Simple Summary
During the past decade, liquid biopsy-related publications have increased exponentially, demonstrating the great potential of screening body fluids for diagnosis, monitoring, and prediction of therapy response in cancer patients. Next to well-established, cancer-associated analytes in the bloodstream, such as circulating tumor cells and cell-free DNA, an increasing number of studies have highlighted extracellular vesicles (EVs) as a promising source of novel tumor biomarkers. In this review, we discuss the advantages, limitations, and challenges of using EVs as cancer biomarkers, with a special focus on solid tumors.
Abstract
Extracellular vesicles (EVs) are secreted by all living cells and are ubiquitous in every human body fluid. They are quite heterogeneous with regard to biogenesis, size, and composition, yet always reflect their parental cells with their cell-of-origin specific cargo loading. Since numerous studies have demonstrated that EV-associated proteins, nucleic acids, lipids, and metabolites can represent malignant phenotypes in cancer patients, EVs are increasingly being discussed as valuable carriers of cancer biomarkers in liquid biopsy samples. However, the lack of standardized and clinically feasible protocols for EV purification and characterization still limits the applicability of EV-based cancer biomarker analysis. This review first provides an overview of current EV isolation and characterization techniques that can be used to exploit patient-derived body fluids for biomarker quantification assays. Secondly, it outlines promising tumor-specific EV biomarkers relevant for cancer diagnosis, disease monitoring, and the prediction of cancer progression and therapy resistance. Finally, we summarize the advantages and current limitations of using EVs in liquid biopsy with a prospective view on strategies for the ongoing clinical implementation of EV-based biomarker screenings.
1. Introduction
Cells in the human body can exchange information through a variety of mechanisms. One that stands out is cell communication via extracellular vesicles (EVs) because they transport proteins, metabolites, lipids, and nucleic acids. All living cells secrete EVs, that promote not only physiological but also pathological processes, such as cancer. As a consequence, EV-based liquid biopsy can be used to gain information about tumor progression and the tumor itself. Next to other blood-derived analytes, such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), cell-free RNA, microRNA, and long non-coding RNA (lncRNA), EVs have received more attention in recent years as a promising source of cancer biomarkers in liquid biopsies.
EVs can be categorized regarding either their cellular origin or size. EVs derived from the intracellular endosomal system are classically denoted as “exosomes”, whereas vesicles that are directly shed from the plasma membrane are known as “ectosomes” or “microvesicles”. Since this distinction was not always clear, EVs with a size up to 200 nm are nowadays simply called “small EVs” (sEVs), and vesicles larger than 200 nm are “large EVs” (lEVs) [1]. Recently, another type of nanoparticle has been described, with a size below 50 nm, the so-called exomeres. These particles are non-membranous, and their function is still unclear [2].
The shedding of lEVs from the plasma membrane is triggered by an increase in intracellular calcium (Ca2+) [3]. High Ca2+ levels lead to transient and local activation of the cysteine protease calpain, which cleaves a panel of cytoskeletal proteins and thus destabilizes the connections of the plasma membrane to the underlying cytoskeleton [4]. Combined with hydrostatic pressure, this results in the outward budding of the membrane and the formation of small blebs [5,6,7]. Proteins of the endosomal sorting complex required for transport (ESCRT) then cut off these blebs and release them as lEVs into the extracellular environment [8] (Figure 1). Furthermore, rising intracellular Ca2+ levels lead to an inactivation of flippases and concordant activation of floppases and scramblases. Under normal conditions, flippases keep the phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) in the inner leaflet of the plasma membrane, thereby controlling membrane asymmetry [5]. Their Ca2+-mediated inactivation consequently facilitates PS and PE externalization, and thus, their expression on the surface of blebbing lEVs.
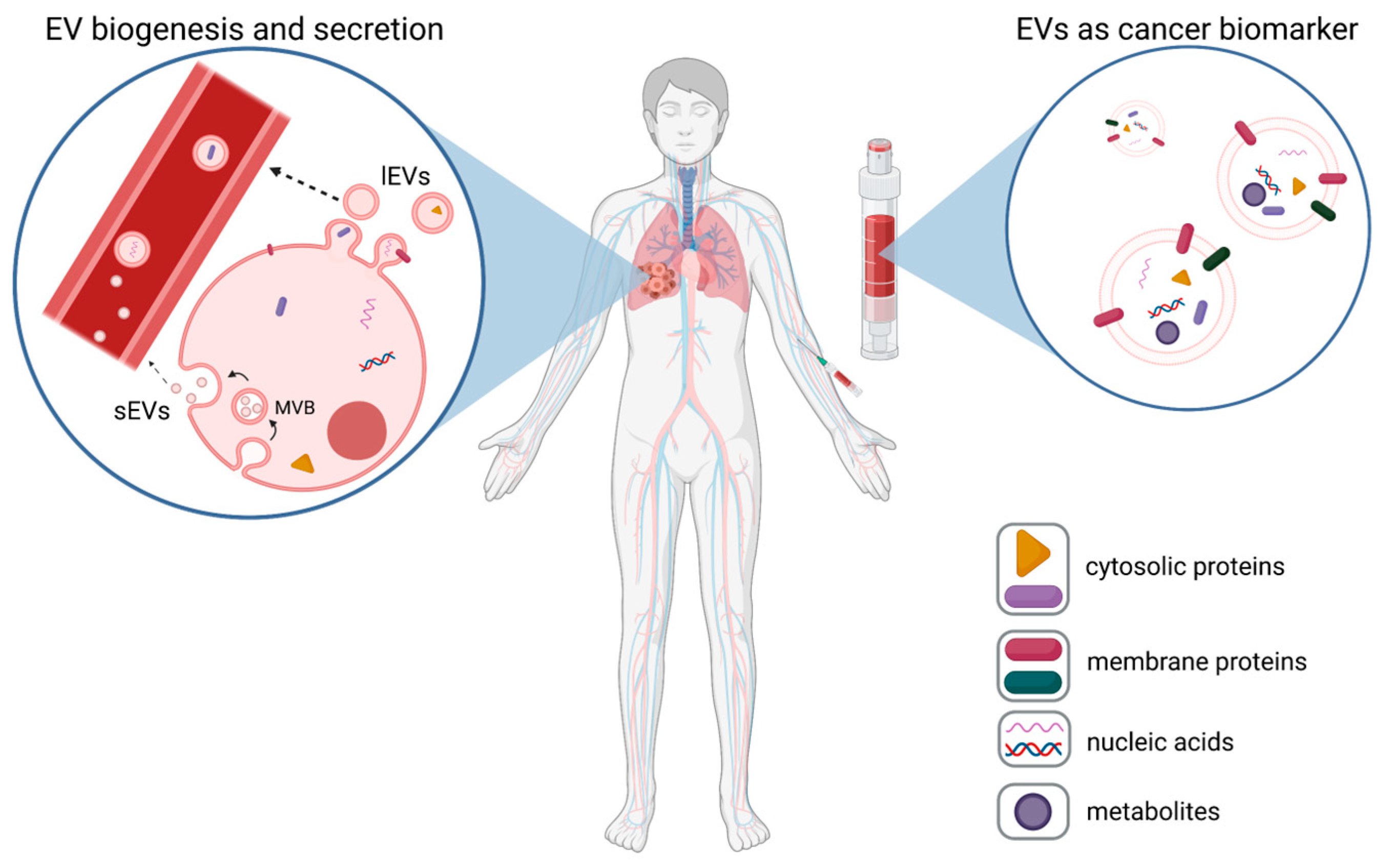
Figure 1.
EVs as cancer biomarkers. Tumor cells release high numbers of plasma membrane-derived large EVs (lEVs) and endosomal-derived small EVs (sEVs)), which are released from multi-vesicular bodies (MVBs) into body fluids, such as blood (left panel). These tumor-EVs can be assessed via liquid biopsies (right panel), and based on the expression of tumor-specific EV proteins, nucleic acids, or metabolites, they can be used as biomarkers for cancer (exemplarily shown for lung cancer).
In contrast to lEVs, sEVs are generated within late endosomes. Intraluminal vesicles (ILVs) are formed by the invagination of late endosomal membranes, which leads to the formation of multivesicular bodies (MVBs) [9]. Comparable to lEV biogenesis, this step involves ESCRT proteins, which are recruited to late endosomal membranes via the ESCRT-binding ALG-2-Interacting protein X (ALIX). In turn, ALIX recruitment was shown to be mediated by the adaptor protein syntenin, which binds to the C-terminus of syndecan heparan sulfate proteoglycans upon their endocytic uptake [10,11]. However, there is also evidence for an ESCRT-independent formation of sEVs in which tetraspanins such as CD9 or CD63 are involved [12]. As a final step, sEVs are released into the extracellular environment upon fusion of the MVBs with the plasma membrane (Figure 1).
Independent of their mechanism of formation, EVs contain components of their parental cells that can be passed on to the recipient cell by various uptake mechanisms. Described mechanisms are clathrin-dependent and -independent endocytosis, macropinocytosis, phagocytosis, and membrane fusion [13].
2. EVs in Cancer
As do healthy cells, tumor cells secrete EVs, and there is growing evidence that such tumor-derived EVs contribute to metastasis, angiogenesis, and chemotherapy resistance [14]. They can affect neighboring cells within the tumor microenvironment, but also distant cells and organs, where they contribute to the formation of pre-metastatic niches [15]. Some proteins, which are overexpressed in tumors, have been found to be involved in EV biogenesis. Examples are the tyrosine kinase SRC, the adapter protein syntenin, and mutated adenomatous polyposis coli (APC) [10,16,17]. These observations are consistent with increased EV secretion rates in tumor cells compared to healthy cells [18]. EVs released by tumor cells have been shown to promote angiogenesis, repress responses of the immune system, and stimulate remodeling of the extracellular matrix (ECM), thus creating a tumor-supportive microenvironment [19,20]. In addition, tumor-derived EVs can transfer drug resistance, and hence, represent promising targets for future therapy approaches [21]. These pathological effects of tumor-derived EVs are mediated by their molecular cargo, which comprises tumor-specific proteins, microRNAs, or mRNAs as well as metabolites [22,23].
Until now, CTCs and cfDNA have been the most comprehensively studied analytes in liquid biopsies for cancer biomarker detection. However, EVs also exhibit enormous diagnostic and prognostic potential in this field. In this review, we discuss the different methods for standardized EV isolation/characterization from peripheral blood, the most frequently used biofluid for liquid biopsy, and focus on the different approaches for using EVs as biomarkers for cancer.
3. EV Isolation and Purification from Biological Fluids
The isolation of EVs from body fluids is challenging as they are prone to contamination with non-EV proteins, lipoproteins, and high-density lipoproteins (HDL) [24]. Such contaminants interfere with the isolation of pure EVs for therapeutic, diagnostic, or prognostic use. A number of isolation methods have been described, which are summarized in Figure 2 and are discussed later in this chapter with regard to their potential applicability in clinical settings. The various EV isolation methods can be subdivided into different categories based on the physical/chemical properties exploited for isolation: centrifugation-based methods, size-based isolation, affinity-based isolation, precipitation, and recently developed microfluidic techniques. Each method has its advantages and disadvantages, and the choice of method largely depends on the intended application of the EVs [25,26]. For instance, for the quantification of established EV-based cancer biomarkers, a high-throughput isolation method with high yields but lower purity might be sufficient. On the other hand, a method providing EVs with high purity but lower yields might be preferable for identifying novel biomarkers found in EVs [27,28]. A promising approach to improve EV purity is to combine different isolation methods to achieve a contamination rate lower than that possible with one-step isolation procedures [29].
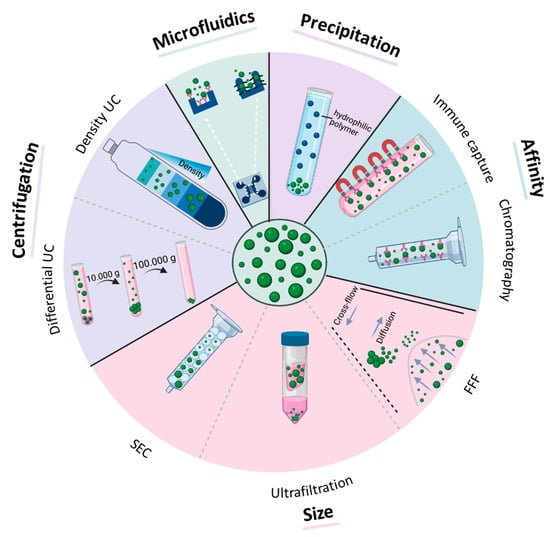
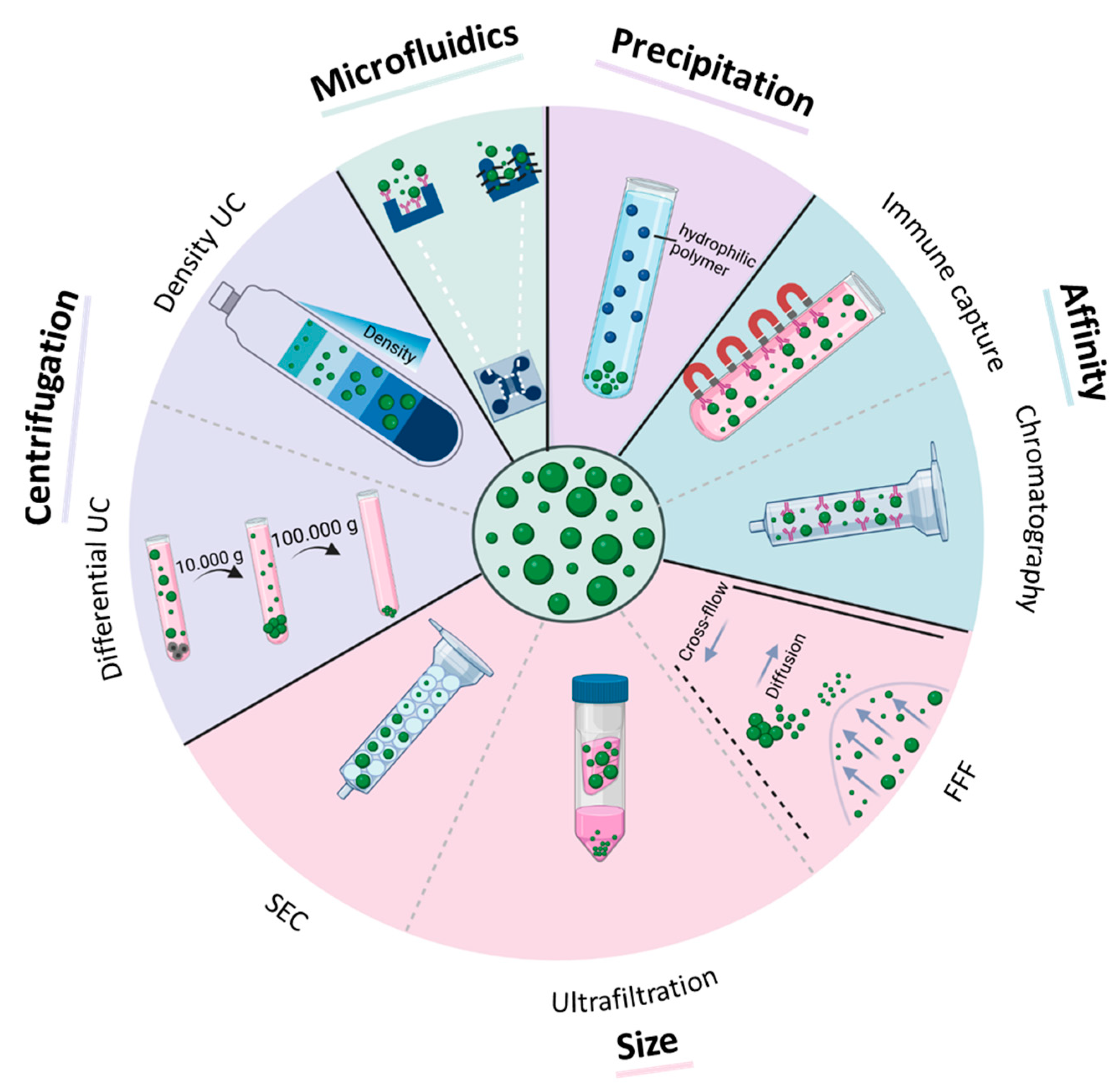
Figure 2.
Methods for EV isolation from biofluids. Purification methods are based on centrifugal force (differential and density ultracentrifugation (UC)), size (size-exclusion chromatography (SEC), ultrafiltration, field-flow fractionation (FFF)), affinity to certain antigens (immune capture, affinity chromatography), precipitation, and microfluidic techniques (size-based microfluidic, immune capture microfluidic).
3.1. Centrifugation-Based Methods
In principle, centrifugation-based methods use a centrifugal force to isolate EVs based on size and density. To isolate EVs from the blood via differential ultracentrifugation, the sample is spun in a sequence of increasing speeds. The protocol usually starts with a spin at 1200× g to pellet the majority of blood cells. The supernatant is then spun at 2000× g for pelleting cell debris, residual platelets, and very large EVs. Finally, two centrifugation steps are performed at 10,000–14,000× g for lEVs and at 100,000× g to pellet sEVs [23,30,31,32]. The quantity of the collected EVs is affected by the centrifugation speed and time [33]. Furthermore, the protocol must be modified according to the source of the biofluid [34]. Although differential ultracentrifugation is still considered to be the gold standard for EV isolation, it is time-consuming, can damage the EVs, and is prone to the co-isolation of protein contaminants [35,36,37]. The isolation of lEVs can be especially challenging due to the large number of platelets or urinary sediments in some biofluids. To reduce vesicle damage and the level of impurities, EVs can be loaded onto a density gradient (i.e., iodixanol or sucrose) prior to ultracentrifugation [35,36,37,38,39]. This results in a separation of EVs in specific fractions according to their buoyant density, while protein contaminants accumulate in different fractions. To further improve EV purity, it is recommended to additionally remove the sample from HDL particles, as they are in a similar density range as EVs. In summary, centrifugation-based methods give high EV yields but low purity and require a time- and labor-consuming isolation procedure, which is not ideal for routine clinical applications.
3.2. Size-Based Isolation
Size-exclusion chromatography (SEC) is a well-established technique used for macromolecule separation based on size. The mobile phase containing the biofluid is loaded onto a column packed with linked porous agarose beads representing the stationary phase [28]. While EVs do not fit into the pores and pass rapidly through the column, protein aggregates do enter the pores, and thus, take longer to elute [40]. Various studies have shown the superiority of SEC over ultracentrifugation [28,41]. As the properties of the EVs are only minimally affected, SEC maintains their biological functions [28]. Furthermore, as with density ultracentrifugation, SEC reduces contaminations with HDL better than conventional ultracentrifugation since HDL particles are smaller than EVs [28]. A major drawback of this method is that it cannot efficiently separate lEVs from sEVs. However, it enables the separation of EVs from small non-EV contaminants.
Filtration is another popular size-based technique for EV isolation, in particular for large-scale EV isolation from diluted samples, such as urine [28,42]. Ultrafiltration utilizes membranes with a defined molecular weight cutoff, usually between 10 and 100 kDa [28]. EVs are retained in the filter, while smaller protein aggregates or lipoparticles pass through. Filtration is faster and easier to handle than ultracentrifugation, and only small amounts of biofluid are required [43]. However, EVs isolated by ultrafiltration suffer from considerable amounts of protein contaminants, loss of EV membrane integrity, and morphological changes, as the EVs are deformed at the filter interface due to pulling forces [44,45]. To solve this problem, tangential flow filtration (TFF), a novel size-based ultrafiltration method, was established in 2018 [46]. In TFF, a stream of biofluid flows tangentially by moving across the membrane. Particles with a molecular weight below a membrane-specific cut-off point are removed, while larger molecules, such as EVs, remain in the system and are concentrated. This method is gentler than traditional ultrafiltration and can avoid filter clogging, thus leading to higher EV yields [46].
Another frequently used size-based EV isolation strategy is field-flow fractionation (FFF), which allows for EV isolation based on the diffusion coefficient [47]. Separation occurs in a thin flow channel, in which the laminar channel flow carries the sample through the channel. Perpendicular to this channel flow, a cross-flow is applied, creating a force field, which drives the particles in the direction of the channel bottom. Due to the diffusion coefficient, small particles diffuse back into the channel faster than large particles and are thereby eluted faster than large particles [47]. In combination with light scattering detectors, FFF can provide accurate information on the EV size, morphology, and aggregation state [28]. This gentle isolation procedure is a major advantage of FFF. However, only small quantities of biofluid can be processed, thereby limiting the application when larger volumes need to be processed [28].
3.3. Affinity-Based Techniques
These methods are based on the highly selective and specific interactions between proteins found on the EV membrane and the corresponding receptors, e.g., antibodies. The receptors are commonly immobilized on a solid medium, such as magnetic beads or chromatography columns [28]. Affinity-based EV isolation is easy to perform, allows for single-step purification even from diluted samples, and can enrich specific EV subsets from complex biofluids. For instance, melanoma-derived sEVs were already successfully captured from plasma using magnetic beads coated with a chondroitin sulfate proteoglycan 4 (CSPG4) antibody [48]. Furthermore, well-established methods, such as enzyme-linked immunosorbent assay (ELISA), can easily be used to quantify the isolated EVs from small amounts (e.g., 100 µL) of plasma, serum, or urine [49]. A major caveat of affinity-based isolation is that it cannot exploit intravesicular antigens. Furthermore, eluted EVs can lose some of their activity and can have different characteristics than those isolated based on size. In addition, the clinical applicability of this method is doubtful as the method is expensive and the EV yield is low [49].
3.4. Polymer Precipitation
Another way to isolate EVs is by polymer precipitation, in which a hydrophilic polymer/reagent is added to the biofluid [50]. The polymer is able to interact with the water surrounding the EVs, thereby causing less soluble components to precipitate from the solution. The sample can then be spun down in order to obtain a collection of EVs [40]. Although this method also creates a pellet similar to ultracentrifugation, no excessive centrifugal forces are necessary [51]. Furthermore, the method is rapid and results in high EV yields. To further minimize the expenses, lower-cost precipitation reagents, such as polyethylene glycol (PEG), can be used. Since this method provides easy three-step EV isolation comprising mixing, incubation, and centrifugation, polymer precipitation is becoming more and more popular. Nevertheless, EV purity can be low as the polymer precipitates not only EVs but also any water-soluble material, including lipoproteins and nucleic acids. In addition to the problem of co-isolation, the reagents added for precipitation are difficult to remove and therefore interfere with EV properties and potential downstream applications [51,52,53].
3.5. Microfluidic Technology
With this novel technology, EVs are captured within micro-sized channels either by specific surface markers (microfluidics-based immunoaffinity capture) or size [54]. Although microfluidic technologies, such as Exo Chip, enable the isolation of various types of EVs and minimize contamination by proteins, they require complicated photolithography fabrication and saturation. Generally, this method integrates both EV isolation and disease detection in one platform and allows for rapid EV isolation from small sample volumes on a single chip. Major advantages are that EVs maintain their morphology, and the cost, processing time, and consumption of reagents are reduced [49]. However, as this is a recently developed method for EV isolation, little is known about its suitability for clinical applications. Thus, further establishment and improvement of microfluidic-based EV isolation are required for a subsequent translation into the clinical routine.
4. EV Analysis Methods
Isolated EVs can be quantified and analyzed by different methods. These methods can be used to measure basic EV characteristics (number, size, and morphology) as well as for quantitative and qualitative analysis of EV cargo (proteomic, transcriptomic, and metabolic).
EVs are commonly characterized morphologically by transmission electron microscopy. The EV number and size can be quantified using light scattering techniques, such as nanoparticle tracking analysis (NTA), or by tunable resistive pulse sensing (TRPS) [55]. NTA is a technique combining laser light scattering microscopy with a charge-coupled device (CCD) camera. Based on the Brownian motion of the particles, the software can track the distance covered by the EVs in a given time and calculate their size with the Stokes–Einstein equation [56]. TRPS forces particles through a pore via pressure and voltage. Each particle causes a resistive pulse signal that is captured and measured [57]. Nevertheless, data from NTA and TRPS need to be considered cautiously, as parameters such as pore sizes and detection sensitivity influence the measurement when analyzing EV populations [58]. The detection with NTA-based analysis is influenced by two parameters: camera-level and detection threshold [59]. Higher camera levels lead to a brighter appearance of the particles, which results in increased detection of weak-scattering proteins. Additionally, an increased detection threshold consequently results in a higher number of detected particles [59]. For TRPS-based quantification, the sensitivity can be variable based on the nanopores used. The sensitivity with different nanopore setups is based on the size of the smallest detectable particle. As such, for EV samples with an unknown size distribution, it is more difficult to obtain the optimal size range to detect all particles [59]. A major advantage of TRPS measurement over the NTA methodology is the possibility to spike biological fluids with polystyrene beads of a known size and concentration to improve the accuracy of EV quantification with TRPS [60]. This method of calibration is not suitable for NTA analysis, as the methodology does not discriminate between EVs and the calibration beads [61].
The evaluation of EV-associated biomarker expression requires methods that specifically detect intravesicular or membrane-associated EV cargo. The expressions of protein-based biomarkers within or on the surface of EVs can be assessed using flow cytometry, ELISA, immunoblotting, or mass spectrometry. The latter two methods are more suitable for the identification of novel EV-based biomarkers than high-throughput screening approaches. While lEVs were already shown to be suitable for the validation of several known cancer biomarkers via flow cytometry [23], similar readouts concerning sEVs remain challenging due to their small size. Compared to conventional flow cytometry, which has a resolution limitation for particles < 300 nm, high-resolution and imaging flow cytometers have been demonstrated to be promising for sEV protein biomarker quantification and subtype characterization [62]. In addition, sEVs can be coupled to larger latex or magnetic beads, which enables bead-assisted flow cytometry [63]. According to the Minimum Information about a Flow Cytometry Experiment (MIFlowCyt) guidelines, relevant steps need to be observed for flow cytometry-based EV analysis, ranging from sample preparation and assay controls to technical guidelines [64]. Another method to quantify the amount of one or more specific proteins in EV samples is ELISA [65]. This method allows for the rapid analysis of specific proteins either generally found on EVs (such as tetraspanins) or tumor-specific antigens, although in a size-independent manner [66].
In addition to the proteomic cargo, EVs can also be analyzed for their metabolic profile using mass spectrometry. In the study performed by Buentzel et al., lEVs derived from the plasma of breast cancer patients or healthy controls were distinguishable based on their distinct metabolome [22]. Furthermore, the metabolomic profiling of lEVs also allowed for patient allocation to the distinct molecular breast cancer subtypes.
A number of approaches can be used to analyze the nucleic acid cargo found in EVs (e.g., DNA, RNA, and microRNA). Dye-assisted analysis of RNA content in EVs was described previously [67]. However, some dyes might also detect non-EV-associated RNA. Thus, real-time polymerase chain reaction (PCR) and next-generation sequencing (NGS) might be more promising analytical techniques for nucleic acid detection and quantification in EVs [68]. Particularly, EV-associated microRNAs are an emerging field of interest, as some microRNAs are tissue- and cell-specific and, as such, might represent the pathophysiological state of the cell of origin more precisely than the proteomic cargo [69,70,71].
To conclude, many different techniques for the quantitative and qualitative analysis of EV number, size, and cargo have been successfully applied for liquid biopsy analysis. However, their clinical implementation still requires standardized protocols that comply with the Minimal Information for Studies of Extracellular Vesicles (MISEV). These guidelines were designed to minimize the influence of different detection and analysis methods as well as confounding factors, including co-isolated non-vesicular contaminants, to pave the way for EV-associated cancer biomarkers in liquid biopsies.
5. EV-Associated Cancer Biomarkers—Translational Studies and Clinical Applications
Until now, multiple translational studies have addressed the question of whether EV levels in the blood of cancer patients or their molecular cargo are suitable for reflecting clinical parameters such as tumor burden, disease progression, or therapy response. Based on these publications, the following chapter focuses on circulating EV-based biomarkers for a variety of solid tumor entities, which have been identified and characterized in a clinical setting. We have not referenced the literature on the importance of EV-based liquid biopsies in hematological malignancies, but this was comprehensively reviewed by others [72].
5.1. Circulating EV Levels in Plasma
The currently available data indicate an increase in sEV abundance in blood samples from patients suffering from various cancer entities compared to healthy donors, as shown, e.g., for melanoma [73], glioblastoma multiforme (GBM) [74], prostate cancer (PC) [66], head and neck cancer (HNC) [75], breast cancer (BC) [76], and colorectal carcinoma (CRC) [77]. The latter two studies showed higher sEV plasma levels in cancer patients to be associated with therapy resistance, disease progression, and shortened overall survival. On the other hand, a study from 2017 found that patients with various types of solid tumors exhibited similar levels of lEVs in their plasma as healthy controls [23]. Different EV isolation protocols and a lack of standardization for EV classification procedures might be the cause for such variations.
The cellular origin of the increased number of EVs in blood from cancer patients is still under debate. Some evidence points to the primary tumor mass as the main driver for exaggerated EV secretion, seeing that markedly lower EV levels have been detected in post-operative plasma samples [74,75,78]. Hence, the measurement of plasma EV concentrations holds promise for monitoring tumor burden in cancer patients after surgery. Chemo- and radiotherapy have triggered EV secretion in patients suffering from different cancer entities [79,80,81]. This observed effect might help to improve the evaluation of therapy responses in the future. Measurements of total plasma EV protein might be a relevant alternative to the quantification of EV concentrations, as the former might reflect both aberrant EV numbers and deregulated EV cargo loading in cancer patients. Indeed, increased EV protein levels were detected in the blood of HNC patients with recurrence [82]. However, numerous other factors are known to influence EV numbers in the blood. Elevated plasma EV concentrations have been found in physiological conditions, such as physical exercise [83] and pregnancy [84], and in pathological processes, including ischemic stroke [85,86,87], Crohn’s disease [86], and diabetes [87]. The diagnostic potential of measuring all EVs present in the blood without further attribution to their cellular origin is therefore likely to be prone to misinterpretations. One way of circumventing these limitations is represented by analyzing the molecular cargo of plasma-derived EVs.
5.2. Molecular Cargo of Circulating EVs in Plasma
5.2.1. Tumor-Specific EV Biomarkers for Cancer Diagnostics
In the field of EV-based liquid biopsy proteins, DNA and RNA are the most comprehensively studied biomarkers for cancer detection, monitoring, and prediction. In addition, EV-associated metabolites are increasingly being discussed as promising biomarkers [22]. Tumor-derived EVs have been shown to reflect the molecular composition of the secreting cancer cells in several studies; by proteomic evaluation of tumor-derived EVs with concomitant hierarchical clustering, EV samples were segregated according to their cellular origin [88]. Similar observations have been made for the RNA and DNA content of EVs secreted by different cancer entities [89,90,91,92]. However, the expression rates of EV-associated cancer biomarkers within plasma-derived liquid biopsy samples do not necessarily reflect the molecular situation of the corresponding tumor because of the large amount of non-tumor EVs in the blood. Due to this, reports about increasing the plasma levels of EV-associated biomarkers in cancer patients alone cannot exclude non-tumor tissue as a driver for these observations [93,94].
A direct strategy for the identification of cancer-specific EV biomarkers is the molecular comparison of plasma EVs with matched tumor tissue biopsies. Based on this approach, Sun et al. demonstrated that vesicular copine-3 (CPNE3) expression in the plasma samples of CRC patients positively correlated with the protein signal from the corresponding tumor tissue and with overall survival [95]. However, the vesicular expression of cancer biomarkers does not always correlate with tumor tissue levels, as was shown for the NH2-terminally truncated P73 (ΔNP73) mRNA [96]. Here, the observed increase in ΔNP73 mRNA expression within plasma-derived sEVs from CRC patients might be a systemic rather than a tumor-driven response. For CD82 even a negative correlation between primary breast cancer and circulating sEV expression have been observed [97]. A study in 2020 took a step further and analyzed the proteomes of sEVs in plasma and those of primary tumor and tumor-adjacent tissue samples from pancreatic and lung cancer patients [98]. When compared to healthy plasma samples, 51 and 19 sEV proteins were exclusively identified in pancreatic and lung cancer patients, respectively. Sixteen of the EV proteins linked to pancreatic cancer were only detected in biopsy samples from the primary tumor and not in tumor-adjacent tissue, which strengthens their usability as cancer-specific biomarkers. Indeed, machine-learning classification of plasma EV cargo detected cancer with a sensitivity and specificity above 90% and was able to differentiate among different tumor entities, which makes analyzing tumor-EV cargo a powerful tool in cancer diagnostics.
Several studies have reported detection sensitivities of 100% after analyzing circulating sEVs regarding their expression of biomarkers, such as miR-1246 for breast cancer [99] and prostate-specific antigen (PSA) for prostate cancer [100]. PSA additionally performed well as an early detection EV biomarker in stage I cancer patients, as did survivin for prostate cancer [101] and Del-1 for breast cancer [102]. In pancreatic ductal adenocarcinoma (PDAC) patients, an EV-associated expression of highly upregulated in liver cancer (HULC) long non-coding RNA (lncRNA) was demonstrated to perform even better than the already established circulating serum biomarkers carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) in terms of discriminating between cancer patients and non-PDAC controls [103]. Not only cancer-specific but also pan-cancer biomarkers have been demonstrated to be diagnostically relevant, as has been shown for miR-21, which has been detected at high levels in circulating sEVs of pancreatic, breast, and lung cancer patients [104,105,106]. In these studies, high vesicular miR-21 expression was also associated with shortened survival and chemoresistance. Similarly, extracellular matrix metalloproteinase inducer (EMMPRIN) was detected in high levels in plasma lEVs from cancer patients with different kinds of solid tumors and showed a robust detection efficiency, especially in combination with epithelial cell adhesion molecule (EpCAM), mucin-1 (MUC1), and epidermal growth factor receptor (EGFR) expression rates [23]. The detection of cancer-specific mutations using EV-associated DNA represents another powerful tool for cancer diagnostics and is described in the following section.
5.2.2. EV-Associated DNA for Mutation Screenings
Recently, an increasing number of studies have addressed the applicability of EV-associated DNA in blood for the detection of cancer-specific mutations, especially in comparison with cfDNA. Data from 2017 indicate that the detection of Kirsten rat sarcoma (KRAS) mutations in PDAC patients via plasma-derived EV-DNA was more sensitive than cfDNA-related detection [107] and additionally predicted patient survival. This finding was confirmed in a later study for KRAS mutations in CRC patients [108]. In turn, Thakur et al. reported similar detection rates when using either circulating EV-DNA or cfDNA for the identification of several mutations in CRC patients [109]. In the future, however, a combination of both EV-derived nucleic acids and cfDNA might represent the most efficient procedure for non-invasive cancer-associated mutation screenings [110,111]. This approach was also applied for the development of a clinical test developed by Exosomes Diagnostics (ExoDx Lung(EGFR T790M)), which efficiently identified different EGFR mutations (L858R, T790M, and exon 19 indels) in plasma sEVs from a large cohort of non-small cell lung carcinoma (NSCLC) patients [112]. Measuring the mutation status of circulating EVs can potentially allow one to monitor parameters such as tumor size [109] and stage [113]. Notably, in the latter study, GBM-associated isocitrate dehydrogenase 1 (IDH1) mutations were detected even in low-grade glioma patients with an intact blood–brain barrier, confirming the high sensitivity of EV-based mutation screenings for cancer diagnostics.
Due to their high diagnostic impact, circulating EV-based biomarkers are also considered to efficiently represent the remaining tumor cells in the blood of cancer patients after therapy. For the monitoring of this so-called minimal or molecular residual disease (MRD) via liquid biopsies, the recent literature has often focused on ctDNA and CTCs [114,115]. However, more and more studies, which are outlined in the following section, have addressed and confirmed the usability of plasma-derived EVs for disease monitoring and relapse prediction.
5.2.3. Therapy Monitoring
In order to investigate the potential of tumor-specific EV biomarkers in the circulation for therapy monitoring, researchers have compared expression levels in matched patient samples before and after therapy. Several protein- and RNA-based biomarkers that were elevated in the plasma-EVs of cancer patients did show reduced expression levels after primary tumor resection or chemotherapy. Some of these proteins with reduced expression after surgery are EMMPRIN [116], 60 kDa heat shock protein (HSP60) [117], and glypican-1 (GPC1) [118] for CRC, cytoskeleton-associated protein 4 (CKAP4) for PDAC [119], and prostate-specific membrane antigen (PSMA) for prostate cancer [120]. Decreased levels of EVs positive for EGFR and its oncogenic variant EGFRvIII were detected in GBM patients after chemotherapy [121]. Similarly, EV-associated RNA molecules, such as the lncRNA HOX antisense intergenic RNA (HOTAIR) and the microRNAs (miR) miR-301 and miR-155, have been proven to be suitable markers for monitoring the response to surgery and chemotherapy, respectively, in breast cancer patients [122,123]. For some markers, even rising expression rates on circulating EVs can reflect a successful response to certain therapies. For example, increasing levels of EpCAM-positive EVs in the plasma of PDAC patients during chemotherapy were associated with longer progression-free survival [124]. This short-term increase in EV-associated EpCAM possibly reflects a therapy-induced stress response of the tumor.
Within the past decade, T-cell-targeted immunomodulators directed against the immune checkpoints of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and its ligand (PD-L1) have revolutionized cancer treatment for several types of tumors [125]. Since the precise identification of responding and non-responding cancer patients before or during certain immunotherapies remains challenging, circulating EV cargo was tested for its ability to predict and monitor therapy responses after immune checkpoint inhibitor treatments. Elevated PD-L1 expression on plasma sEVs during anti-PD-1 immunotherapy was linked to successful therapy response in melanoma patients [126], and high PD-L1 mRNA expression on circulating sEVs from melanoma and NSCLC patients before anti-PD-1 treatment was correlated with partial or complete remission after therapy [127]. Vesicular miR-320b, -c and –d, in turn, seem to be increasingly expressed in the blood of NSCLC patients identified as non-responders to antiPD-1 therapy [128].
5.2.4. Prediction of Disease Progression and Therapy Resistance
Apart from the promising application of EV-based liquid biopsy for cancer detection and the monitoring of therapy responses, the prediction of disease progression and resistance to certain therapies represents another area for its clinical use. The majority of EV biomarkers associated with chemoresistance exhibited high plasma levels in the blood of non-responding cancer patients as shown for several RNA molecules [129,130] and protein-based markers [131,132]. However, other studies also identified significantly downregulated levels of EV-associated miRNAs in the circulation of non-responders to chemo- and radiotherapy [133,134].
Regarding the potential use of plasma-derived EVs in the prediction of disease progression, miR-1247-3p for hepatocellular carcinoma (HCC) and the entire mRNA profile for osteosarcoma were shown to be associated with metastasis formation [135,136], while miR-17-5p and miR-92a-3p for CRC and miR-638 for HCC were correlated with cancer stage [137,138]. The expressions of miR-217 and lncRNA colorectal neoplasia differentially expressed (CRNDE) were linked to both metastasis and stage [139]. In addition, high plasma levels of Treg-derived CD3 and PD-L1 double-positive sEVs predicted recurrence in HNC patients after a combination therapy [82], which underlines the importance of also considering non-tumor-derived EV biomarkers for the prediction of disease states and therapy outcome. Figure 3 gives an overview of most of the biomarkers described above. All given information is additionally listed in the Supplementary Materials, Table S1.
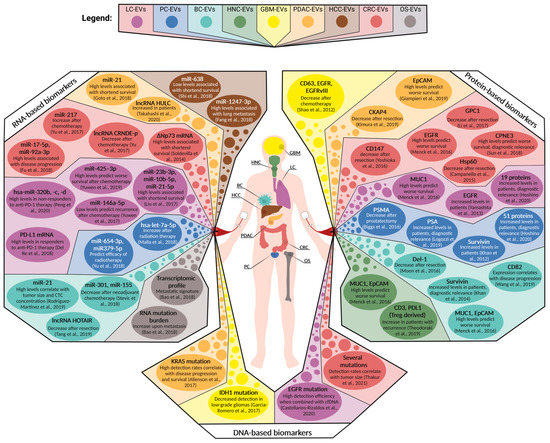
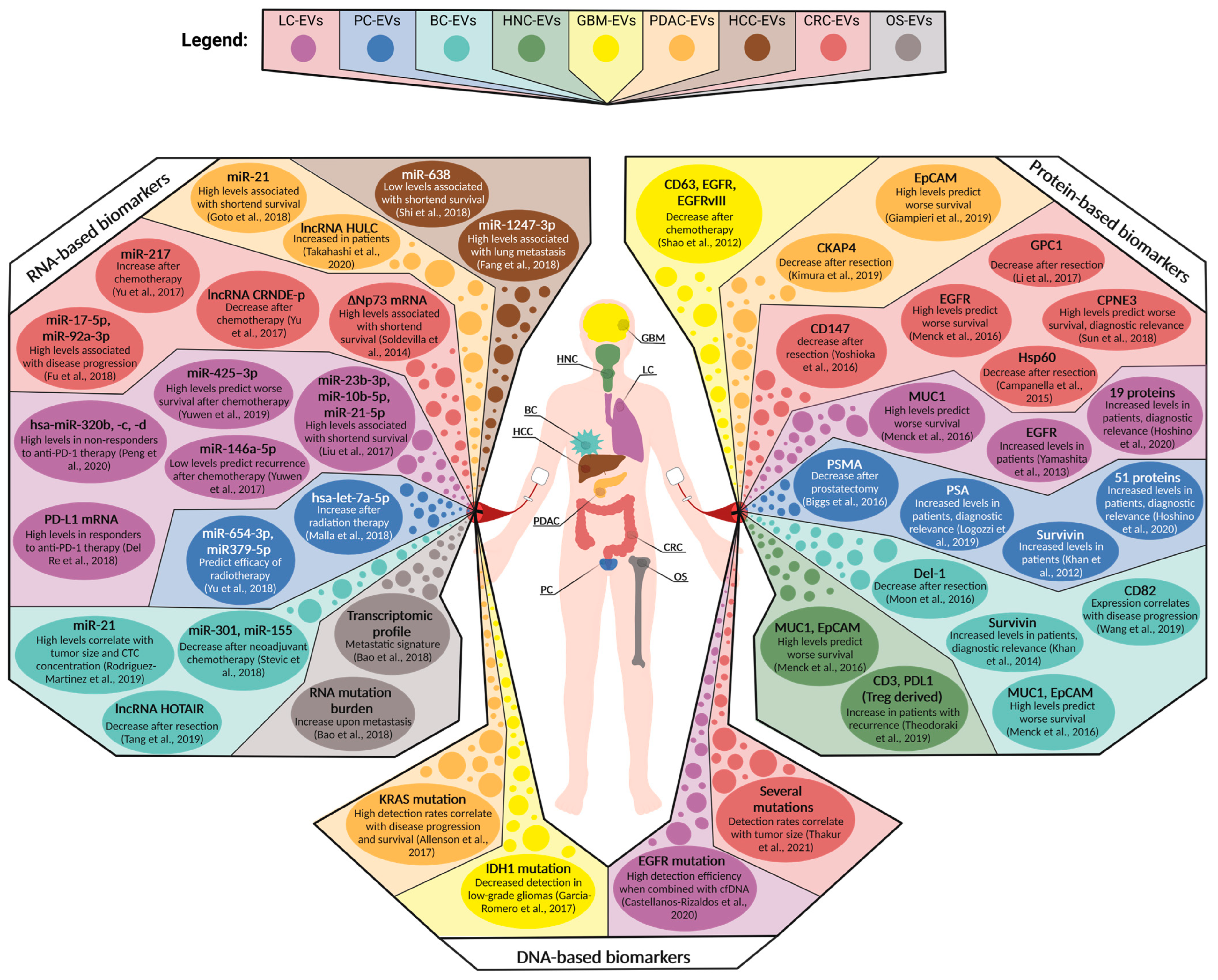
Figure 3.
Overview of circulating EV-associated biomarkers for selected solid tumor entities tested in a clinical setting. Depicted are the protein-, RNA-, and DNA-based biomarkers that have been clinically assessed in patient cohorts suffering from glioblastoma multiforme (GBM), head and neck cancer (HNC), lung cancer (LC), breast cancer (BC), hepatocellular carcinoma (HCC), pancreatic ductal adenocarcinoma (PDAC), colorectal carcinoma (CRC), prostate cancer (PC), and osteosarcoma (OS) [23,80,82,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,109,112,116,117,118,119,120,121,122,123,124,127,128,130,133,134,135,136,137,138,139,140].
5.2.5. Commercially Available Tests Exploiting EVs as Cancer Biomarkers
In spite of the large number of potential biomarkers for EV-driven cancer diagnostics, only three clinical tests that exploit EVs from liquid biopsies for cancer patient stratification have been commercially launched. All were developed by the Clinical Laboratory Improvement Amendment (CLIA)-certified laboratories of Exosome Diagnostics. ExoDx™ Lung (ALK) and ExoDx Prostate IntelliScore (EPI) were designed for the quantification of certain sEV-associated mRNAs as biomarkers for NSCLC and prostate cancer, respectively. The ExoDx™ Lung (ALK) test detects EML4-ALK mutations via quantitative PCR analysis of plasma EVs from NSCLC patients with 88% sensitivity and 100% specificity [141]. In turn, the EPI test uses urine as an EV source and is described in the following section. Furthermore, Exosome Diagnostics recently announced MedOncAlyzer 170 as a test, which also utilizes a combination of blood-derived EV-RNA/DNA and cfDNA for detecting a panel of mutations in multiple cancer entities [111].
5.3. Clinical Relevance of EVs from Other Body Fluids
In addition to blood as an EV-based liquid biopsy source, other body fluids hold great promise for the diagnosis, prognosis, and monitoring of cancer patients as well. One of the first commercially available tests using sEVs for cancer patient stratification is urine-based and called ExoDx Prostate IntelliScore (EPI), which detects vesicular mRNA levels of prostate cancer antigen 3 (PCA3), ETS transcription factor ERG (ERG), and SAM-pointed domain containing ETS transcription factor (SPDEF). In clinical studies, the EPI test has been demonstrated to efficiently discriminate high-grade (Gleason score ≥ 7) from low-grade (Gleason score 6) prostate cancer and benign disease in men ≥ 50 years with equivocal PSA levels (2–10 ng/mL), thereby reducing the number of unnecessary tissue biopsies [142,143]. In general, urine-derived EVs have been investigated in multiple studies for the assessment of urological malignancies such as prostate, bladder, and kidney cancers [144].
Further examples of the predictive ability of EV-based cancer biomarkers in other body fluids are vesicular miR-21 in the cerebrospinal fluid of GBM patients, which was associated with poor prognosis and tumor recurrence [145], and EGFR mutations detected by the EV-DNA of NSCLC-derived bronchial wash fluid, which was correlated with disease progression [146]. A study in 2016 observed that even epigenetic modifications of EV-DNA can function as clinically relevant biomarkers for cancer detection. The researchers used gastric juice samples for the quantification of EV-associated BarH, such as homeobox 2 (BARHL2) DNA methylation, which discriminated gastric cancer (GC) patients from non-GC controls with 90% sensitivity and 100% specificity [147]. Additionally, salivary gland fluid seems to be a promising source for EV-based liquid biopsies with regard to oral cancer, although it is prone to contamination with bacterial EV [148].
For the detection of cholangiocarcinoma, claudin-3 was reported to be enriched on bile-derived EVs, showing a sensitivity and specificity of 87.5% [149]. Consistently, this tight junction protein was previously shown to be differentially expressed in tumor cells from CRC patients, leading to the hyperactivation of Wnt-signaling [150].
6. Advantages and Limitations of EVs as Liquid Biopsy Biomarkers
Despite their underrepresentation in liquid biopsy-based cancer diagnostics, EVs are undoubtedly suitable for non-invasive biomarker detection processes. Similar to CTCs, they can be used as a multi-analyte platform for the detection and quantification of different kinds of cancer-related molecules. Whereas CTCs suffer from rather low numbers in blood, EVs are highly abundant in all human body fluids and provide a wide range of possibilities for their diagnostic use [140]. EV cargo is also less prone to degradation due to the protective function of the vesicular lipid membranes, and is, hence, more stable than tumor-associated cfDNA and soluble proteins in body fluids [151]. Probably as a result of the combination of their abundance and their cargo protection, EVs have shown high detection sensitivities and have frequently been observed to be even superior to detection strategies involving cfDNA or soluble proteins as tumor antigens [100,103,107]. EV-based liquid biopsies have shown convincing results in clinical studies, particularly for early cancer diagnostics and conclusive monitoring of minimal-residual disease in follow-up patients after therapy [100,101,102]. However, their translation into clinical practice still faces several obstacles. Since most current characterization techniques use bulk analyses of the EVs, which comprise multiple subpopulations from many different cellular sources, the resulting parameters, such as the concentration or expression profiles, are prone to biological variations. This is further aggravated by the lack of standardized EV isolation and characterization protocols. The effects of pre-analytical variables, such as the storage time, the number of freeze-and-thaw cycles, conditions during transport, etc., must be seen as sources of technical variations. Furthermore, most clinical studies have been performed with rather small patient cohorts, which weakens the power of the studies and the reliability of the respective observations.
Modern approaches that provide EV quantification and molecular characterization on a single particle level, such as advanced imaging flow cytometry or fluorescence-based NTA, could help to improve EV classification, thereby reducing biological variations [152,153]. As a solution for the technical variations, the International Society for Extracellular Vesicles (ISEV) released the MISEV guidelines in order to lay the foundation for achieving comparability of EV-based studies using different protocols [1]. Moreover, EV characterization protocols for the direct analysis of body fluids without prior isolation steps represent a promising strategy to achieve standardized high-throughput screenings for cancer diagnostics. As an example, whole-blood EV characterization using alternating current electrokinetic (ACE) microarray chips with subsequent on-chip immunofluorescence analysis was shown to be suitable for detecting PDAC via GPC1 and CD63 expression [154]. Taken together, EV-based liquid biopsy holds great promise for cancer diagnosis, prognosis, and therapy monitoring but still requires precise EV subtype classification, standardized protocols, and larger patient cohorts for its further clinical translation.
7. Conclusions
EVs have emerged as critical mediators of intercellular communication in cancer. Tumor-EVs circulating in body fluids, such as blood, harbor the malignant traits of their cells of origin, which makes them ideal biomarker candidates for detecting, assessing, and monitoring tumor growth in liquid biopsies. In this context, not only tumor-EVs but also EVs derived from non-malignant cells reacting to the growing tumor contribute to the vesiculome measurable in body fluids and can provide valuable information. However, the use of EVs as cancer biomarkers in routine diagnostics is still hampered by the lack of standardization, the lack of data from large patient cohorts, and methodological challenges. Once these problems have been solved, EVs have great potential to become valuable tools in liquid biopsies, either alone or in combination with other body fluid components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15041307/s1, Table S1: Overview of circulating EV-associated biomarkers for selected solid tumor entities tested in a clinical setting. Listed are protein-, RNA-, and DNA-based biomarkers that were clinically assessed in patient cohorts suffering from glioblastoma multiforme (GBM), head and neck cancer (HNC), lung cancer (LC), breast cancer (BC), hepatocellular carcinoma (HCC), pancreatic ductal adenocarcinoma (PDAC), colorectal carcinoma (CRC), prostate cancer (PC) and osteosarcoma (OS).
Author Contributions
Writing—original draft preparation, B.I., S.C., L.M. and K.M.; writing—review and editing, B.I., S.C., L.M., A.B. and K.M.; visualization, B.I., S.C. and L.M.; supervision, K.M.; funding acquisition, A.B. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation—project 424252458), the Else Kröner-Fresenius-Stiftung (project 2019_A162), the Volkswagen Foundation within the research project MTB-Report (ZN3424), and the Innovative Medical Research (IMF) of the Medical Faculty, University of Münster (project ME 12 19 14).
Acknowledgments
Barnabas Irmer and Suganja Sivaloganathan are members of CiM-IMPRS, the joint graduate school of the Cells-in-Motion Interfaculty Center, University of Münster, Germany, and the International Max Planck Research School—Molecular Biomedicine, Münster, Germany. We acknowledge support from the Open Access Publication Fund of the University of Muenster. We thank Thomas Crozier for his language revision. All figures were created with Biorender.com (accessed on 10 January 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Bucki, R.; Bachelot-Loza, C.; Zachowski, A.; Giraud, F.; Sulpice, J.-C. Calcium Induces Phospholipid Redistribution and Microvesicle Release in Human Erythrocyte Membranes by Independent Pathways. Biochemistry 1998, 37, 15383–15391. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Ma, B. Calpain–calpastatin system and cancer progression. Biol. Rev. 2021, 96, 961–975. [Google Scholar] [CrossRef]
- Bern, M.M. Extracellular vesicles: How they interact with endothelium, potentially contributing to metastatic cancer cell implants. Clin. Transl. Med. 2017, 6, 33. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Taylor, J.; Bebawy, M. Proteins Regulating Microvesicle Biogenesis and Multidrug Resistance in Cancer. Proteomics 2018, 19, e1800165. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Dignat-George, F. Microparticles: An Introduction. Arter. Thromb. Vasc. Biol. 2011, 31, 2–3. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Fares, J.; Kashyap, R.S.; Zimmermann, P. Syntenin: Key player in cancer exosome biogenesis and uptake? Cell Adhes. Migr. 2016, 11, 124–126. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.P.; Gilligan, K.E.; Dwyer, R.M. Role of Extracellular Vesicles (EVs) in Cell Stress Response and Resistance to Cancer Therapy. Cancers 2019, 11, 136. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Nitadori-Hoshino, A.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Imjeti, N.S.; Menck, K.; Egea-Jimenez, A.L.; Lecointre, C.; Lembo, F.; Bouguenina, H.; Badache, A.; Ghossoub, R.; David, G.; Roche, S.; et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2017, 114, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Szvicsek, Z.; Oszvald, Á.; Szabó, L.; Sándor, G.O.; Kelemen, A.; Soós, A.Á.; Pálóczi, K.; Harsányi, L.; Tölgyes, T.; Dede, K.; et al. Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell. Mol. Life Sci. 2019, 76, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Ginestra, A.; La Placa, M.D.; Saladino, F.; Cassarà, D.; Nagase, H.; Vittorelli, M.L. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998, 18, 3433–3437. [Google Scholar] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Buentzel, J.; Klemp, H.G.; Kraetzner, R.; Schulz, M.; Dihazi, G.H.; Streit, F.; Bleckmann, A.; Menck, K.; Wlochowitz, D.; Binder, C. Metabolomic Profiling of Blood-Derived Microvesicles in Breast Cancer Patients. Int. J. Mol. Sci. 2021, 22, 13540. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Bleckmann, A.; Wachter, A.; Hennies, B.; Ries, L.; Schulz, M.; Balkenhol, M.; Pukrop, T.; Schatlo, B.; Rost, U.; et al. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J. Extracell. Vesicles 2017, 6, 1340745. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Nazarenko, I. Extracellular Vesicles: Recent Developments in Technology and Perspectives for Cancer Liquid Biopsy. Recent Results Cancer Res. 2020, 215, 319–344. [Google Scholar] [CrossRef]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef]
- Friedhoff, A.J.; VAN Winkle, E. Isolation and Characterization of a Compound from the Urine of Schizophrenics. Nature 1962, 194, 897–898. [Google Scholar] [CrossRef]
- Revenfeld, A.L.S.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and Prognostic Potential of Extracellular Vesicles in Peripheral Blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, J.; Park, J. Methods to isolate extracellular vesicles for diagnosis. Micro Nano Syst. Lett. 2017, 5, 15. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Yamada, T.; Inoshima, Y.; Matsuda, T.; Ishiguro, N. Comparison of Methods for Isolating Exosomes from Bovine Milk. J. Vet. Med. Sci. 2012, 74, 1523–1525. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; La Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch, D.; Gu, D.; Benito-Martin, A.; Calzaferri, G.; Aherne, S.; Holthofer, H. A Simplified Method to Recover Urinary Vesicles for Clinical Applications and Sample Banking. Sci. Rep. 2014, 4, 7532. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Powell, D.W.; Wilkey, D.W.; Cummins, T.D.; Deegens, J.K.; Rood, I.M.; McAfee, K.J.; Fleischer, C.; Klein, E.; Klein, J.B. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteom. Clin. Appl. 2010, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- Rood, I.M.; Deegens, J.K.J.; Merchant, M.L.; Tamboer, W.P.M.; Wilkey, D.W.; Wetzels, J.F.M.; Klein, J.B. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010, 78, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, E273. [Google Scholar] [CrossRef]
- Sitar, S.; Kejžar, A.; Pahovnik, D.; Kogej, K.; Tušek-Žnidarič, M.; Lenassi, M.; Žagar, E. Size Characterization and Quantification of Exosomes by Asymmetrical-Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar] [CrossRef]
- Mondal, S.K.; Whiteside, T.L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol. Biol. 2021, 2265, 305–321. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Pei, F.; Zeng, C.; Yao, Y.; Liao, W.; Zhao, Z. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. BioMed Res. Int. 2021, 2021, 6611244. [Google Scholar] [CrossRef]
- Niu, Z.; Pang, R.T.K.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S.B. Polymer-based precipitation preserves biological activities of extracellular vesicles from an endometrial cell line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef]
- Brown, P.N.; Yin, H. Polymer-Based Purification of Extracellular Vesicles. Methods Mol. Biol. 2017, 1660, 91–103. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Zacharias, W.; Gercel-Taylor, C. Exosome Isolation for Proteomic Analyses and RNA Profiling. Methods Mol. Biol. 2011, 728, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12, 1600699. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Coumans, F.A.W.; Maltesen, R.G.; Böing, A.N.; Bonnington, K.E.; Broekman, M.L.; Broom, M.F.; Buzás, E.I.; Christiansen, G.; Hajji, N.; et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles 2016, 5, 31242. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Vogel, R.; Minelli, C. Chapter 3.1.4—Tunable Resistive Pulse Sensing (TRPS). In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–136. ISBN 978-0-12-814182-3. [Google Scholar]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Maas, S.L.; de Vrij, J.; van der Vlist, E.J.; Geragousian, B.; van Bloois, L.; Mastrobattista, E.; Schiffelers, R.M.; Wauben, M.H.M.; Broekman, M.L.D.; Hoen, E.N.N. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J. Control. Release 2015, 200, 87–96. [Google Scholar] [CrossRef]
- de Vrij, J.; Maas, S.L.; van Nispen, M.; Sena-Esteves, M.; Limpens, R.W.A.; Koster, A.J.; Leenstra, S.; Lamfers, M.L.; Broekman, M.L.D. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine 2013, 8, 1443–1458. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.G.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- Van Der Vlist, E.J.; Nolte-’t Hoen, E.N.M.; Stoorvogel, W.; Arkesteijn, G.J.A.; Wauben, M.H.M. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 2012, 7, 1311–1326. [Google Scholar] [CrossRef]
- Suárez, H.; Gámez-Valero, A.; Reyes, R.; López-Martín, S.; Rodríguez, M.J.; Carrascosa, J.L.; Cabañas, C.; Borràs, F.E.; Yáñez-Mó, M. A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles. Sci. Rep. 2017, 7, 11271. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; van der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Zeng, Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 2016, 16, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Duijvesz, D.; Versluis, C.Y.L.; van der Fels, C.A.M.; Berg, M.S.V.-V.D.; Leivo, J.; Peltola, M.T.; Bangma, C.H.; Pettersson, K.S.; Jenster, G. Immuno-based detection of extracellular vesicles in urine as diagnostic marker for prostate cancer. Int. J. Cancer 2015, 137, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- De Rond, L.; van der Pol, E.; Hau, C.M.; Varga, Z.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R.; Coumans, F.A.W. Comparison of Generic Fluorescent Markers for Detection of Extracellular Vesicles by Flow Cytometry. Clin. Chem. 2018, 64, 680–689. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Wang, X. Extracellular vesicle-based liquid biopsy holds great promise for the management of ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188395. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Petrasek, J.; Mundkur, S.; Catalano, D.; Levin, I.; Ward, J.; Alao, H.; Kodys, K.; Szabo, G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012, 56, 1946–1957. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Laterza, O.F.; Lim, L.; Garrett-Engele, P.W.; Vlasakova, K.; Muniappa, N.; Tanaka, W.K.; Johnson, J.M.; Sina, J.F.; Fare, T.L.; Sistare, F.D.; et al. Plasma MicroRNAs as Sensitive and Specific Biomarkers of Tissue Injury. Clin. Chem. 2009, 55, 1977–1983. [Google Scholar] [CrossRef]
- Trino, S.; Lamorte, D.; Caivano, A.; De Luca, L.; Sgambato, A.; Laurenzana, I. Clinical relevance of extracellular vesicles in hematological neoplasms: From liquid biopsy to cell biopsy. Leukemia 2021, 35, 661–678. [Google Scholar] [CrossRef]
- Alegre, E.; Zubiri, L.; Perez-Gracia, J.L.; González-Cao, M.; Soria, L.; Martín-Algarra, S.; González, A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin. Chim. Acta 2016, 454, 28–32. [Google Scholar] [CrossRef]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 25, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, S.R.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Torreira, M.G.; García, A.G. A Pilot Clinical Study on the Prognostic Relevance of Plasmatic Exosomes Levels in Oral Squamous Cell Carcinoma Patients. Cancers 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Kasimir-Bauer, S.; Bittner, A.-K.; Hoffmann, O.; Wagner, B.; Manvailer, L.F.S.; Kimmig, R.; Horn, P.A.; Rebmann, V. Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. Oncoimmunology 2017, 7, e1376153. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosom. Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Zhou, Y.; Jie, S. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma. Cancer Biomark. 2013, 13, 351–357. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef]
- Malla, B.; Aebersold, D.M.; Pra, A.D. Protocol for serum exosomal miRNAs analysis in prostate cancer patients treated with radiotherapy. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, e1593805. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jørgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Peña, E.; Padró, T.; Jiménez-Xarrié, E.; Martí-Fàbregas, J.; Badimon, L. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016, 11, e0148176. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, D.; Reimund, J.-M.; Tesse, A.; Viennot, S.; Martínez, M.C.; Bretagne, A.-L.; Andriantsitohaina, R. Circulating Microparticles from Crohn’s Disease Patients Cause Endothelial and Vascular Dysfunctions. PLoS ONE 2013, 8, e73088. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Imaizumi, M.; Nomura, S.; Shozu, A.; Arichi, M.; Matsuoka, M.; Matsumura, M. Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2005, 68, 193–201. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef]
- Lucas, F.A.S.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. 2016, 27, 635–641. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Khan, S.; Bennit, H.F.; Turay, D.; Perez, M.; Mirshahidi, S.; Yuan, Y.; Wall, N.R. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kamada, H.; Kanasaki, S.; Maeda, Y.; Nagano, K.; Abe, Y.; Inoue, M.; Yoshioka, Y.; Tsutsumi, Y.; Katayama, S.; et al. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Die Pharm. 2013, 68, 969–973. [Google Scholar]
- Sun, B.; Li, Y.; Zhou, Y.; Ng, T.K.; Zhao, C.; Gan, Q.; Gu, X.; Xiang, J. Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J. Cell. Physiol. 2018, 234, 1416–1425. [Google Scholar] [CrossRef]
- Soldevilla, B.; Rodríguez, M.; San Millán, C.; García, V.; Fernández-Periañez, R.; Gil-Calderón, B.; Martín, P.; García-Grande, A.; Silva, J.; Bonilla, F.; et al. Tumor-derived exosomes are enriched in ΔNp73, which promotes oncogenic potential in acceptor cells and correlates with patient survival. Hum. Mol. Genet. 2014, 23, 467–478. [Google Scholar] [CrossRef]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, W.; Bu, J.; Li, Y.; Li, R.; Nie, R.; Xiao, C.; Ma, K.; Huang, X.; Li, Y. Exosomal Protein CD82 as a Diagnostic Biomarker for Precision Medicine for Breast Cancer. Mol. Carcinog. 2019, 58, 674–685. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Zhai, L.-Y.; Li, M.-X.; Pan, W.-L.; Chen, Y.; Pang, J.-X.; Zheng, L.; Chen, J.-X.; Duan, W.-J. In Situ Detection of Plasma Exosomal MicroRNA-1246 for Breast Cancer Diagnostics by a Au Nanoflare Probe. ACS Appl. Mater. Interfaces 2018, 10, 39478–39486. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Angelini, D.F.; Giuliani, A.; Mizzoni, D.; Di Raimo, R.; Maggi, M.; Gentilucci, A.; Marzio, V.; Salciccia, S.; Borsellino, G.; et al. Increased Plasmatic Levels of PSA-Expressing Exosomes Distinguish Prostate Cancer Patients from Benign Prostatic Hyperplasia: A Prospective Study. Cancers 2019, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jutzy, J.M.S.; Valenzuela, M.M.A.; Turay, D.; Aspe, J.R.; Ashok, A.; Mirshahidi, S.; Mercola, D.; Lilly, M.B.; Wall, N.R. Plasma-Derived Exosomal Survivin, a Plausible Biomarker for Early Detection of Prostate Cancer. PLoS ONE 2012, 7, e46737. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.-G.; Lee, J.-E.; Cho, Y.-E.; Lee, S.J.; Jung, J.H.; Chae, Y.S.; Bae, H.-I.; Kim, Y.-B.; Kim, I.-S.; Park, H.Y.; et al. Identification of Developmental Endothelial Locus-1 on Circulating Extracellular Vesicles as a Novel Biomarker for Early Breast Cancer Detection. Clin. Cancer Res. 2016, 22, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ota, Y.; Kogure, T.; Suzuki, Y.; Iwamoto, H.; Yamakita, K.; Kitano, Y.; Fujii, S.; Haneda, M.; Patel, T.; et al. Circulating extracellular vesicle-encapsulated HULC is a potential biomarker for human pancreatic cancer. Cancer Sci. 2020, 111, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An elevated expression of serum exosomal microRNA-191, −21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, A.; De Miguel-Pérez, D.; Ortega, F.G.; García-Puche, J.L.; Robles-Fernández, I.; Exposito, J.; Martorell-Marugan, J.; Carmona-Sáez, P.; Garrido-Navas, M.D.C.; Rolfo, C.; et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019, 21, 21. [Google Scholar] [CrossRef]
- Allenson, K.; Castillo, J.; Lucas, F.S.; Scelo, G.; Kim, D.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.; et al. High prevalence of mutantKRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef]
- Galvano, A.; Castiglia, M.; Guarini, A.; Gristina, V.; Cutaia, S.; Bazan, V.; Russo, A.; Carreca, I.U. Cell-free DNA and exoDNA analysis in metastatic colorectal cancer patients (mCRC). J. Clin. Oncol. 2020, 38, e16093. [Google Scholar] [CrossRef]
- Thakur, K.; Singh, M.S.; Feldstein-Davydova, S.; Hannes, V.; Hershkovitz, D.; Tsuriel, S. Extracellular Vesicle-Derived DNA vs. CfDNA as a Biomarker for the Detection of Colon Cancer. Genes 2021, 12, 1171. [Google Scholar] [CrossRef]
- Krug, A.; Enderle, D.; Karlovich, C.; Priewasser, T.; Bentink, S.; Spiel, A.; Brinkmann, K.; Emenegger, J.; Grimm, D.G.; Castellanos-Rizaldos, E.; et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann. Oncol. 2018, 29, 700–706. [Google Scholar] [CrossRef]
- Möhrmann, L.; Huang, H.J.; Hong, D.S.; Tsimberidou, A.M.; Fu, S.; Piha-Paul, S.A.; Subbiah, V.; Karp, D.D.; Naing, A.; Krug, A.; et al. Liquid Biopsies Using Plasma Exosomal Nucleic Acids and Plasma Cell-Free DNA Compared with Clinical Outcomes of Patients with Advanced Cancers. Clin. Cancer Res. 2018, 24, 181–188. [Google Scholar] [CrossRef]
- Castellanos-Rizaldos, E.; Zhang, X.; Tadigotla, V.R.; Grimm, D.G.; Karlovich, C.; Raez, L.E.; Skog, J.K. Exosome-based detection of activating and resistance EGFR mutations from plasma of non-small cell lung cancer patients. Oncotarget 2019, 10, 2911–2920. [Google Scholar] [CrossRef]
- García-Romero, N.; Carrión-Navarro, J.; Esteban-Rubio, S.; Lázaro-Ibáñez, E.; Peris-Celda, M.; Alonso, M.M.; Guzmán-De-Villoria, J.; Fernández-Carballal, C.; de Mendivil, A.O.; García-Duque, S.; et al. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 2017, 8, 1416–1428. [Google Scholar] [CrossRef]
- Gristina, V.; La Mantia, M.; Peri, M.; Iacono, F.; Barraco, N.; Perez, A.; Viscardi, G.; Cutaia, S.; Russo, T.D.B.; Anwar, Z.; et al. Navigating the liquid biopsy Minimal Residual Disease (MRD) in non-small cell lung cancer: Making the invisible visible. Crit. Rev. Oncol. 2023, 182, 103899. [Google Scholar] [CrossRef] [PubMed]
- Gristina, V.; Barraco, N.; La Mantia, M.; Castellana, L.; Insalaco, L.; Bono, M.; Perez, A.; Sardo, D.; Inguglia, S.; Iacono, F.; et al. Clinical Potential of Circulating Cell-Free DNA (cfDNA) for Longitudinally Monitoring Clinical Outcomes in the First-Line Setting of Non-Small-Cell Lung Cancer (NSCLC): A Real-World Prospective Study. Cancers 2022, 14, 6013. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Gammazza, A.M.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Msc, C.C.B.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Guo, X.; Zhou, L.; Jia, Z.; Peng, Z.; Tang, Y.; Liu, W.; Zhu, B.; Wang, L.; et al. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J. Cell. Mol. Med. 2017, 21, 838–847. [Google Scholar] [CrossRef]
- Kimura, H.; Yamamoto, H.; Harada, T.; Fumoto, K.; Osugi, Y.; Sada, R.; Maehara, N.; Hikita, H.; Mori, S.; Eguchi, H.; et al. CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clin. Cancer Res. 2019, 25, 1936–1947. [Google Scholar] [CrossRef]
- Biggs, C.N.; Siddiqui, K.M.; Al-Zahrani, A.A.; Pardhan, S.; Brett, S.I.; Guo, Q.Q.; Yang, J.; Wolf, P.; Power, N.E.; Durfee, P.N.; et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget 2016, 7, 8839–8849. [Google Scholar] [CrossRef]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef]
- Stevic, I.; Müller, V.; Weber, K.; Fasching, P.A.; Karn, T.; Marmé, F.; Schem, C.; Stickeler, E.; Denkert, C.; Van Mackelenbergh, M.; et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, K.; Tang, Y.; Li, Z.; Zou, T.; Liu, D. Overexpression of serum exosomal HOTAIR is correlated with poor survival and poor response to chemotherapy in breast cancer patients. J. Biosci. 2019, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Piva, F.; Occhipinti, G.; Bittoni, A.; Righetti, A.; Pagliaretta, S.; Murrone, A.; Bianchi, F.; Amantini, C.; Giulietti, M.; et al. Clinical impact of different exosomes’ protein expression in pancreatic ductal carcinoma patients treated with standard first line palliative chemotherapy. PLoS ONE 2019, 14, e0215990. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Peng, X.-X.; Yu, R.; Wu, X.; Wu, S.-Y.; Pi, C.; Chen, Z.-H.; Zhang, X.-C.; Gao, C.-Y.; Shao, Y.W.; Liu, L.; et al. Correlation of Plasma Exosomal MicroRNAs with the Efficacy of Immunotherapy in EGFR/ALK Wild-Type Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer 2020, 8, e000376. [Google Scholar] [CrossRef]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef]
- Shao, H.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999. [Google Scholar] [CrossRef]
- Yuwen, D.; Ma, Y.; Wang, D.; Gao, J.; Li, X.; Xue, W.; Fan, M.; Xu, Q.; Shen, Y.; Shu, Y. Prognostic Role of Circulating Exosomal miR-425-3p for the Response of NSCLC to Platinum-Based Chemotherapy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 163–173. [Google Scholar] [CrossRef]
- Kato, T.; Mizutani, K.; Kameyama, K.; Kawakami, K.; Fujita, Y.; Nakane, K.; Kanimoto, Y.; Ehara, H.; Ito, H.; Seishima, M.; et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 385.e15–385.e20. [Google Scholar] [CrossRef]
- Wang, T.; Ning, K.; Lu, T.-X.; Sun, X.; Jin, L.; Qi, X.; Jin, J.; Hua, D. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017, 108, 448–454. [Google Scholar] [CrossRef]
- Yu, Q.; Li, P.; Weng, M.; Wu, S.; Zhang, Y.; Chen, X.; Zhang, Q.; Shen, G.; Ding, X.; Fu, S. Nano-Vesicles are a Potential Tool to Monitor Therapeutic Efficacy of Carbon Ion Radiotherapy in Prostate Cancer. J. Biomed. Nanotechnol. 2018, 14, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, D.-L.; Sheng, B.-B.; Liu, J.; Wenyu, W.; Shu, Y.-Q. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 2650–2658. [Google Scholar]
- Bao, Q.; Gong, L.; Wang, J.; Wen, J.; Shen, Y.; Zhang, W. Extracellular Vesicle RNA Sequencing Reveals Dramatic Transcriptomic Alterations Between Metastatic and Primary Osteosarcoma in a Liquid Biopsy Approach. Ann. Surg. Oncol. 2018, 25, 2642–2651. [Google Scholar] [CrossRef]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, Y.; Yang, L.; Yan, S.; Wang, Y.-G.; Lu, X.-J. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kumar, B.; Chen, Z.; Chen, X.; Müller, D.; Lele, S.M.; Washington, M.K.; Batra, S.K.; Dhawan, P.; Singh, A.B. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/β-catenin signaling. Oncogene 2017, 36, 6592–6604. [Google Scholar] [CrossRef]
- Yu, B.; Du, Q.; Li, H.; Liu, H.-Y.; Ye, X.; Zhu, B.; Zhai, Q.; Li, X.-X. Diagnostic potential of serum exosomal colorectal neoplasia differentially expressed long non-coding RNA (CRNDE-p) and microRNA-217 expression in colorectal carcinoma. Oncotarget 2017, 8, 83745–83753. [Google Scholar] [CrossRef]
- Brinkmann, K.; Enderle, D.; Flinspach, C.; Meyer, L.; Skog, J.; Noerholm, M. Exosome liquid biopsies of NSCLC patients for longitudinal monitoring of ALK fusions and resistance mutations. J. Clin. Oncol. 2018, 36, e24090. [Google Scholar] [CrossRef]
- Margolis, E.; Brown, G.; Partin, A.; Carter, B.; McKiernan, J.; Tutrone, R.; Torkler, P.; Fischer, C.; Tadigotla, V.; Noerholm, M.; et al. Predicting high-grade prostate cancer at initial biopsy: Clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies. Prostate Cancer Prostatic Dis. 2021, 25, 296–301. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- Lourenço, C.; Constâncio, V.; Henrique, R.; Carvalho, A.; Jerónimo, C. Urinary Extracellular Vesicles as Potential Biomarkers for Urologic Cancers: An Overview of Current Methods and Advances. Cancers 2021, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. miR-21 in the Extracellular Vesicles (EVs) of Cerebrospinal Fluid (CSF): A Platform for Glioblastoma Biomarker Development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, C.; Eom, J.S.; Kim, M.-H.; Cho, Y.-K. Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma. Cancers 2020, 12, 2822. [Google Scholar] [CrossRef]
- Yamamoto, H.; Watanabe, Y.; Oikawa, R.; Morita, R.; Yoshida, Y.; Maehata, T.; Yasuda, H.; Itoh, F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker for Early Detection of Gastric Cancer in an H. pylori -Independent Manner. Clin. Transl. Gastroenterol. 2016, 7, e184. [Google Scholar] [CrossRef] [PubMed]
- Chiabotto, G.; Gai, C.; Deregibus, M.C.; Camussi, G. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers 2019, 11, 891. [Google Scholar] [CrossRef]
- Ikeda, C.; Haga, H.; Makino, N.; Inuzuka, T.; Kurimoto, A.; Ueda, T.; Matsuda, A.; Kakizaki, Y.; Ishizawa, T.; Kobayashi, T.; et al. Utility of Claudin-3 in extracellular vesicles from human bile as biomarkers of cholangiocarcinoma. Sci. Rep. 2021, 11, 1195. [Google Scholar] [CrossRef]
- Garcia-Romero, N.; Esteban-Rubio, S.; Rackov, G.; Carrión-Navarro, J.; Belda-Iniesta, C.; Ayuso-Sacido, A. Extracellular vesicles compartment in liquid biopsies: Clinical application. Mol. Asp. Med. 2018, 60, 27–37. [Google Scholar] [CrossRef]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1775, 181–232. [Google Scholar] [CrossRef]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of Extracellular Vesicles Using Fluorescence Nanoparticle Tracking Analysis. Methods Mol. Biol. 2017, 1660, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Mastoridis, S.; Bertolino, G.M.; Whitehouse, G.; Dazzi, F.; Sanchez-Fueyo, A.; Martinez-Llordella, M. Multiparametric Analysis of Circulating Exosomes and Other Small Extracellular Vesicles by Advanced Imaging Flow Cytometry. Front. Immunol. 2018, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).