schott, D.S.; Pizon, M.; Pachmann, U.; Pachmann, K. Sensitive Detection of PD-L1 Expression on Circulating Epithelial Tumor Cells (CETCs) Could Be a Potential Biomarker to Select Patients for Treatment with PD-1/PD-L1 Inhibitors in Early and Metastatic Solid Tumors. Oncotarget 2017, 8, 72755–72772. [Google Scholar] [CrossRef]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 Expression in Circulating Tumor Cells of Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef]
- Darga, E.P.; Dolce, E.M.; Fang, F.; Kidwell, K.M.; Gersch, C.L.; Kregel, S.; Thomas, D.G.; Gill, A.; Brown, M.E.; Gross, S.; et al. PD-L1 Expression on Circulating Tumor Cells and Platelets in Patients with Metastatic Breast Cancer. PLoS ONE 2021, 16, e0260124. [Google Scholar] [CrossRef]
- Boffa, D.J.; Graf, R.P.; Salazar, M.C.; Hoag, J.; Lu, D.; Krupa, R.; Louw, J.; Dugan, L.; Wang, Y.; Landers, M.; et al. Cellular Expression of PD-L1 in the Peripheral Blood of Lung Cancer Patients Is Associated with Worse Survival. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1139–1145. [Google Scholar] [CrossRef]
- Sinoquet, L.; Jacot, W.; Gauthier, L.; Pouderoux, S.; Viala, M.; Cayrefourcq, L.; Quantin, X.; Alix-Panabières, C. Programmed Cell Death Ligand 1-Expressing Circulating Tumor Cells: A New Prognostic Biomarker in Non-Small Cell Lung Cancer. Clin. Chem. 2021, 67, 1503–1512. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 Positive Circulating Tumor Cells in Non-Small Cell Lung Cancer Patients Treated with the PD-1 Inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Liu, Q.; Wang, C.; Yao, R.; Wang, Y. CTC Immune Escape Mediated by PD-L1. Med. Hypotheses 2016, 93, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.-H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in Circulating Tumor Cells and White Blood Cells from Patients with Advanced Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.-L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.-A.; Böttcher, L.-M.; et al. Determination of PD-L1 Expression in Circulating Tumor Cells of NSCLC Patients and Correlation with Response to PD-1/PD-L1 Inhibitors. Cancers 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Yagi, S.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Higuchi, M.; Kanbara, H.; Nakanishi, M.; et al. Heterogeneous Expression of Programmed Death Receptor-Ligand 1 on Circulating Tumor Cells in Patients With Lung Cancer. Clin. Lung Cancer 2019, 20, 270–277.e1. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Todd, N.W.; Xing, L.; Xie, Y.; Zhang, H.; Liu, Z.; Fang, H.; Zhang, J.; Katz, R.L.; Jiang, F. Early Detection of Lung Adenocarcinoma in Sputum by a Panel of MicroRNA Markers. Int. J. Cancer 2010, 127, 2870–2878. [Google Scholar] [CrossRef]
- Xie, Y.; Todd, N.W.; Liu, Z.; Zhan, M.; Fang, H.; Peng, H.; Alattar, M.; Deepak, J.; Stass, S.A.; Jiang, F. Altered MiRNA Expression in Sputum for Diagnosis of Non-Small Cell Lung Cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Todd, N.W.; Yu, L.; Fang, H.; Jiang, F. Early Detection of Squamous Cell Lung Cancer in Sputum by a Panel of MicroRNA Markers. Mod. Pathol. 2010, 23, 1157–1164. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Z.; Todd, N.W.; Zhang, H.; Liao, J.; Yu, L.; Guarnera, M.A.; Li, R.; Cai, L.; Zhan, M.; et al. Diagnosis of Lung Cancer in Individuals with Solitary Pulmonary Nodules by Plasma MicroRNA Biomarkers. BMC Cancer 2011, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Yanaihara, N.; Caplen, N.; Bowman, E.; Seike, M.; Kumamoto, K.; Yi, M.; Stephens, R.M.; Okamoto, A.; Yokota, J.; Tanaka, T.; et al. Unique MicroRNA Molecular Profiles in Lung Cancer Diagnosis and Prognosis. Cancer Cell 2006, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Kannisto, E.; Knudsen, S.; Yendamuri, S. Evaluation of MicroRNA Expression Profiles That May Predict Recurrence of Localized Stage I Non–Small Cell Lung Cancer after Surgical Resection. Cancer Res. 2010, 70, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lebanony, D.; Benjamin, H.; Gilad, S.; Ezagouri, M.; Dov, A.; Ashkenazi, K.; Gefen, N.; Izraeli, S.; Rechavi, G.; Pass, H.; et al. Diagnostic Assay Based on Hsa-MiR-205 Expression Distinguishes Squamous from Nonsquamous Non–Small-Cell Lung Carcinoma. JCO 2009, 27, 2030–2037. [Google Scholar] [CrossRef]
- Liu, X.-G.; Zhu, W.-Y.; Huang, Y.-Y.; Ma, L.-N.; Zhou, S.-Q.; Wang, Y.-K.; Zeng, F.; Zhou, J.-H.; Zhang, Y.-K. High Expression of Serum MiR-21 and Tumor MiR-200c Associated with Poor Prognosis in Patients with Lung Cancer. Med. Oncol. 2012, 29, 618–626. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, X.; Zhao, Y.; Tian, T.; Jin, G.; Shu, Y.; Chen, Y.; Xu, L.; Zen, K.; Zhang, C.; et al. Serum MicroRNA Signatures Identified in a Genome-Wide Serum MicroRNA Expression Profiling Predict Survival of Non–Small-Cell Lung Cancer. JCO 2010, 28, 1721–1726. [Google Scholar] [CrossRef]
- Han, F.; He, J.; Li, F.; Yang, J.; Wei, J.; Cho, W.C.; Liu, X. Emerging Roles of MicroRNAs in EGFR-Targeted Therapies for Lung Cancer. BioMed. Res. Int. 2015, 2015, 672759. [Google Scholar] [CrossRef]
- Pasini, L.; Ulivi, P. Liquid Biopsy for the Detection of Resistance Mechanisms in NSCLC: Comparison of Different Blood Biomarkers. JCM 2019, 8, 998. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Xu, F.; Kong, J.; Yu, H.; Qian, B. Circulating MicroRNAs in Relation to EGFR Status and Survival of Lung Adenocarcinoma in Female Non-Smokers. PLoS ONE 2013, 8, e81408. [Google Scholar] [CrossRef]
- Li, B.; Ren, S.; Li, X.; Wang, Y.; Garfield, D.; Zhou, S.; Chen, X.; Su, C.; Chen, M.; Kuang, P.; et al. MiR-21 Overexpression Is Associated with Acquired Resistance of EGFR-TKI in Non-Small Cell Lung Cancer. Lung Cancer 2014, 83, 146–153. [Google Scholar] [CrossRef]
- Jasinski-Bergner, S.; Mandelboim, O.; Seliger, B. The Role of MicroRNAs in the Control of Innate Immune Response in Cancer. JNCI J. Natl. Cancer Inst. 2014, 106, dju257. [Google Scholar] [CrossRef] [PubMed]
- Augustus, E.; Zwaenepoel, K.; Siozopoulou, V.; Raskin, J.; Jordaens, S.; Baggerman, G.; Sorber, L.; Roeyen, G.; Peeters, M.; Pauwels, P. Prognostic and Predictive Biomarkers in Non-Small Cell Lung Cancer Patients on Immunotherapy—The Role of Liquid Biopsy in Unraveling the Puzzle. Cancers 2021, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, N.; Carrión-Navarro, J.; Esteban-Rubio, S.; Lázaro-Ibáñez, E.; Peris-Celda, M.; Alonso, M.M.; Guzmán-De-Villoria, J.; Fernández-Carballal, C.; de Mendivil, A.O.; García-Duque, S.; et al. DNA Sequences within Glioma-Derived Extracellular Vesicles Can Cross the Intact Blood-Brain Barrier and Be Detected in Peripheral Blood of Patients. Oncotarget 2017, 8, 1416–1428. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal MiRNA as Potential Diagnostic Biomarkers for Early-Stage Non–Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Garcia, V.; Zaballos, A.; Provencio, M.; Lombardia, L.; Almonacid, L.; Garcia, J.M.; Dominguez, G.; Pena, C.; Diaz, R.; et al. Vesicle-Related MicroRNAs in Plasma of Nonsmall Cell Lung Cancer Patients and Correlation with Survival. Eur. Respir. J. 2011, 37, 617–623. [Google Scholar] [CrossRef]
- Möhrmann, L.; Huang, H.J.; Hong, D.S.; Tsimberidou, A.M.; Fu, S.; Piha-Paul, S.A.; Subbiah, V.; Karp, D.D.; Naing, A.; Krug, A.; et al. Liquid Biopsies Using Plasma Exosomal Nucleic Acids and Plasma Cell-Free DNA Compared with Clinical Outcomes of Patients with Advanced Cancers. Clin. Cancer Res. 2018, 24, 181–188. [Google Scholar] [CrossRef]
- Castellanos-Rizaldos, E.; Grimm, D.G.; Tadigotla, V.; Hurley, J.; Healy, J.; Neal, P.L.; Sher, M.; Venkatesan, R.; Karlovich, C.; Raponi, M.; et al. Exosome-Based Detection of EGFR T790M in Plasma from Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 2944–2950. [Google Scholar] [CrossRef]
- Cazzoli, R.; Buttitta, F.; Di Nicola, M.; Malatesta, S.; Marchetti, A.; Rom, W.N.; Pass, H.I. MicroRNAs Derived from Circulating Exosomes as Noninvasive Biomarkers for Screening and Diagnosing Lung Cancer. J. Thorac. Oncol. 2013, 8, 1156–1162. [Google Scholar] [CrossRef]
- Giallombardo, M.; Jorge Chacartegui, J.; Reclusa, P.; Van Meerbeeck, J.P.; Alessandro, R.; Peeters, M.; Pauwels, P.; Rolfo, C.D. Follow up Analysis by Exosomal MiRNAs in EGFR Mutated Non-Small Cell Lung Cancer (NSCLC) Patients during Osimertinib (AZD9291) Treatment: A Potential Prognostic Biomarker Tool. JCO 2016, 34, e23035. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Gunasekaran, M.; Russo, A.; Cardona, A.F.; de Miguel Perez, D.; Lapidus, R.; Cooper, B.; Kaushal, S.; Adamo, V.; Rolfo, C. Exosomal PD-L1 Expression as Non-Invasive Biomarker for Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. J. Immunol. 2020, 204, 90–100. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical Significance of PD-L1 Expression in Serum-Derived Exosomes in NSCLC Patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shi, M.; Feng, J. Expression of PD-L1 in Plasma Exosomes of NSCLC Patients and Its Associations with PD-L1 Expression of Corresponding Tumour Tissues. Ann. Oncol. 2019, 30, v43. [Google Scholar] [CrossRef]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 MRNA Expression in Plasma-Derived Exosomes Is Associated with Response to Anti-PD-1 Antibodies in Melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef]
- Fleitas, T.; Martínez-Sales, V.; Vila, V.; Reganon, E.; Mesado, D.; Martín, M.; Gómez-Codina, J.; Montalar, J.; Reynés, G. Circulating Endothelial Cells and Microparticles as Prognostic Markers in Advanced Non-Small Cell Lung Cancer. PLoS ONE 2012, 7, e47365. [Google Scholar] [CrossRef]
- Menck, K.; Bleckmann, A.; Wachter, A.; Hennies, B.; Ries, L.; Schulz, M.; Balkenhol, M.; Pukrop, T.; Schatlo, B.; Rost, U.; et al. Characterisation of Tumour-Derived Microvesicles in Cancer Patients’ Blood and Correlation with Clinical Outcome. J. Extracell. Vesicles 2017, 6, 1340745. [Google Scholar] [CrossRef]
- Krug, A.K.; Enderle, D.; Karlovich, C.; Priewasser, T.; Bentink, S.; Spiel, A.; Brinkmann, K.; Emenegger, J.; Grimm, D.G.; Castellanos-Rizaldos, E.; et al. Improved EGFR Mutation Detection Using Combined Exosomal RNA and Circulating Tumor DNA in NSCLC Patient Plasma. Ann. Oncol. 2018, 29, 700–706. [Google Scholar] [CrossRef]
- Hur, J.Y.; Kim, H.J.; Lee, J.S.; Choi, C.-M.; Lee, J.C.; Jung, M.K.; Pack, C.G.; Lee, K.Y. Extracellular Vesicle-Derived DNA for Performing EGFR Genotyping of NSCLC Patients. Mol. Cancer 2018, 17, 15. [Google Scholar] [CrossRef]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and Noncoding Landscape of Extracellular RNA Released by Human Glioma Stem Cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef]
- Kwok, H.-H.; Ning, Z.; Chong, P.; Wan, T.; Ng, M.; Ho, G.; Ip, M.; Lam, D. Transfer of Extracellular Vesicle-Associated-RNAs Induces Drug Resistance in ALK-Translocated Lung Adenocarcinoma. Cancers 2019, 11, 104. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular Transfer of the Oncogenic Receptor EGFRvIII by Microvesicles Derived from Tumour Cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating Genomic Features for Non-Invasive Early Lung Cancer Detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct Detection of Early-Stage Cancers Using Circulating Tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers with a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Killock, D. CancerSEEK and Destroy—A Blood Test for Early Cancer Detection. Nat. Rev. Clin. Oncol. 2018, 15, 133. [Google Scholar] [CrossRef]
- Beer, T.M. Novel Blood-Based Early Cancer Detection: Diagnostics in Development. Am. J. Manag. Care 2020, 26, S292–S299. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of Blood Testing Combined with PET-CT to Screen for Cancer and Guide Intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Pantel, K.; Denève, E.; Nocca, D.; Coffy, A.; Vendrell, J.-P.; Maudelonde, T.; Riethdorf, S.; Alix-Panabières, C. Circulating Epithelial Cells in Patients with Benign Colon Diseases. Clin. Chem. 2012, 58, 936–940. [Google Scholar] [CrossRef]
- Bobek, V.; Kolostova, K.; Kucera, E. Circulating Endometrial Cells in Peripheral Blood. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 267–274. [Google Scholar] [CrossRef]
- Kuessel, L.; Kasimir-Bauer, S.; Zeillinger, R.; Pateisky, P.; Ott, J.; Zeisler, H.; Birdir, C. Detection of Circulating Trophoblast Particles in Maternal Blood Using Density Gradient Centrifugation in Preeclampsia and in Normotensive Pregnancies. Hypertens. Pregnancy 2016, 35, 323–329. [Google Scholar] [CrossRef]
- He, Y.; Shi, J.; Shi, G.; Xu, X.; Liu, Q.; Liu, C.; Gao, Z.; Bai, J.; Shan, B. Using the New CellCollector to Capture Circulating Tumor Cells from Blood in Different Groups of Pulmonary Disease: A Cohort Study. Sci. Rep. 2017, 7, 9542. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Hofman, V.; Long-Mira, E.; Selva, E.; Vignaud, J.-M.; Padovani, B.; Mouroux, J.; Marquette, C.-H.; Hofman, P. “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef] [PubMed]
- Leroy, S.; Benzaquen, J.; Mazzetta, A.; Marchand-Adam, S.; Padovani, B.; Israel-Biet, D.; Pison, C.; Chanez, P.; Cadranel, J.; Mazières, J.; et al. Circulating Tumour Cells as a Potential Screening Tool for Lung Cancer (the AIR Study): Protocol of a Prospective Multicentre Cohort Study in France. BMJ Open 2017, 7, e018884. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Lou, F.; Ma, Y.; Li, J.; Yang, B.; Chen, W.; Ye, H.; Zhang, J.-B.; Zhao, M.-Y.; Wu, W.-J.; et al. Circulating Tumor DNA Detection in Lung Cancer Patients before and after Surgery. Sci. Rep. 2016, 6, 33519. [Google Scholar] [CrossRef]
- Skougaard, K.; Østrup, O.; Guldbrandsen, K.; Sørensen, B.; Meldgaard, P.; Saghir, Z.; Gørtz, P.; Lonsdale, M.N.; Frank, M.S.; Gerke, O.; et al. Surveillance With PET/CT and Liquid Biopsies of Stage I-III Lung Cancer Patients After Completion of Definitive Therapy: A Randomized Controlled Trial (SUPER). Clin. Lung Cancer 2020, 21, e61–e64. [Google Scholar] [CrossRef]
- Newman, A.M.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.W.; Modlin, L.A.; Liu, C.L.; Neal, J.W.; Wakelee, H.A.; Merritt, R.E.; et al. An Ultrasensitive Method for Quantitating Circulating Tumor DNA with Broad Patient Coverage. Nat. Med. 2014, 20, 548–554. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- The TRACERx Consortium; The PEACE Consortium; Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; et al. Phylogenetic CtDNA Analysis Depicts Early-Stage Lung Cancer Evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Moding, E.J.; Liu, Y.; Nabet, B.Y.; Chabon, J.J.; Chaudhuri, A.A.; Hui, A.B.; Bonilla, R.F.; Ko, R.B.; Yoo, C.H.; Gojenola, L.; et al. Circulating Tumor DNA Dynamics Predict Benefit from Consolidation Immunotherapy in Locally Advanced Non-Small-Cell Lung Cancer. Nat. Cancer 2020, 1, 176–183. [Google Scholar] [CrossRef]
- Herbreteau, G.; Vallée, A.; Charpentier, S.; Normanno, N.; Hofman, P.; Denis, M.G. Circulating Free Tumor DNA in Non-Small Cell Lung Cancer (NSCLC): Clinical Application and Future Perspectives. J. Thorac. Dis. 2019, 11, S113–S126. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Topaloglu, U.; Petty, W.J.; Pagni, M.; Foley, K.L.; Grant, S.C.; Robinson, M.; Bitting, R.L.; Thomas, A.; Alistar, A.T.; et al. Circulating Mutational Portrait of Cancer: Manifestation of Aggressive Clonal Events in Both Early and Late Stages. J. Hematol. Oncol. 2017, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Buttitta, F.; Felicioni, L.; Lorito, A.D.; Cortellini, A.; Irtelli, L.; Brocco, D.; Marino, P.D.; Traisci, D.; D’Ostilio, N.; Paolo, A.D.; et al. Early Prediction of Resistance to Tyrosine Kinase Inhibitors by Plasma Monitoring of EGFR Mutations in NSCLC: A New Algorithm for Patient Selection and Personalized Treatment. Oncotarget 2020, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Taus, Á.; Camacho, L.; Rocha, P.; Hardy-Werbin, M.; Pijuan, L.; Piquer, G.; López, E.; Dalmases, A.; Longarón, R.; Clavé, S.; et al. Dynamics of EGFR Mutation Load in Plasma for Prediction of Treatment Response and Disease Progression in Patients With EGFR-Mutant Lung Adenocarcinoma. Clin. Lung Cancer 2018, 19, 387–394.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Imamura, F.; Cheng, Y.; Okamoto, I.; Cho, B.C.; Lin, M.C.; Majem, M.; Gautschi, O.; Gray, J.E.; Boyer, M.J.; et al. Early Clearance of Plasma EGFR Mutations as a Predictor of Response to Osimertinib and Comparator EGFR-TKIs in the FLAURA Trial. JCO 2019, 37, 9020. [Google Scholar] [CrossRef]
- Mack, P.C.; Redman, M.W.; Moon, J.; Goldberg, S.B.; Herbst, R.S.; Melnick, M.A.C.; Walther, Z.; Hirsch, F.R.; Politi, K.A.; Kelly, K.; et al. Residual Circulating Tumor DNA (CtDNA) after Two Months of Therapy to Predict Progression-Free and Overall Survival in Patients Treated on S1403 with Afatinib +/− Cetuximab. JCO 2020, 38, 9532. [Google Scholar] [CrossRef]
- Gray, J.E.; Okamoto, I.; Sriuranpong, V.; Vansteenkiste, J.; Imamura, F.; Lee, J.S.; Pang, Y.-K.; Cobo, M.; Kasahara, K.; Cheng, Y.; et al. Tissue and Plasma EGFR Mutation Analysis in the FLAURA Trial: Osimertinib versus Comparator EGFR Tyrosine Kinase Inhibitor as First-Line Treatment in Patients with EGFR-Mutated Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6644–6652. [Google Scholar] [CrossRef] [PubMed]
- Vrankar, M.; Unk, M. Immune RECIST Criteria and Symptomatic Pseudoprogression in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy. Radiol. Oncol. 2018, 52, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Jones, G.; Beeler, J.F.; Plagnol, V.; Morris, C.; Mourlanette, J.; Delaunay, M.; Keller, L.; Rouquette, I.; Favre, G.; et al. Targeted Sequencing of Plasma Cell-Free DNA to Predict Response to PD1 Inhibitors in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2019, 137, 1–6. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M.; et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized Circulating Tumor DNA Analysis as a Predictive Biomarker in Solid Tumor Patients Treated with Pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Forde, P.M.; White, J.R.; Niknafs, N.; Hruban, C.; Naidoo, J.; Marrone, K.; Sivakumar, I.K.A.; Bruhm, D.C.; Rosner, S.; et al. Dynamics of Tumor and Immune Responses during Immune Checkpoint Blockade in Non–Small Cell Lung Cancer. Cancer Res. 2019, 79, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Rolfo, C.D.; Oxnard, G.R.; Gray, J.E.; Sholl, L.M.; Gandara, D.R. Strategies for the Successful Implementation of Plasma-Based NSCLC Genotyping in Clinical Practice. Nat. Rev. Clin. Oncol. 2021, 18, 56–62. [Google Scholar] [CrossRef]
- Cho, M.-S.; Park, C.H.; Lee, S.; Park, H.S. Clinicopathological Parameters for Circulating Tumor DNA Shedding in Surgically Resected Non-Small Cell Lung Cancer with EGFR or KRAS Mutation. PLoS ONE 2020, 15, e0230622. [Google Scholar] [CrossRef]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.-M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Wang, J.; Zhou, S.; Wang, C.L.; Ye, M.Z.; Wang, X.Y.; Song, Y.; Wang, Y.Q.; Zhang, L.T.; et al. Biological Background of the Genomic Variations of Cf-DNA in Healthy Individuals. Ann. Oncol. 2019, 30, 464–470. [Google Scholar] [CrossRef]
- Bauml, J.; Levy, B. Clonal Hematopoiesis: A New Layer in the Liquid Biopsy Story in Lung Cancer. Clin. Cancer Res. 2018, 24, 4352–4354. [Google Scholar] [CrossRef]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-Intensity Sequencing Reveals the Sources of Plasma Circulating Cell-Free DNA Variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef]
- Chan, H.T.; Nagayama, S.; Chin, Y.M.; Otaki, M.; Hayashi, R.; Kiyotani, K.; Fukunaga, Y.; Ueno, M.; Nakamura, Y.; Low, S. Clinical Significance of Clonal Hematopoiesis in the Interpretation of Blood Liquid Biopsy. Mol. Oncol. 2020, 14, 1719–1730. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Xie, H.; Urrutia, R.; Mahipal, A. The Promise of Circulating Tumor DNA (CtDNA) in the Management of Early-Stage Colon Cancer: A Critical Review. Cancers 2020, 12, 2808. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The Prevalence of EGFR Mutation in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Crosbie, P.A.J.; Shah, R.; Krysiak, P.; Zhou, C.; Morris, K.; Tugwood, J.; Booton, R.; Blackhall, F.; Dive, C. Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. J. Thorac. Oncol. 2016, 11, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.P.; Husereau, D.; Leighl, N.B.; Melosky, B.; Nam, J. Health and Budget Impact of Liquid-Biopsy-Based Comprehensive Genomic Profile (CGP) Testing in Tissue-Limited Advanced Non-Small Cell Lung Cancer (ANSCLC) Patients. Curr. Oncol. 2021, 28, 5278–5294. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).