PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology
Abstract
Simple Summary
Abstract
1. Introduction
2. ICIs Therapy, PCSK9, and Risk of Atherosclerotic Cardiovascular Diseases
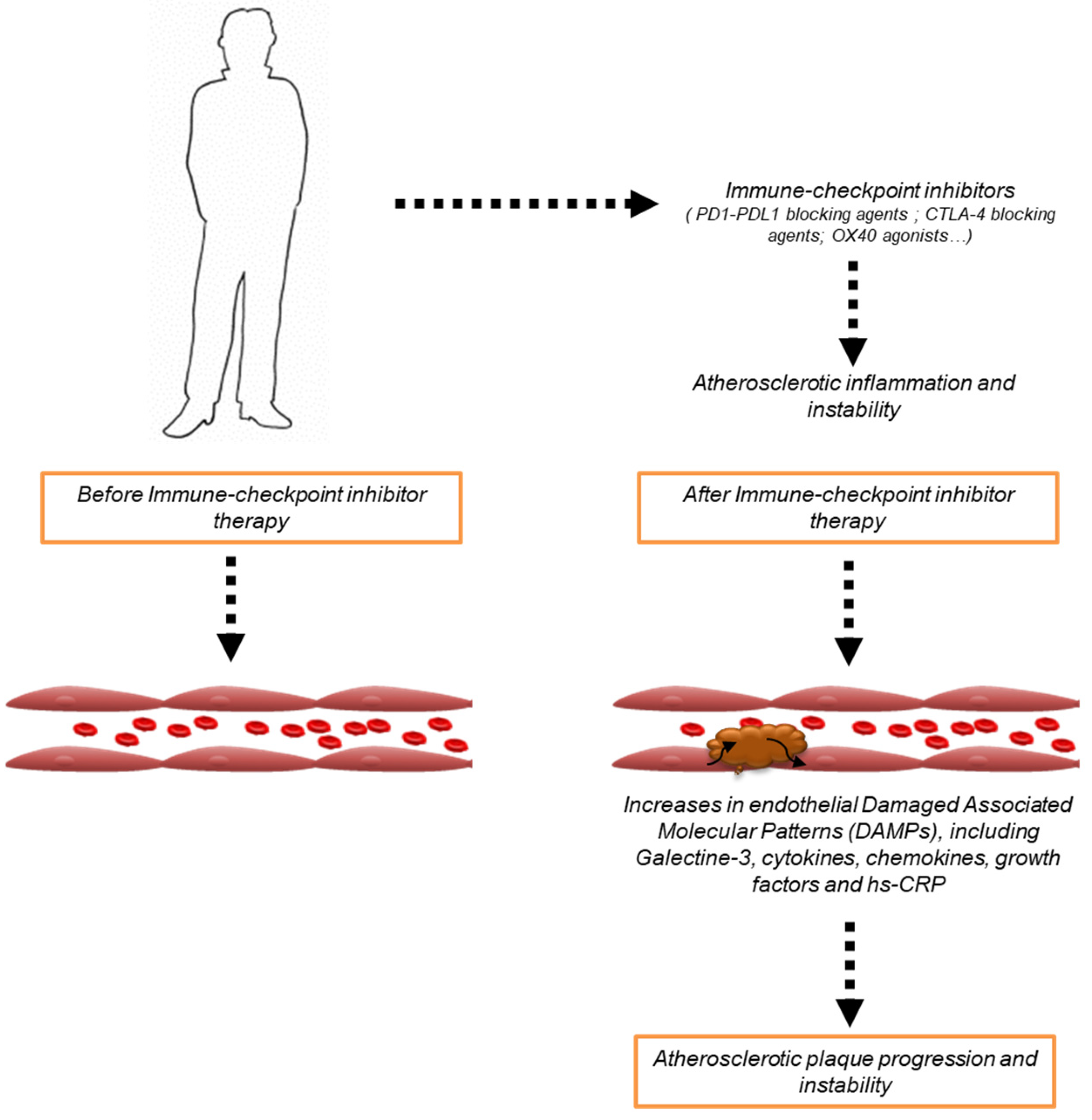
2.1. ICIs-Mediated Atherosclerosis
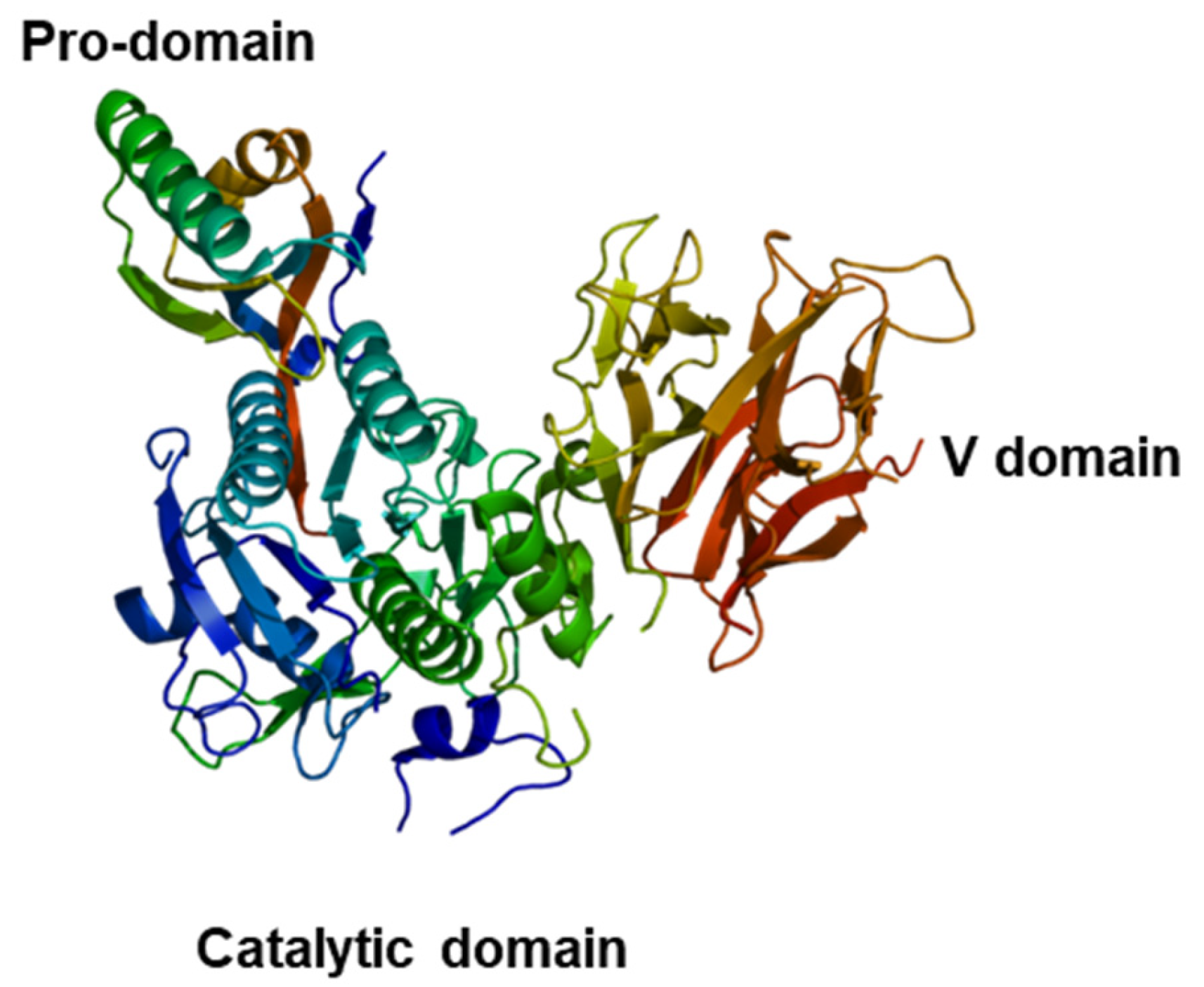
2.2. PCSK9 Role in Atherosclerotic Pathogenesis
2.3. PCSK9 in Cardiomyocyte and Endothelial Metabolism
3. PCSK9i in Cardiovascular Outcome Trials
- -
- Evolocumab
- -
- Alirocumab
- -
- Bococizumab
- -
- Inclisiran
| Drug | Chemistry | Cardiovascular Benefits | References |
|---|---|---|---|
| Evolocumab | Antibody |
| [79] |
| [80] [81] [81] [82] [83] | ||
| Alirocumab | Antibody |
| [84] [85] [85] [86] |
| Bococizumab | Antibody |
| [88] |
| [89] | ||
| Inclisiran | siRNA |
| [94] |
| [95,96] [97] |
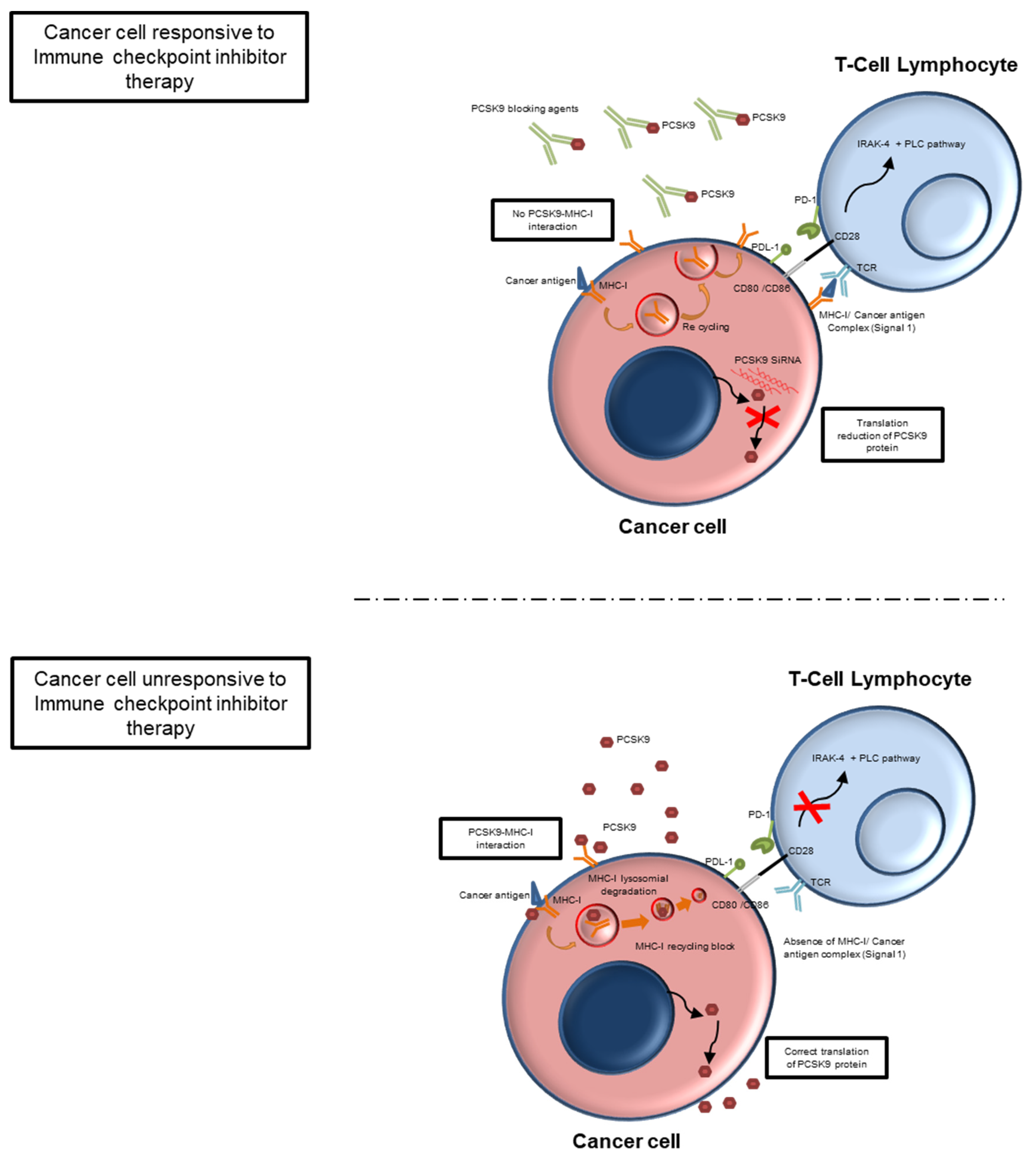
4. PCSK9i in Oncology: Mechanisms and Potential Application
5. PCSK9 Inhibitors in Cardio-Oncology: A Potential Therapy in ASCVD Cancer Patients Treated with ICIs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 579–597. [Google Scholar] [CrossRef]
- Li, H.; Zhao, A.; Li, M.; Shi, L.; Han, Q.; Hou, Z. Targeting T-cell metabolism to boost immune checkpoint inhibitor therapy. Front. Immunol. 2022, 13, 1046755. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Vyungura, O.; Zhao, Y. Molecular subtyping and IMScore based on immune-related pathways, oncogenic pathways, and DNA damage repair pathways for guiding immunotherapy in hepatocellular carcinoma patients. J. Gastrointest. Oncol. 2022, 13, 3135–3153. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458, Erratum in Lancet Oncol. 2022, 23, e492. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; Zhou, D.; He, A.; Ge, W.; Xu, X. Assessing Potential Factors Influencing the Efficacy of Immune Checkpoint Inhibitors with Radiation in Advanced Non-Small-Cell Lung Cancer Patients: A Systematic Review and Meta-Analysis. J. Oncol. 2023, 2023, 4477263. [Google Scholar] [CrossRef]
- Røssevold, A.H.; Andresen, N.K.; Bjerre, C.A.; Gilje, B.; Jakobsen, E.H.; Raj, S.X.; Falk, R.S.; Russnes, H.G.; Jahr, T.; Mathiesen, R.R.; et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: The randomized, double-blind phase 2b ALICE trial. Nat. Med. 2022, 28, 2573–2583. [Google Scholar] [CrossRef]
- Tao, Y.; Biau, J.; Sun, X.; Sire, C.; Martin, L.; Alfonsi, M.; Prevost, J.; Modesto, A.; Lafond, C.; Tourani, J.; et al. Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015-01 PembroRad): A multicenter, randomized, phase II trial. Ann. Oncol. 2023, 34, 101–110. [Google Scholar] [CrossRef]
- Padmanabhan, R.; Kheraldine, H.; Gupta, I.; Meskin, N.; Hamad, A.; Vranic, S.; Al Moustafa, A.-E. Quantification of the growth suppression of HER2+ breast cancer colonies under the effect of trastuzumab and PD-1/PD-L1 inhibitor. Front. Oncol. 2022, 12, 977664. [Google Scholar] [CrossRef]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef]
- Versluis, J.; Menzies, A.; Sikorska, K.; Rozeman, E.; Saw, R.; van Houdt, W.; Eriksson, H.; Klop, W.; Ch’Ng, S.; van Thienen, J.; et al. Survival Update of Neoadjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage III Melanoma in the OpACIN and OpACIN-Neo Trials. Ann. Oncol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, X.; Ren, Y.; Xu, Q.; Liu, X.; Song, S.; Wang, Y. Research progress of abnormal lactate metabolism and lactate modification in immunotherapy of hepatocellular carcinoma. Front. Oncol. 2023, 12, 1063423. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Maqsood, R. Review to Understand the Crosstalk between Immunotherapy and Tumor Metabolism. Molecules 2023, 28, 862. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Sacher, A. A New Risk Factor for Cardiovascular Events in Patients Receiving Immune Checkpoint Inhibitor Therapy? JACC CardioOncol. 2022, 4, 670–672. [Google Scholar] [CrossRef]
- Quinaglia, T.; Gongora, C.; Awadalla, M.; Hassan, M.Z.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Zhang, L.; Coelho-Filho, O.R.; Suero-Abreu, G.A.; et al. Global Circumferential and Radial Strain Among Patients With Immune Checkpoint Inhibitor Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Chen, Y.; Procureur, A.; Gradone, A.; Azam, T.U.; Perry, D.; Shadid, H.; Anderson, E.; Catalan, T.; Blakely, P.; et al. Biomarker Trends, Incidence, and Outcomes of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC CardioOncol. 2022, 4, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Oishi, H.; Morimoto, R.; Shimoyama, Y.; Kuroda, K.; Urata, T.; Kondo, T.; Okumura, T.; Bando, Y.K.; Akiyama, M.; Murohara, T. Myocardial Vasculitis Associated With the Immune Checkpoint Inhibitor Pembrolizumab. JACC Case Rep. 2020, 2, 1937–1941. [Google Scholar] [CrossRef]
- Shalit, A.; Sarantis, P.; Koustas, E.; Trifylli, E.M.; Matthaios, D.; Karamouzis, M.V. Predictive Biomarkers for Immune-Related Endocrinopathies following Immune Checkpoint Inhibitors Treatment. Cancers 2023, 15, 375. [Google Scholar] [CrossRef]
- Iwamuro, M.; Tanaka, T.; Kono, Y.; Kawano, S.; Okada, H. Multiple White Plaques in the Esophagus: A Possible Case of Esophageal Mucosal Alteration Associated With Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Cureus 2022, 14, e32710. [Google Scholar] [CrossRef]
- Reid, P.; Cappelli, L.C. Treatment of Rheumatic Adverse Events of Cancer Immunotherapy. Best Pract. Res. Clin. Rheumatol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Di Mauro, A.; Cipullo, C.; Paccone, A.; Barbieri, A.; Palma, G.; Luciano, A.; Buccolo, S.; Bisceglia, I.; et al. Immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. Front. Cardiovasc. Med. 2022, 9, 930797. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Rea, D.; Barbieri, A.; Iovine, M.; Bonelli, A.; Caronna, A.; Botti, G.; De Lorenzo, C.; Maurea, N. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. J. Pers. Med. 2020, 10, 179. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Zanni, M.V.; Neilan, T.G. Atherosclerosis with Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 598–615. [Google Scholar] [CrossRef]
- Thuny, F.; Naidoo, J.; Neilan, T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022, 43, 4458–4468. [Google Scholar] [CrossRef]
- Poels, K.; van Leent, M.M.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.; Reiche, M.E.; Kusters, P.J.; Malinova, T.; et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell–Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncol. 2020, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Ministrini, S.; Bonaventura, A.; Vecchié, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Serum levels of VCAM-1 are associated with survival in patients treated with nivolumab for NSCLC. Eur. J. Clin. Investig. 2022, 52, e13668. [Google Scholar] [CrossRef]
- Tajiri, K.; Sekine, I. Atherosclerotic cardiovascular events associated with immune checkpoint inhibitors in cancer patients. Jpn. J. Clin. Oncol. 2022, 52, 659–664, Erratum in Jpn. J. Clin. Oncol. 2022, 52, 1358. [Google Scholar] [CrossRef]
- Sotler, T.; Šebeštjen, M. PCSK9 as an Atherothrombotic Risk Factor. Int. J. Mol. Sci. 2023, 24, 1966. [Google Scholar] [CrossRef]
- Luquero, A.; Vilahur, G.; Casani, L.; Badimon, L.; Borrell-Pages, M. Differential cholesterol uptake in liver cells: A role for PCSK9. FASEB J. 2022, 36, e22291. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.; Andresyuk, E.; Porozov, Y.; Tarasov, V.; Samsonov, M.; Preferanskaya, N.; Veselov, V.; Alyautdin, R. PCSK9 as a Target for Development of a New Generation of Hypolipidemic Drugs. Molecules 2022, 27, 434. [Google Scholar] [CrossRef]
- Wilkins, J.T.; Lloyd-Jones, D.M. Novel Lipid-Lowering Therapies to Reduce Cardiovascular Risk. JAMA 2021, 326, 266–267, Erratum in JAMA 2021, 326, 1637. [Google Scholar] [CrossRef]
- Rohrbach, S.; Li, L.; Novoyatleva, T.; Niemann, B.; Knapp, F.; Molenda, N.; Schulz, R. Impact of PCSK9 on CTRP9-Induced Metabolic Effects in Adult Rat Cardiomyocytes. Front. Physiol. 2021, 12, 593862. [Google Scholar] [CrossRef]
- Schmid, J.A. PCSK9 inhibition might increase endothelial inflammation. Atherosclerosis 2022, 362, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Alannan, M.; Fatrouni, H.; Trézéguet, V.; Dittrich-Domergue, F.; Moreau, P.; Siegfried, G.; Liet, B.; Khatib, A.-M.; Grosset, C.F.; Badran, B.; et al. Targeting PCSK9 in Liver Cancer Cells Triggers Metabolic Exhaustion and Cell Death by Ferroptosis. Cells 2022, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hyung, S.; Lee, J.; Choi, S.-H. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: A retrospective cohort study. J. Immunother. Cancer 2022, 10, e005226. [Google Scholar] [CrossRef]
- E Sise, M.; Wang, Q.; Seethapathy, H.; Moreno, D.; Harden, D.; Smith, R.N.; A Rosales, I.; Colvin, R.B.; Chute, S.; Cornell, L.D.; et al. Soluble and cell-based markers of immune checkpoint inhibitor-associated nephritis. J. Immunother. Cancer 2023, 11, e006222. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, T.; Niu, M.; Wu, Y.; Zhao, Z.; Wu, K. TGF-β: A novel predictor and target for anti-PD-1/PD-L1 therapy. Front. Immunol. 2022, 13, 1061394. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, X.; Chen, L. Immune myocarditis related to sintilimab treatment in a patient with advanced lung adenocarcinoma: A case report. Front. Cardiovasc. Med. 2022, 9, 955527. [Google Scholar] [CrossRef]
- Kurozumi, A.; Sakamoto, K.; Nakagawa, T.; Matsunaga, F.; Shimomura, A.; Shimizu, C.; Hara, H.; Hiroi, Y. Atherosclerotic Progression Is Related to Immune-Related Adverse Events. Int. Heart J. 2022, 63, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.N.; Ben Zadok, O.I.; Kornowski, R. The Potential Cardiotoxicity of Immune Checkpoint Inhibitors. J. Clin. Med. 2022, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Chiampan, A.; Lanzoni, L.; Verzè, M.; Molon, G.; Gori, S. Immune Checkpoint Inhibitors and Atherosclerotic Vascular Events in Cancer Patients. Front. Cardiovasc. Med. 2021, 8, 652186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Chen, X.; Zhang, R.; Li, J.; Zhang, X.; Zuo, P.; Ma, G. Effects of PCSK9 Inhibition on Coronary Atherosclerosis Regression of Nontarget Lesions after Primary Percutaneous Coronary Intervention in Acute Coronary Syndrome Patients. J. Interv. Cardiol. 2022, 2022, 4797529. [Google Scholar] [CrossRef]
- Luo, J.; Liao, W.; Wang, X.; Xu, R.; Li, W.; Li, W.; Liu, K.; Huang, K.; Ma, Y.; Wang, T.; et al. PCSK9 inhibitors for anti-inflammation in atherosclerosis: Protocol for a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e062046. [Google Scholar] [CrossRef]
- Noto, D.; Arca, M.; Tarugi, P.; Cefalù, A.B.; Barbagallo, C.M.; Averna, M.R. Association between familial hypobetalipoproteinemia and the risk of diabetes. Is this the other side of the cholesterol–diabetes connection? A systematic review of literature. Acta Diabetol. 2017, 54, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction during Sepsis via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation, 2022; ahead of print. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, Y.-M.; He, N.-Q.; Gao, R.; Xu, W.-X.; Zhuo, X.-J.; Ren, Z.; Wu, C.-Y.; Liu, L.-S. PCSK9: A emerging participant in heart failure. Biomed. Pharmacother. 2023, 158, 114106. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ballester, M.; Hurtado-Genovés, G.; Taberner-Cortés, A.; Herrero-Cervera, A.; Martínez-Hervás, S.; González-Navarro, H. Therapies for the Treatment of Cardiovascular Disease Associated with Type 2 Diabetes and Dyslipidemia. Int. J. Mol. Sci. 2021, 22, 660. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Koutagiar, I.; Terentes-Printzios, D.; Skoumas, I.; Rigatou, A.; Miliou, A.; Skliros, A.-N.; Pantou, S.; Filis, K.; Tousoulis, D. Relationship of PCSK9 levels with indices of vascular function and subclinical atherosclerosis in patients with familial dyslipidemias. Hell. J. Cardiol. 2018, 60, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cacciottolo, P.J.; Kostapanos, M.S.; Sancho, E.H.; Pavey, H.; Kaloyirou, F.; Vamvaka, E.; Helmy, J.; Hubsch, A.; McEniery, C.M.; Wilkinson, I.B.; et al. Investigating the Lowest Threshold of Vascular Benefits from LDL Cholesterol Lowering with a PCSK9 mAb Inhibitor (Alirocumab) in Patients with Stable Cardiovascular Disease (INTENSITY-HIGH): Protocol and study rationale for a randomised, open label, parallel group, mechanistic study. BMJ Open 2021, 11, e037457. [Google Scholar] [CrossRef]
- Sawaguchi, J.; Saeki, Y.; Oda, M.; Takamura, T.-A.; Fujibayashi, K.; Wakasa, M.; Akao, H.; Kitayama, M.; Kawai, Y.; Kajinami, K. The circulating furin-cleaved/mature PCSK9 ratio has a potential prognostic significance in statin-naïve patients with acute ST elevation myocardial infarction. Atheroscler. Plus 2022, 50, 50–56. [Google Scholar] [CrossRef]
- Wang, S.; Fu, D.; Liu, H.; Peng, D. Independent association of PCSK9 with platelet reactivity in subjects without statin or antiplatelet agents. Front. Cardiovasc. Med. 2022, 9, 934914. [Google Scholar] [CrossRef]
- Papotti, B.; Adorni, M.P.; Marchi, C.; Zimetti, F.; Ronda, N.; Panighel, G.; Lupo, M.G.; Vilella, A.; Giuliani, D.; Ferri, N.; et al. PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12192. [Google Scholar] [CrossRef]
- Gratton, J.; Finan, C.; Hingorani, A.D.; Humphries, S.E.; Futema, M. LDL-C Concentrations and the 12-SNP LDL-C Score for Polygenic Hypercholesterolaemia in Self-Reported South Asian, Black and Caribbean Participants of the UK Biobank. Front. Genet. 2022, 13, 845498. [Google Scholar] [CrossRef]
- Ostadal, P.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; A Bittner, V.; Chua, T.; Diaz, R.; Goodman, S.G.; Huo, Y.; Jukema, J.W.; et al. Metabolic risk factors and effect of alirocumab on cardiovascular events after acute coronary syndrome: A post-hoc analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2022, 10, 330–340. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef]
- Merleev, A.; Ji-Xu, A.; Toussi, A.; Tsoi, L.C.; Le, S.T.; Luxardi, G.; Xing, X.; Wasikowski, R.; Liakos, W.; Brüggen, M.-C.; et al. Proprotein convertase subtilisin/kexin type 9 is a psoriasis-susceptibility locus that is negatively related to IL36G. J. Clin. Investig. 2022, 7, 141193. [Google Scholar] [CrossRef]
- Seidah, N.G. The PCSK9 discovery, an inactive protease with varied functions in hypercholesterolemia, viral infections, and cancer. J. Lipid Res. 2021, 62, 100130. [Google Scholar] [CrossRef] [PubMed]
- Krempf, M.; Hopkins, P.N.; Bruckert, E.; Lee, S.; Donahue, S. Efficacy and Safety of Alirocumab in Patients With Autosomal Dominant Hypercholesterolemia Associated With Proprotein Convertase Subtilisin/Kexin Type 9 Gain-of-Function or Apolipoprotein B Loss-of-Function Mutations. Am. J. Cardiol. 2019, 125, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, B.; Tai, S.; Zhou, S.; Zheng, X.-L. PCSK9: Associated with cardiac diseases and their risk factors? Arch. Biochem. Biophys. 2020, 704, 108717. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; McSweeney, C.M.; Maneechote, C.; Moisescu, D.M.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischaemia/reperfusion injury: Benefits beyond lipid-lowering effects. J. Cell. Mol. Med. 2019, 23, 7310–7319. [Google Scholar] [CrossRef]
- Li, X.; Dai, F.; Wang, H.; Wei, G.; Jiang, Q.; Yin, P.; Wang, S.; Ge, J.; Yang, C.; Wu, J.; et al. PCSK9 participates in oxidized-low density lipoprotein-induced myocardial injury through mitochondrial oxidative stress and Drp1-mediated mitochondrial fission. Clin. Transl. Med. 2022, 12, e729. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Shahanawaz, J.; Theus, S.; Fan, Y.; Deng, X.; Zhou, S.; Mehta, J. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 2018, 114, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef]
- Huang, G.; Lu, X.; Duan, Z.; Zhang, K.; Xu, L.; Bao, H.; Xiong, X.; Lin, M.; Li, C.; Li, Y.; et al. PCSK9 Knockdown Can Improve Myocardial Ischemia/Reperfusion Injury by Inhibiting Autophagy. Cardiovasc. Toxicol. 2022, 22, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Cantin, C.; Garchitorena, M.J.; Escalona, R.; Carvajal, J.A.; Illanes, S.E.; Gutierrez, J.; Leiva, A. Increased Circulating Levels of PCSK9 and Pro-Atherogenic Lipoprotein Profile in Pregnant Women with Maternal Supraphysiological Hypercholesterolemia. Antioxidants 2022, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Vecchione, R.; De Capua, A.; Lagreca, E.; Iaffaioli, R.V.; Botti, G.; A Netti, P.; Maurea, N. Nano-Encapsulation of Coenzyme Q10 in Secondary and Tertiary Nano-Emulsions for Enhanced Cardioprotection and Hepatoprotection in Human Cardiomyocytes and Hepatocytes During Exposure to Anthracyclines and Trastuzumab. Int. J. Nanomed. 2020, 15, 4859–4876. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Coppola, C.; Mita, D.; Piscopo, G.; Iaffaioli, R.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Byun, J.H.; Lebeau, P.F.; Platko, K.; Carlisle, R.E.; Faiyaz, M.; Chen, J.; MacDonald, M.E.; Makda, Y.; Yousof, T.; Lynn, E.G.; et al. Inhibitory Antibodies against PCSK9 Reduce Surface CD36 and Mitigate Diet-Induced Renal Lipotoxicity. Kidney360 2022, 3, 1394–1410. [Google Scholar] [CrossRef]
- Lüscher, T.F. Cholesterol production, accumulation, reverse transport, and excretion: Opportunities for statins, PPAR-α agonists, and PCSK9 inhibitors. Eur. Heart J. 2015, 36, 2965–2967. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Nanna, M.G.; Nelson, A.J.; Haynes, K.; Shambhu, S.; Eapen, Z.; Cziraky, M.J.; Calvert, S.B.; Pagidipati, N.J.; Granger, C.B. Lipid-Lowering Treatment among Older Patients with Atherosclerotic Cardiovascular Disease. J. Am. Geriatr. Soc. 2022; ahead of print. [Google Scholar] [CrossRef]
- Likozar, A.R.; Šebeštjen, M. Smoking and diabetes attenuate beneficial effects of PSCK9 inhibitors on arterial wall properties in patients with very high lipoprotein (a) levels. Atheroscler. Plus 2022, 50, 1–9. [Google Scholar] [CrossRef]
- Ahamad, S.; Bhat, S.A. Recent Update on the Development of PCSK9 Inhibitors for Hypercholesterolemia Treatment. J. Med. Chem. 2022, 65, 15513–15539. [Google Scholar] [CrossRef]
- Goonewardena, S.N.; Rosenson, R.S. PCSK9: The Nexus of Lipoprotein Metabolism and Inflammation in COVID-19. J. Am. Coll. Cardiol. 2023, 81, 235–236. [Google Scholar] [CrossRef]
- Paré, G.; Chong, M.; Mohammadi-Shemirani, P. Lipoprotein(a) Cholesterol Masquerading as Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1047–1049. [Google Scholar] [CrossRef]
- Dai, N.; Chen, Z.; Zhou, F.; Zhou, Y.; Hu, N.; Duan, S.; Wang, W.; Yu, Y.; Zhang, L.; Qian, J.; et al. Association of Lipoprotein (a) With Coronary-Computed Tomography Angiography-Assessed High-Risk Coronary Disease Attributes and Cardiovascular Outcomes. Circ. Cardiovasc. Imaging 2022, 15, e014611. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; Daghem, M.; Tzolos, E.; Meah, M.N.; Doris, M.K.; Moss, A.J.; Kwiecinski, J.; Kroon, J.; Nurmohamed, N.S.; van der Harst, P.; et al. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J. Am. Coll. Cardiol. 2022, 79, 223–233. [Google Scholar] [CrossRef]
- Sheikhy, M.; Schrieber, S.J.; Sun, Q.; Gershuny, V.; Matta, M.K.; Bai, J.P.; Du, X.; Vegesna, G.; Shah, A.; Prentice, K.; et al. Considerations for Use of Pharmacodynamic Biomarkers to Support Biosimilar Development—(I) A Randomized Trial with PCSK9 Inhibitors. Clin. Pharmacol. Ther. 2022, 113, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Parham, J.S.; Goldberg, A.C. Review of recent clinical trials and their impact on the treatment of hypercholesterolemia. Prog. Cardiovasc. Dis. 2022, 75, 90–96. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, F.; Lei, C.; Qi, T.; Xue, X.; Meng, Y.; Zhang, W.; Zhang, H.; Wang, J.; Zhu, H.; et al. Effect of evolocumab on the progression of intraplaque neovascularization of the carotid based on contrast-enhanced ultrasonography (EPIC study): A prospective single-arm, open-label study. Front. Pharmacol. 2023, 13, 999224. [Google Scholar] [CrossRef]
- Erviti, J.; Wright, J.; Bassett, K.; Ben-Eltriki, M.; Jauca, C.; Saiz, L.C.; Leache, L.; Gutiérrez-Valencia, M.; Perry, T.L. Restoring mortality data in the FOURIER cardiovascular outcomes trial of evolocumab in patients with cardiovascular disease: A reanalysis based on regulatory data. BMJ Open 2022, 12, e060172. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.C.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nissen, S.E.; Prati, F.; Windecker, S.; Kataoka, Y.; Puri, R.; Hucko, T.; Kassahun, H.; Liao, J.; Somaratne, R.; et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: Rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 2021, 11, 120–129. [Google Scholar] [CrossRef]
- Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.; Zafar, S.; Panthangi, V.; Kurupp, A.R.C.; Raju, A.; Khan, S. Exploring the Efficacy of Alirocumab and Evolocumab in Reducing Low-Density Lipoprotein (LDL) Cholesterol Levels in Patients With Familial Hypercholesterolemia: A Systematic Review. Cureus 2022, 14, e28930. [Google Scholar] [CrossRef]
- Nolain, P.; Djebli, N.; Brunet, A.; Fabre, D.; Khier, S. Combined Semi-mechanistic Target-Mediated Drug Disposition and Pharmacokinetic–Pharmacodynamic Models of Alirocumab, PCSK9, and Low-Density Lipoprotein Cholesterol in a Pooled Analysis of Randomized Phase I/II/III Studies. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 789–802. [Google Scholar] [CrossRef]
- Mahmood, T.; Minnier, J.; Ito, M.K.; Li, Q.H.; Koren, A.; Kam, I.W.; Fazio, S.; Shapiro, M.D. Discordant responses of plasma low-density lipoprotein cholesterol and lipoprotein(a) to alirocumab: A pooled analysis from 10 ODYSSEY Phase 3 studies. Eur. J. Prev. Cardiol. 2020, 28, 816–822. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Q.; Bukowski, J.F.; Yunis, C.; Shear, C.L.; Ridker, P.M.; Schwartz, P.F.; Baltrukonis, D. Assessing the Potential Risk of Cross-Reactivity Between Anti-Bococizumab Antibodies and Other Anti-PCSK9 Monoclonal Antibodies. Biodrugs 2019, 33, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rose, L.M.; Kastelein, J.J.; Santos, R.D.; Wei, C.; Revkin, J.; Yunis, C.; Tardif, J.-C.; Shear, C.L. Cardiovascular event reduction with PCSK9 inhibition among 1578 patients with familial hypercholesterolemia: Results from the SPIRE randomized trials of bococizumab. J. Clin. Lipidol. 2018, 12, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Amarenco, P.; Brunell, R.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.; Koenig, W.; Nissen, S.; Revkin, J.; Santos, R.D.; et al. Evaluating bococizumab, a monoclonal antibody to PCSK9, on lipid levels and clinical events in broad patient groups with and without prior cardiovascular events: Rationale and design of the Studies of PCSK9 Inhibition and the Reduction of vascular Events (SPIRE) Lipid Lowering and SPIRE Cardiovascular Outcomes Trials. Am. Heart J. 2016, 178, 135–144. [Google Scholar] [CrossRef]
- Fazio, S.; Robertson, D.G.; Joh, T.; Wan, H.; Riel, T.; Forgues, P.; Baum, C.M.; Garzone, P.D.; Gumbiner, B. Effects of 12 weeks of treatment with intravenously administered bococizumab, a humanized monoclonal antibody blocking proprotein convertase subtilisin/kexin type 9, in hypercholesterolemic subjects on high-dose statin. Cardiovasc. Ther. 2018, 36, e12308. [Google Scholar] [CrossRef]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients With Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef]
- Scheen, A.J.; Wallemacq, C.; Lancellotti, P. Le médicament du mois. L’inclisiran (Leqvio®), hypocholestérolémiant puissant inhibant la synthèse de PCSK9 par la technique innovante de l’ARN interférent [Inclisiran (Leqvio®), a potent cholesterol-lowering agent by inhibiting PCSK9 using small interfering RNA-based innovative therapy]. Rev. Med. Liege. 2022, 77, 745–751. (In French) [Google Scholar]
- Ray, K.K.; Stoekenbroek, R.M.; Kallend, D.; Nishikido, T.; Leiter, L.A.; Landmesser, U.; Wright, R.S.; Wijngaard, P.L.J.; Kastelein, J.J.P. Effect of 1 or 2 Doses of Inclisiran on Low-Density Lipoprotein Cholesterol Levels: One-Year Follow-up of the ORION-1 Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 1067–1075. [Google Scholar] [CrossRef]
- Raal, F.; Ableson, M.; Blignaut, S.; Burgess, L.; Coetzer, S.; Ebrahim, I.; Gibbon, A.; van Rensburg, D.J.; Jaros, M.; Lombard, L.; et al. Safety and efficacy of inclisiran in South African patients at high cardiovascular risk: A subanalysis of the ORION phase III clinical trials. S. Afr. Med. J. 2022, 112, 426–432. [Google Scholar] [CrossRef]
- Koenig, W.; Conde, L.G.; Landmesser, U.; Leiter, L.A.; Ray, K.K.; Schwartz, G.G.; Wright, R.S.; Han, J.; Raal, F.J. Efficacy and Safety of Inclisiran in Patients with Polyvascular Disease: Pooled, Post Hoc Analysis of the ORION-9, ORION-10, and ORION-11 Phase 3 Randomized Controlled Trials. Cardiovasc. Drugs Ther. 2022; ahead of print. [Google Scholar] [CrossRef]
- Ray, K.K.; Kallend, D.; Leiter, L.A.; Raal, F.J.; Koenig, W.; Jaros, M.J.; Schwartz, G.G.; Landmesser, U.; Garcia Conde, L.; Wright, R.S.; et al. Effect of inclisiran on lipids in primary prevention: The ORION-11 trial. Eur. Heart J. 2022, 43, 5047–5057. [Google Scholar] [CrossRef]
- Warden, B.A.; Duell, P.B. Inclisiran: A Novel Agent for Lowering Apolipoprotein B–containing Lipoproteins. J. Cardiovasc. Pharmacol. 2021, 78, e157–e174. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03705234 (accessed on 25 January 2023).
- Mehranzadeh, E.; Crende, O.; Badiola, I.; Garcia-Gallastegi, P. What Are the Roles of Proprotein Convertases in the Immune Escape of Tumors? Biomedicines 2022, 10, 3292. [Google Scholar] [CrossRef]
- Seidah, N.G.; Prat, A. The Multifaceted Biology of PCSK9. Endocr. Rev. 2021, 43, 558–582. [Google Scholar] [CrossRef]
- Seidah, N.G.; Garçon, D. Expanding Biology of PCSK9: Roles in Atherosclerosis and Beyond. Curr. Atheroscler. Rep. 2022, 24, 821–830. [Google Scholar] [CrossRef]
- Fu, T.; Guan, Y.; Xu, J.; Wang, Y. APP, APLP2 and LRP1 interact with PCSK9 but are not required for PCSK9-mediated degradation of the LDLR in vivo. Biochim. et Biophys. Acta BBA-Mol. Cell Biol. Lipids 2017, 1862, 883–889. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Carey, S.T.; Bridgeman, C.; Jewell, C.M. Biomaterial Strategies for Selective Immune Tolerance: Advances and Gaps. Adv. Sci. 2023, 2, e2205105. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Wei, J.; Cun, X.; Lu, Z.; Qiu, Y.; Zhang, Z.; He, Q. Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8+ T cells. Nanomedicine 2018, 14, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.-X.; Wu, D.-F.; Miao, L.; Aung, L.H.H.; Cao, X.-L.; Yan, T.-T.; Long, X.-J.; Liu, W.-Y.; Zhang, L.; Li, M. Several genetic polymorphisms interact with overweight/obesity to influence serum lipid levels. Cardiovasc. Diabetol. 2012, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.M.; Li, C.M.; Tu, Y.M.; Li, Z.M.; Zhang, M. Additive effects of ezetimibe, evolocumab, and alirocumab on plaque burden and lipid content as assessed by intravascular ultrasound: A PRISMA-compliant meta-analysis. Medicine 2022, 101, e31199. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Meng, Z. Immunomodulatory Effect of Locoregional Therapy in the Tumor Microenvironment. Mol. Ther. 2023; ahead of print. [Google Scholar] [CrossRef]
- Goksøyr, L.; Skrzypczak, M.; Sampson, M.; Nielsen, M.A.; Salanti, A.; Theander, T.G.; Remaley, A.T.; De Jongh, W.A.; Sander, A.F. A cVLP-Based Vaccine Displaying Full-Length PCSK9 Elicits a Higher Reduction in Plasma PCSK9 Than Similar Peptide-Based cVLP Vaccines. Vaccines 2022, 11, 2. [Google Scholar] [CrossRef]
- Mbikay, M.; Chrétien, M. The biological relevance of PCSK9: When less is better…. Biochem. Cell Biol. 2022, 100, 189–198. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Zhang, X.; Yu, J.; Xu, H.; Cui, J.; Li, Y.; Niu, Y.; Zhou, C.; Xia, J.; et al. Targeting PCSK9 Ameliorates Graft Vascular Disease in Mice by Inhibiting NLRP3 Inflammasome Activation in Vascular Smooth Muscle Cells. Front. Immunol. 2022, 13, 894789. [Google Scholar] [CrossRef]
- Charbe, N.B.; Lagos, C.F.; Ortiz, C.A.V.; Tambuwala, M.; Palakurthi, S.S.; Zacconi, F.C. PCSK9 conjugated liposomes for targeted delivery of paclitaxel to the cancer cell: A proof-of-concept study. Biomed. Pharmacother. 2022, 153, 113428. [Google Scholar] [CrossRef]
- Piao, M.-X.; Bai, J.-W.; Zhang, P.-F.; Zhang, Y.-Z. PCSK9 regulates apoptosis in human neuroglioma u251 cells via mitochondrial signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 2787–2794. [Google Scholar] [PubMed]
- Douna, H.; Smit, V.; van Puijvelde, G.H.; Kiss, M.G.; Binder, C.J.; Bot, L.; Kuchroo, V.K.; Lichtman, A.H.; Kuiper, J.; Foks, A.C. Tim-1 mucin domain-mutant mice display exacerbated atherosclerosis. Atherosclerosis 2022, 352, 1–9. [Google Scholar] [CrossRef]
- Abdelwahed, K.S.; Siddique, A.B.; Qusa, M.H.; King, J.A.; Souid, S.; Elmageed, Z.Y.A.; El Sayed, K.A. PCSK9 Axis-Targeting Pseurotin A as a Novel Prostate Cancer Recurrence Suppressor Lead. ACS Pharmacol. Transl. Sci. 2021, 4, 1771–1781. [Google Scholar] [CrossRef]
- Saha, S.; Singh, A.; Kumar, P.; Sonkar, A.B.; Gautam, A.K.; Verma, A.; Maity, B.; Tiwari, H.; Sahoo, N.G.; Keshari, A.K.; et al. A Comprehensive Review on PCSK9 as Mechanistic Target Approach in Cancer Therapy. Mini-Rev. Med. Chem. 2021; ahead of print. [Google Scholar] [CrossRef]
- Xia, X.-D.; Peng, Z.-S.; Gu, H.-M.; Wang, M.; Wang, G.-Q.; Zhang, D.-W. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Wei, J.; Nguyen, T.-L.M.; Shi, H.; Su, W.; Palacios, G.; Dhungana, Y.; Chapman, N.M.; Long, L.; Saravia, J.; et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 2021, 591, 306–311. [Google Scholar] [CrossRef]
- McBrearty, N.; Cho, C.; Chen, J.; Zahedi, F.; Peck, A.R.; Radaelli, E.; Assenmacher, C.A.; Pavlak, C.; Devine, A.; Yu, P.; et al. Tumor Suppressive and Immune-Stimulating Roles of Cholesterol 25-Hydroxylase in Pancreatic Cancer Cells. Mol. Cancer Res. 2022; OF1–OF12, ahead of print. [Google Scholar] [CrossRef]
- Yuan, J.; Cai, T.; Zheng, X.; Ren, Y.; Qi, J.; Lu, X.; Chen, H.; Lin, H.; Chen, Z.; Liu, M.; et al. Correction to: Potentiating CD8+ T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell 2022, 13, 694–700, Erratum in Protein Cell 2021, 12, 240–260. [Google Scholar] [CrossRef]
- Mahboobnia, K.; Pirro, M.; Marini, E.; Grignani, F.; Bezsonov, E.E.; Jamialahmadi, T.; Sahebkar, A. PCSK9 and cancer: Rethinking the link. Biomed. Pharmacother. 2021, 140, 111758. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N. HMGB1/RAGE/TLR4 axis and glutamate as novel targets for PCSK9 inhibitor in high fat cholesterol diet induced cognitive impairment and amyloidosis. Life Sci. 2021, 273, 119310. [Google Scholar] [CrossRef]
- Nishikawa, R.; Furuhashi, M.; Hori, M.; Ogura, M.; Harada-Shiba, M.; Okada, T.; Koseki, M.; Kujiraoka, T.; Hattori, H.; Ito, R.; et al. A Resuscitated Case of Acute Myocardial Infarction with both Familial Hypercholesterolemia Phenotype Caused by Possibly Oligogenic Variants of the PCSK9 and ABCG5 Genes and Type I CD36 Deficiency. J. Atheroscler. Thromb. 2022, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Luo, H.; Lu, Q.; Yu, S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef]
- Chong, E.W.; Joncas, F.-H.; Seidah, N.G.; Calon, F.; Diorio, C.; Gangloff, A. Circulating levels of PCSK9, ANGPTL3 and Lp(a) in stage III breast cancers. BMC Cancer 2022, 22, 1049. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; He, P.; An, D.; Guo, X.; Zhang, X.; Feng, M. Inhibition of PCSK9 enhances the antitumor effect of PD-1 inhibitor in colorectal cancer by promoting the infiltration of CD8+ T cells and the exclusion of Treg cells. Front. Immunol. 2022, 13, 947756. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Meng, J.; Guo, F.; Ren, D.; Wu, H.; Jin, X. S-palmitoylation of PCSK9 induces sorafenib resistance in liver cancer by activating the PI3K/AKT pathway. Cell Rep. 2022, 40, 111194. [Google Scholar] [CrossRef]
- Wong, C.C.; Wu, J.-L.; Ji, F.; Kang, W.; Bian, X.; Chen, H.; Chan, L.-S.; Luk, S.T.Y.; Tong, S.; Xu, J.; et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat. Commun. 2022, 13, 3971. [Google Scholar] [CrossRef]
- Shu, X.; Wu, J.; Zhang, T.; Ma, X.; Du, Z.; Xu, J.; You, J.; Wang, L.; Chen, N.; Luo, M.; et al. Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance. Nutrients 2022, 14, 5314. [Google Scholar] [CrossRef] [PubMed]
- Tchéoubi, S.E.R.; Akpovi, C.D.; Coppée, F.; Declèves, A.-E.; Laurent, S.; Agbangla, C.; Burtea, C. Molecular and cellular biology of PCSK9: Impact on glucose homeostasis. J. Drug Target. 2022, 30, 948–960. [Google Scholar] [CrossRef]
- Sun, X.; Essalmani, R.; Day, R.; Khatib, A.M.; Seidah, N.G.; Prat, A. Proprotein Convertase Subtilisin/Kexin Type 9 Deficiency Reduces Melanoma Metastasis in Liver. Neoplasia 2012, 14, 1122–1131. [Google Scholar] [CrossRef]
- Sun, L.; Ding, H.; Jia, Y.; Shi, M.; Guo, D.; Yang, P.; Wang, Y.; Liu, F.; Zhang, Y.; Zhu, Z. Associations of genetically proxied inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with breast cancer and prostate cancer. Breast Cancer Res. 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Sanz, D.J.; Raivola, J.; Karvonen, H.; Arjama, M.; Barker, H.; Murumägi, A.; Ungureanu, D. Evaluating Targeted Therapies in Ovarian Cancer Metabolism: Novel Role for PCSK9 and Second Generation mTOR Inhibitors. Cancers 2021, 13, 3727. [Google Scholar] [CrossRef]
- Bonaventura, A.; Grossi, F.; Carbone, F.; Vecchié, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Serum PCSK9 levels at the second nivolumab cycle predict overall survival in elderly patients with NSCLC: A pilot study. Cancer Immunol. Immunother. 2019, 68, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, H.; Li, H.; Ge, S.; Zhang, F.; Wang, L.; Shi, H.; Han, A. Self-Assembly of a Multifunction DNA Tetrahedron for Effective Delivery of Aptamer PL1 and Pcsk9 siRNA Potentiate Immune Checkpoint Therapy for Colorectal Cancer. ACS Appl. Mater. Interfaces 2022, 14, 31634–31644. [Google Scholar] [CrossRef]
- Liu, X.; Bao, X.; Hu, M.; Chang, H.; Jiao, M.; Cheng, J.; Xie, L.; Huang, Q.; Li, F.; Li, C.-Y. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020, 588, 693–698. [Google Scholar] [CrossRef]
- Carbone, A.; Bottino, R.; Russo, V.M.; D’Andrea, A.M.; Liccardo, B.; Maurea, N.; Quagliariello, V.; Cimmino, G.M.; Golino, P.M. Takotsubo Cardiomyopathy as Epiphenomenon of Cardiotoxicity in Patients With Cancer: A Meta-summary of Case Reports. J. Cardiovasc. Pharmacol. 2021, 78, e20–e29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, X.; Li, H.; Chen, X. Comparative efficacy and safety of immune checkpoint inhibitors for unresectable advanced melanoma: A systematic review and network meta-analysis. Int. Immunopharmacol. 2023, 115, 109657. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04586894 (accessed on 26 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT03709771 (accessed on 26 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04115410 (accessed on 26 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. https://doi.org/10.3390/cancers15051397
Quagliariello V, Bisceglia I, Berretta M, Iovine M, Canale ML, Maurea C, Giordano V, Paccone A, Inno A, Maurea N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers. 2023; 15(5):1397. https://doi.org/10.3390/cancers15051397
Chicago/Turabian StyleQuagliariello, Vincenzo, Irma Bisceglia, Massimiliano Berretta, Martina Iovine, Maria Laura Canale, Carlo Maurea, Vienna Giordano, Andrea Paccone, Alessandro Inno, and Nicola Maurea. 2023. "PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology" Cancers 15, no. 5: 1397. https://doi.org/10.3390/cancers15051397
APA StyleQuagliariello, V., Bisceglia, I., Berretta, M., Iovine, M., Canale, M. L., Maurea, C., Giordano, V., Paccone, A., Inno, A., & Maurea, N. (2023). PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers, 15(5), 1397. https://doi.org/10.3390/cancers15051397










