P21 Overexpression Promotes Cell Death and Induces Senescence in Human Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Radiation Induces Senescent-like Gene Expression in a Dose-Dependent Manner
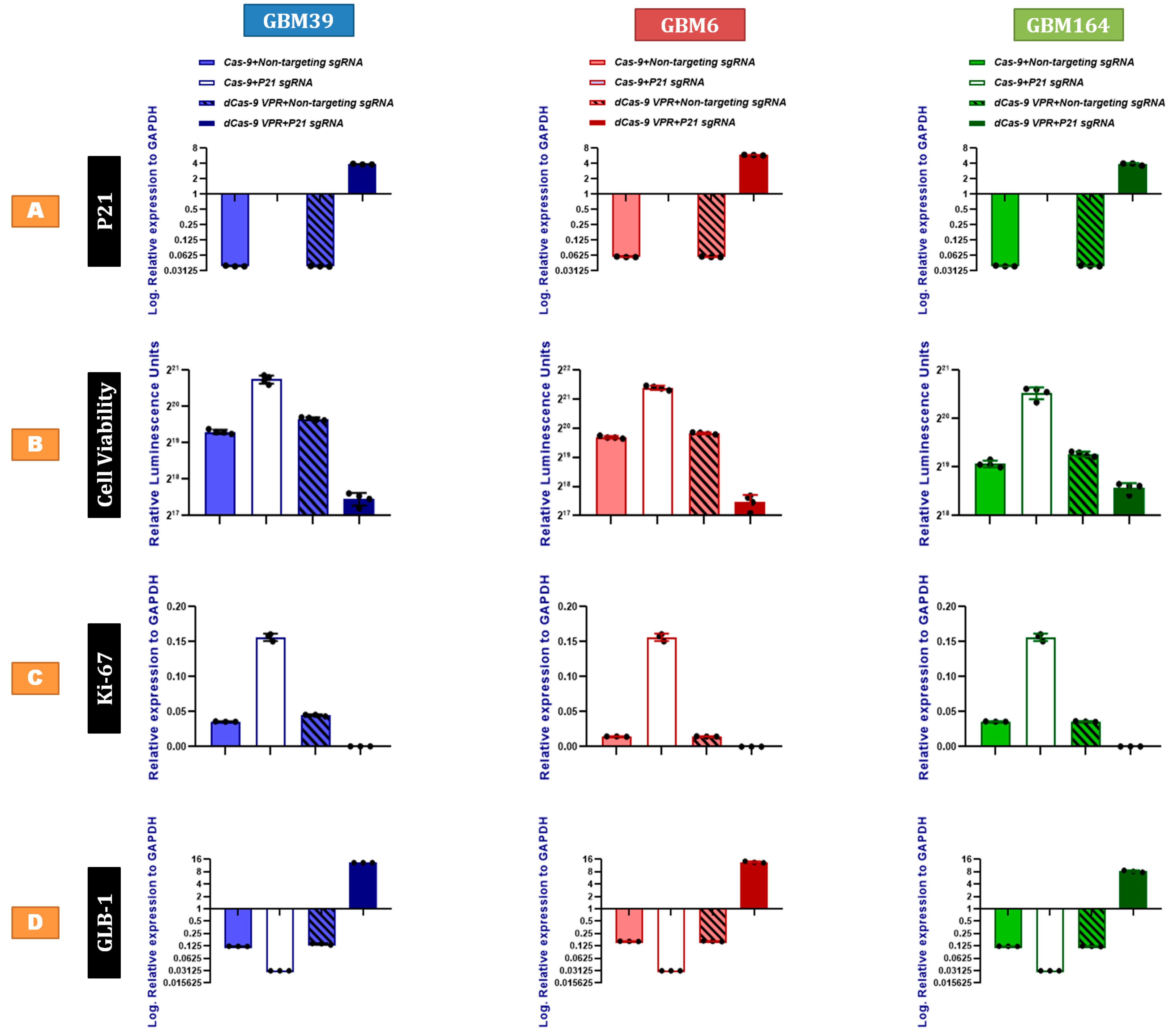
2.2. P21 Overexpression Inhibits Human GBM Proliferation and Promotes Cellular Senescence
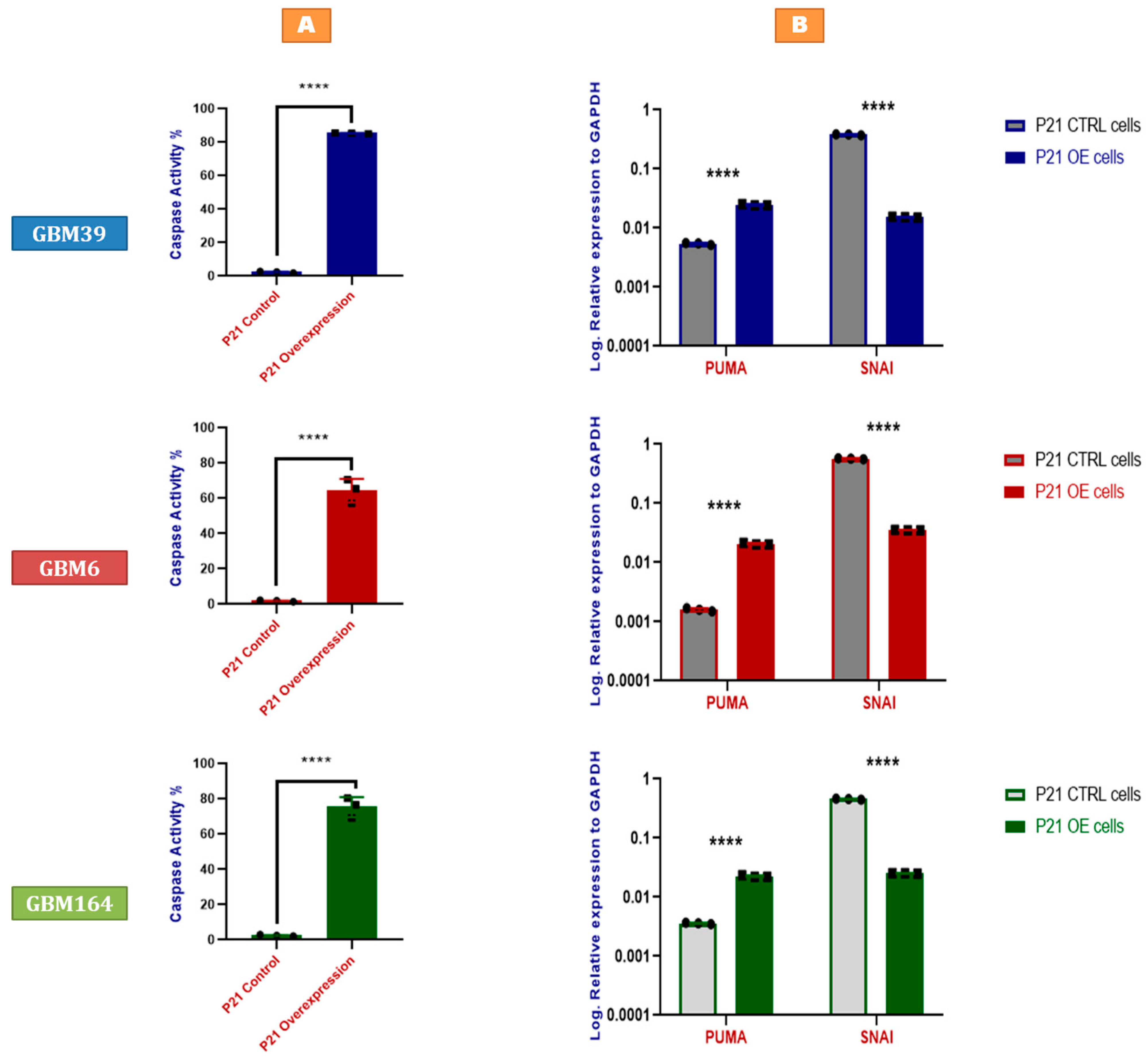
2.3. P21 Overexpression Induces Tumor Cell Apoptosis by Activating the p53-Upregulated Modulator of Apoptosis
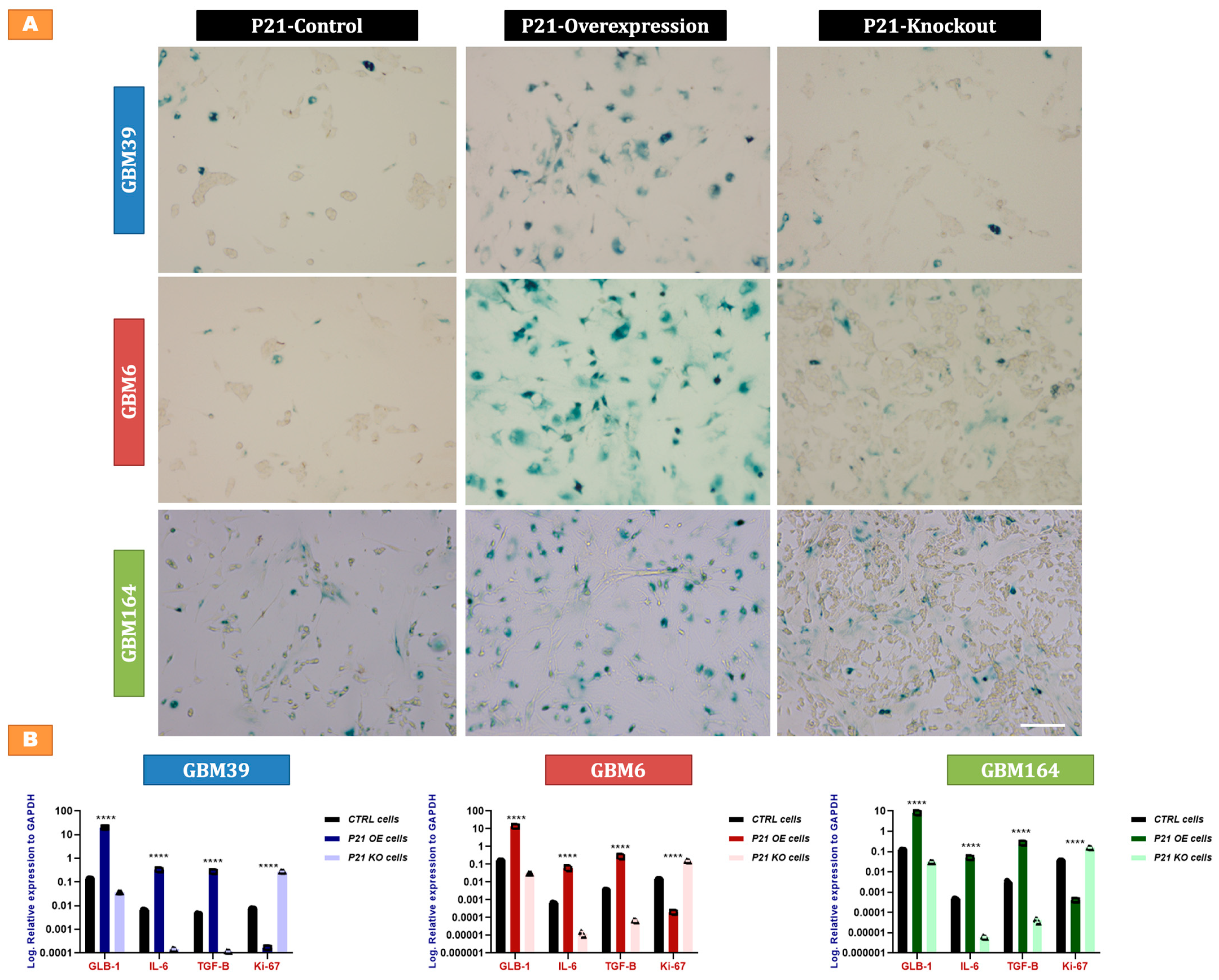
2.4. P21-Mediated Senescence in GBM Cells
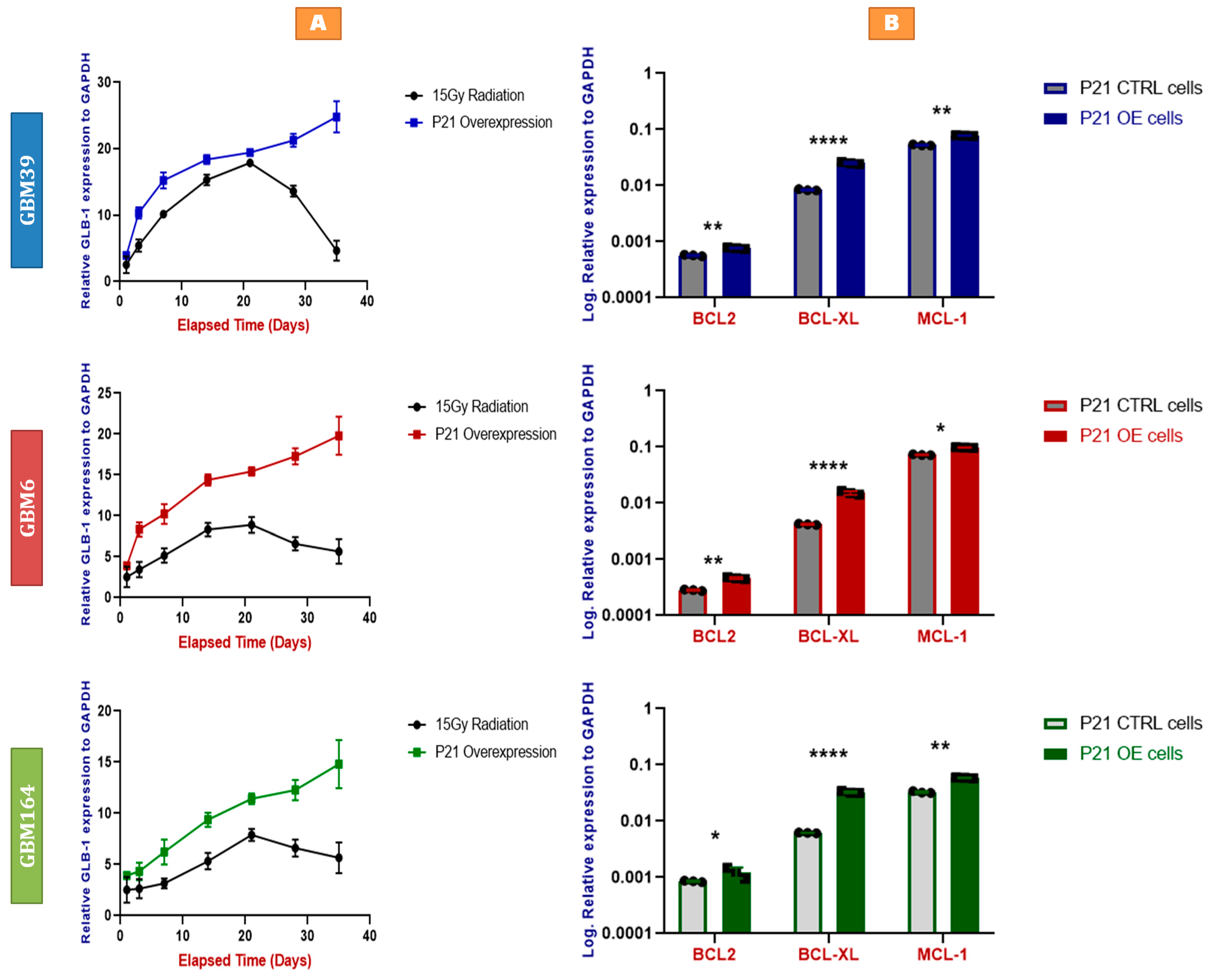
2.5. Faster and More Stable Senescence with P21 Overexpression than Irradiation
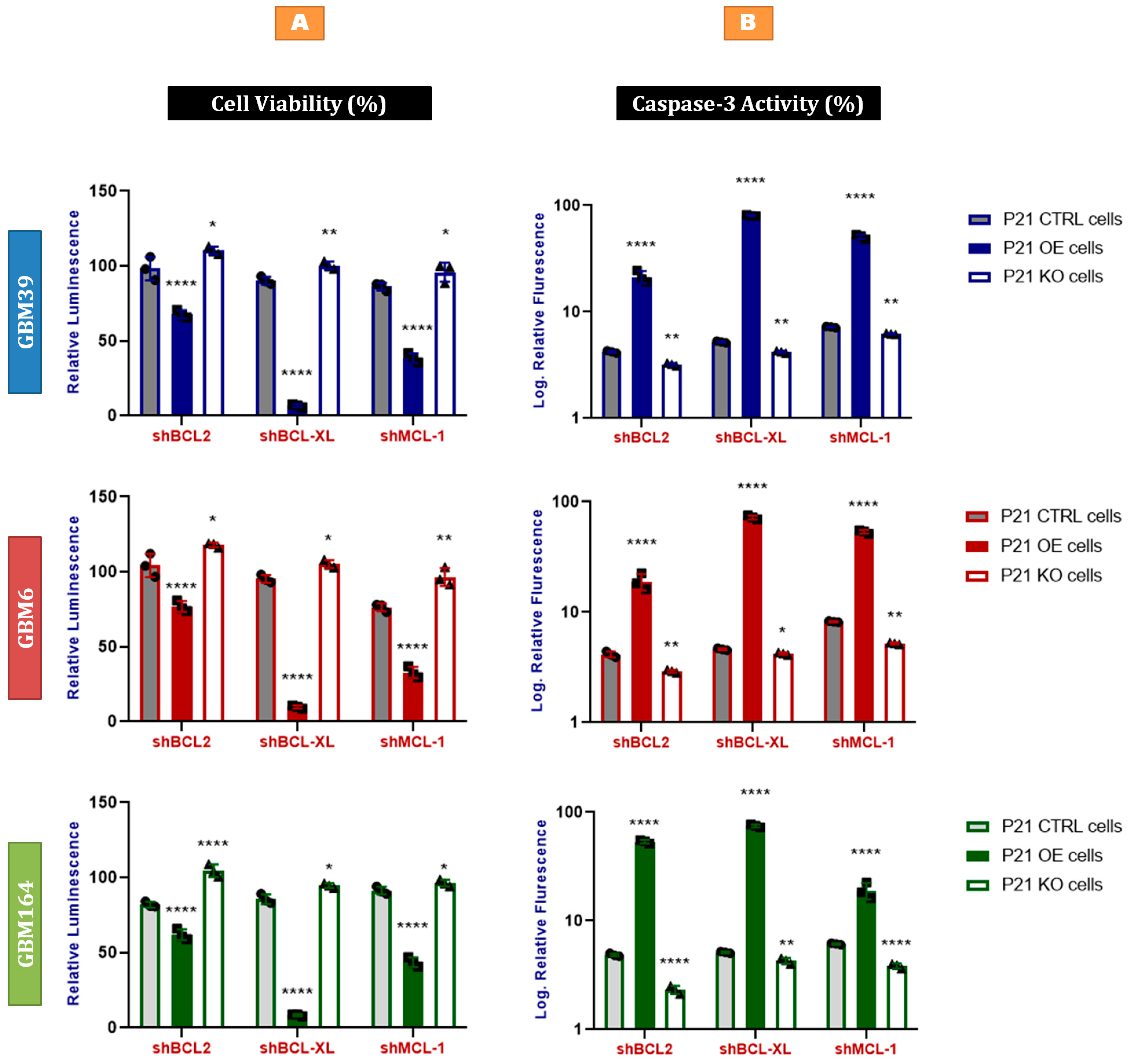
2.6. P21-Induced Senescence Relies on Bcl-xL for Survival
2.7. Bcl-xL Knock-Down Induces Apoptosis in P21-Induced Senescent Glioblastoma Cells
3. Methods
3.1. Cell Culture and Growth Conditions
3.2. Irradiation of Cells
3.3. Cell Viability Assay
3.4. Caspase-3 Assay
3.5. Quantitative Real-Time PCR (qRT-PCR)
3.6. Viral Packaging and Gene Knock-Down by shRNA
3.7. Gene Knock-Out by CRISPR/Cas9
3.8. Gene Knock-In (Overexpression) by dCas-VPR
3.8.1. Generating Cell Lines That Constitutively Express dCas9-VPR
3.8.2. Inducing P21 Overexpression
3.9. Senescence-Associated β-Galactosidase Staining
3.10. Protein Analysis by Western Blotting (WB)
3.11. Statistical Analysis
3.12. Reagents and Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, C.J.; Keister, B.A.; Stairs, D.B.; Boxer, R.B.; Moody, S.E.; Chodosh, L.A. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007, 9, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M.; Lezina, L.; Carrera, S.; Jukes-Jones, R.; Giblett, S.M.; Antonov, A.; Barlev, N.; Saldanha, G.S.; Pritchard, C.A.; Cain, K.; et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Collado, M.; Gil, J.; Efeyan, A.; Guerra, C.; Schuhmacher, A.J.; Barradas, M.; Benguría, A.; Zaballos, A.; Flores, J.M.; Barbacid, M.; et al. Tumour biology: Senescence in premalignant tumours. Nature 2005, 436, 642. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 325, 987–996. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Rocco, J.W.; Sidransky, D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp. Cell Res. 2001, 264, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Radhakrishnan, S.K. Lost in transcription: P21 repression, mechanisms, and consequences. Cancer Res. 2005, 65, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking P21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.L.; Pokorny, J.L.; Schroeder, M.A.; Sarkaria, J.N. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr. Protoc. Pharmacol. 2011, 52, 14–16. [Google Scholar] [CrossRef]
- Zilfou, J.T.; Spector, M.S.; Lowe, S.W. Slugging it out: Fine tuning the p53-PUMA death connection. Cell 2005, 123, 545–548. [Google Scholar] [CrossRef]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 2016, 8, 1294–1315. [Google Scholar] [CrossRef]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.I.; et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 2017, 9, 1867–1884. [Google Scholar] [CrossRef]
- Rahman, M.; Olson, I.; Mansour, M.; Carlstrom, L.P.; Sutiwisesak, R.; Saber, R.; Rajani, K.; Warrington, A.E.; Howard, A.; Schroeder, M.; et al. Selective Vulnerability of Senescent Glioblastoma Cells to BCL-XL Inhibition. Mol. Cancer Res. 2022, 20, 938–948. [Google Scholar] [CrossRef]
- Yeh, A.C.; Ramaswamy, S. Mechanisms of Cancer Cell Dormancy—Another Hallmark of Cancer? Cancer Res. 2015, 75, 5014–5022. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Auffinger, B.; Lesniak, M.S. Understanding glioma stem cells: Rationale, clinical relevance and therapeutic strategies. Expert Rev. Neurother. 2013, 13, 545–555. [Google Scholar] [CrossRef]
- Almog, N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010, 294, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gulaia, V.; Kumeiko, V.; Shved, N.; Cicinskas, E.; Rybtsov, S.; Ruzov, A.; Kagansky, A. Molecular Mechanisms Governing the Stem Cell’s Fate in Brain Cancer: Factors of Stemness and Quiescence. Front. Cell Neurosci. 2018, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martínez, F.; Loza, M.I.; Domínguez, E. Beyond Tumor Suppression: Senescence in Cancer Stemness and Tumor Dormancy. Cells 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Zucchinetti, C.; Buda, G.; Orciuolo, E. Tumor dormancy as an alternative step in the development of chemoresistance and metastasis—Clinical implications. Cell Oncol. 2020, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.L.; Cappell, S.D.; Tsai, F.C.; Overton, K.W.; Wang, C.L.; Meyer, T. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 2013, 155, 369–383. [Google Scholar] [CrossRef]
- Stewart-Ornstein, J.; Lahav, G. Dynamics of CDKN1A in Single Cells Defined by an Endogenous Fluorescent Tagging Toolkit. Cell Rep. 2016, 14, 1800–1811. [Google Scholar] [CrossRef]
- Kumar, R.; Paul, A.M.; Amjesh, R.; George, B.; Pillai, M.R. Coordinated dysregulation of cancer progression by the HER family and P21-activated kinases. Cancer Metastasis Rev. 2020, 39, 583–601. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Gruber, A.D. Differential expression of cell cycle regulators P21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res. Vet. Sci. 2009, 87, 91–96. [Google Scholar] [CrossRef]
- Klopfleisch, R.; von Euler, H.; Sarli, G.; Pinho, S.S.; Gärtner, F.; Gruber, A.D. Molecular carcinogenesis of canine mammary tumors: News from an old disease. Vet. Pathol. 2011, 48, 98–116. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. P21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Mosieniak, G.; Sliwinska, M.A. Morphological and Functional Characteristic of Senescent Cancer Cells. Curr. Drug Targets 2016, 17, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Prata, L.G.P.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Ito, Y.; Hoare, M.; Narita, M. Spatial and Temporal Control of Senescence. Trends Cell Biol. 2017, 27, 820–832. [Google Scholar] [CrossRef]
- Nacarelli, T.; Lau, L.; Fukumoto, T.; Zundell, J.; Fatkhutdinov, N.; Wu, S.; Aird, K.M.; Iwasaki, O.; Kossenkov, A.V.; Schultz, D.; et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 2019, 21, 397–407. [Google Scholar] [CrossRef]

| Sex | Male | Male | Female |
|---|---|---|---|
| Age | 65 | 51 | 38 |
| Recurrence Status | Primary | Primary | Primary |
| MGMT Methylation | U | M | M |
| Sub-type | Classical | Mesenchymal | Proneural |
| IDH-1 | - | - | Mh |
| CDKN2A | LL | L | LL |
| PTEN | L | LM | L |
| EGFR | A (V3) | A (V3) | - |
| TP53 | M | - | - |
| Met. | A | A | A |
| TERT Prom. | M | M | - |
| Others | - | MDM4 & PIKC32B (A) | PDGFR (A); NF1 (L); ATRX (T) |
| Reagent | Manufacturer | Catalogue Number |
|---|---|---|
| DMEM (Dulbecco’s Modified Eagle’s Medium) | Corning | 10-013-CV |
| Trypsin EDTA 1X | Corning | 25-052-CI |
| Opti-MEM™ I Reduced Serum Medium | Gibco | 31-985-070 |
| Blasticidin S HCl | ThermoFisher | A1113903 |
| Puromycin Dihydrochloride | ThermoFisher | A1113803 |
| Guide-it Cas9 Polyclonal Antibody | TaKaRa | 632606 & 632607 |
| Guide-it Mutation Detection kit | TaKaRa | 631448 |
| PureLink™ HiPure Plasmid Miniprep kit | Invitrogen | K210002 and K210003 |
| LB Broth | Gibco | 10855021 |
| M-MLV reverse transcriptase kit | ThermoFisher | 28025013 and 28025021 |
| The Cell Titer-Glo® | Promega | G7570, G7571, G7572, and G7573 |
| Caspase-3 Activity Assay Kit | Cell Signaling Technology (CST) | 5723 |
| TRIzol™ Reagent | Invitrogen | 15596018 |
| Senescence-associated β-Galactosidase Staining Kit | Cell Signaling Technology (CST) | 9860 |
| Pierce™ BCA Protein Assay Kit | ThermoFisher | 23225 |
| NuPAGE™ 4 to 12%, Bis-Tris, 1.0–1.5 mm, Mini Protein Gels | Invitrogen | NP0322BOX & NP0321BOX |
| Immun-Blot PVDF Membrane | Bio-Rad | 1620177 |
| Non-fat Dry Milk | Cell Signaling Technology (CST) | 9999 |
| P21 Antibody for WB | Cell Signaling Technology (CST) | 2947 |
| GAPDH | Cell Signaling Technology (CST) | 8884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, M.A.; Rahman, M.; Ayad, A.A.; Warrington, A.E.; Burns, T.C. P21 Overexpression Promotes Cell Death and Induces Senescence in Human Glioblastoma. Cancers 2023, 15, 1279. https://doi.org/10.3390/cancers15041279
Mansour MA, Rahman M, Ayad AA, Warrington AE, Burns TC. P21 Overexpression Promotes Cell Death and Induces Senescence in Human Glioblastoma. Cancers. 2023; 15(4):1279. https://doi.org/10.3390/cancers15041279
Chicago/Turabian StyleMansour, Moustafa A., Masum Rahman, Ahmad A. Ayad, Arthur E. Warrington, and Terry C. Burns. 2023. "P21 Overexpression Promotes Cell Death and Induces Senescence in Human Glioblastoma" Cancers 15, no. 4: 1279. https://doi.org/10.3390/cancers15041279
APA StyleMansour, M. A., Rahman, M., Ayad, A. A., Warrington, A. E., & Burns, T. C. (2023). P21 Overexpression Promotes Cell Death and Induces Senescence in Human Glioblastoma. Cancers, 15(4), 1279. https://doi.org/10.3390/cancers15041279






