The Timing of Surgery Following Stereotactic Body Radiation Therapy Impacts Local Control for Borderline Resectable or Locally Advanced Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Pancreatic Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 18 July 2022).
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; et al. Influence of Resection Margins on Survival for Patients with Pancreatic Cancer Treated by Adjuvant Chemoradiation and/or Chemotherapy in the ESPAC-1 Randomized Controlled Trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Shi, Q.; Meyers, J.; Herman, J.M.; Chuong, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.; Schwartz, L.; Behr, S.; et al. Efficacy of Preoperative MFOLFIRINOX vs. MFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 Gene Status of the Primary Carcinoma Correlates with Patterns of Failure in Patients with Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 1806. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.; Van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled after 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Hill, C.S.; Rosati, L.M.; Hu, C.; Fu, W.; Sehgal, S.; Hacker-Prietz, A.; Wolfgang, C.L.; Weiss, M.J.; Burkhart, R.A.; Hruban, R.H.; et al. Neoadjuvant Stereotactic Body Radiotherapy after Upfront Chemotherapy Improves Pathologic Outcomes Compared with Chemotherapy Alone for Patients with Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma without Increasing Perioperative Toxicity. Ann. Surg. Oncol. 2022, 29, 2456–2468. [Google Scholar] [CrossRef]
- Faris, J.E.; Blaszkowsky, L.S.; McDermott, S.; Guimaraes, A.R.; Szymonifka, J.; Huynh, M.A.; Ferrone, C.R.; Wargo, J.A.; Allen, J.N.; Dias, L.E.; et al. FOLFIRINOX in Locally Advanced Pancreatic Cancer: The Massachusetts General Hospital Cancer Center Experience. Oncologist 2013, 18, 543–548. [Google Scholar] [CrossRef]
- Lefevre, J.H.; Mineur, L.; Kotti, S.; Rullier, E.; Rouanet, P.; De Chaisemartin, C.; Meunier, B.; Mehrdad, J.; Cotte, E.; Desrame, J.; et al. Effect of Interval (7 or 11 Weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J. Clin. Oncol. 2016, 34, 3773–3780. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal Fractionation of Preoperative Radiotherapy and Timing to Surgery for Rectal Cancer (Stockholm III): A Multicentre, Randomised, Non-Blinded, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Hill, C.; Sehgal, S.; Fu, W.; Hu, C.; Reddy, A.; Thompson, E.; Hacker-Prietz, A.; Le, D.; De Jesus-Acosta, A.; Lee, V.; et al. High Local Failure Rates despite High Margin-Negative Resection Rates in a Cohort of Borderline Resectable and Locally Advanced Pancreatic Cancer Patients Treated with Stereotactic Body Radiation Therapy Following Multi-Agent Chemotherapy. Cancer Med. 2022, 11, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; de Marsh, R.W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, C.A.; Friess, H.; Büchler, M.W. Curative Resection Is the Single Most Important Factor Determining Outcome in Patients with Pancreatic Adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar] [CrossRef]
- Allema, J.H.; Reinders, M.E.; van Gulik, T.M.; Koelemay, M.J.; Van Leeuwen, D.J.; de Wit, L.T.; Gouma, D.J.; Obertop, H. Prognostic Factors for Survival after Pancreaticoduodenectomy for Patients with Carcinoma of the Pancreatic Head Region. Cancer 1995, 75, 2069–2076. [Google Scholar] [CrossRef]
- Raut, C.P.; Tseng, J.F.; Sun, C.C.; Wang, H.; Wolff, R.A.; Crane, C.H.; Hwang, R.; Vauthey, J.N.; Abdalla, E.K.; Lee, J.E.; et al. Impact of Resection Status on Pattern of Failure and Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Ann. Surg. 2007, 246, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Uchida, Y.; Terajima, H. Clinical Impact of Margin Status on Survival and Recurrence Pattern after Curative-Intent Surgery for Pancreatic Cancer. Asian J. Surg. 2019, 42, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Del Castillo, C.F.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic Ductal Adenocarcinoma: Is There a Survival Difference for R1 Resections versus Locally Advanced Unresectable Tumors? What Is a “True” R0 Resection? Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 Resection in Pancreatic Cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Johns, A.L.; Merrett, N.D.; Gill, A.J.; Colvin, E.K.; Scarlett, C.J.; Nguyen, N.Q.; Leong, R.W.L.; Cosman, P.H.; Kelly, M.I.; et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Pach, R.; Kulig, J.; Richter, P.; Gach, T.; Szura, M.; Kowalska, T. Randomized Clinical Trial on Preoperative Radiotherapy 25 Gy in Rectal Cancer—Treatment Results at 5-Year Follow-Up. Langenbeck’s Arch. Surg. 2012, 397, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.L.; Nagtegaal, I.D. The Importance of the Pathologist’s Role in Assessment of the Quality of the Mesorectum. Curr. Color. Cancer Rep. 2012, 8, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Van der Geest, L.G.M.; van Rijssen, L.B.; Molenaar, I.Q.; de Hingh, I.H.; Groot Koerkamp, B.; Busch, O.R.C.; Lemmens, V.E.P.P.; Besselink, M.G.H. Volume–Outcome Relationships in Pancreatoduodenectomy for Cancer. HPB 2016, 18, 317. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Van de Velde, C.J.H.; Van Der Worp, E.; Kapiteijn, E.; Quirke, P.; Van Krieken, J.H.J.M. Macroscopic Evaluation of Rectal Cancer Resection Specimen: Clinical Significance of the Pathologist in Quality Control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Lillemoe, K.D.; Talamonti, M.S.; Ko, C.Y. Assessment of Pancreatic Cancer Care in the United States Based on Formally Developed Quality Indicators. JNCI J. Natl. Cancer Inst. 2009, 101, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, J.M.; Reames, B.N.; Klute, K.A.; Hall, W.A.; Baine, M.J.; Abdel-Wahab, M.; Lin, C. The Timing and Design of Stereotactic Radiotherapy Approaches as a Part of Neoadjuvant Therapy in Pancreatic Cancer: Is It Time for Change? Clin. Transl. Radiat. Oncol. 2021, 28, 124–128. [Google Scholar] [CrossRef] [PubMed]
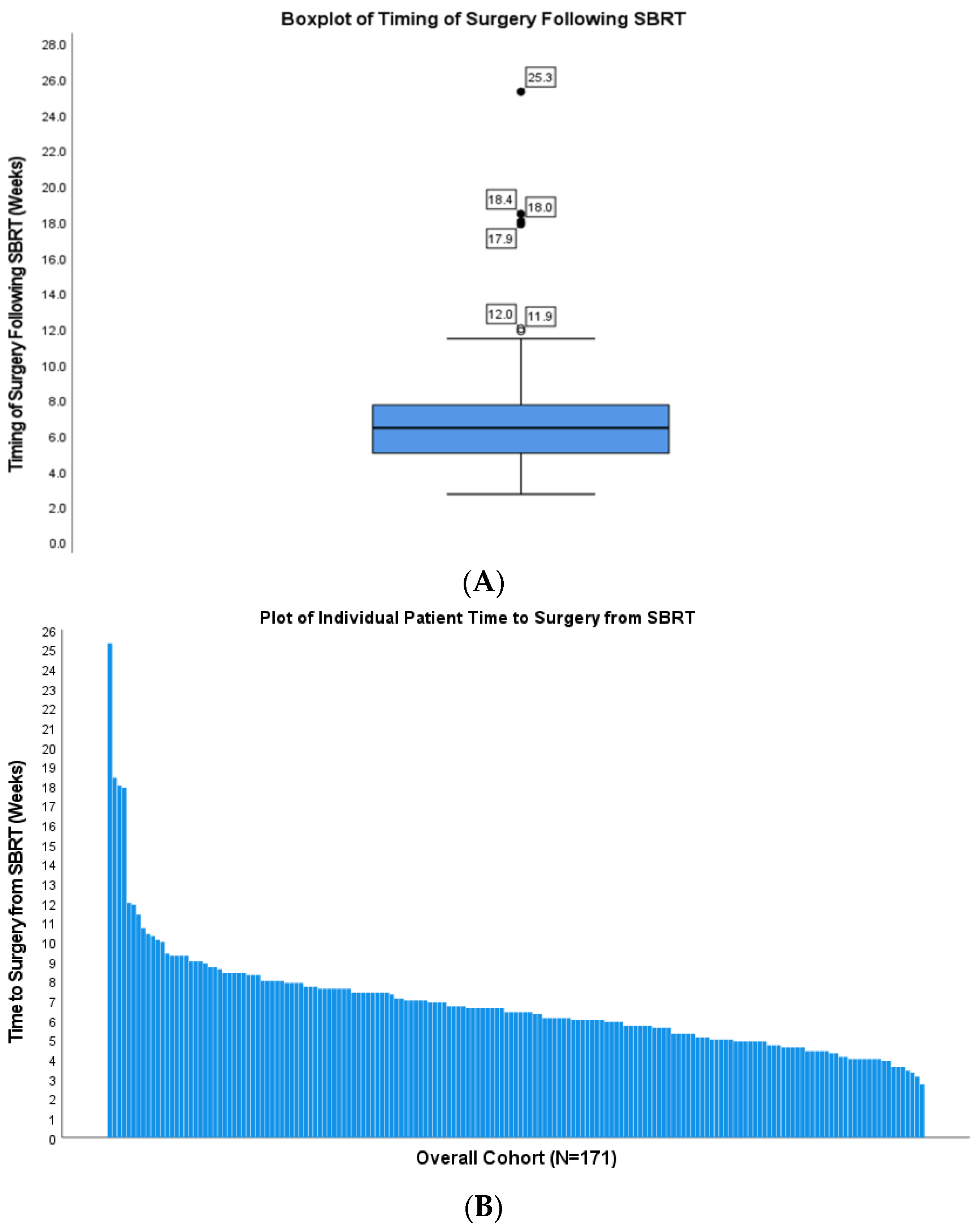
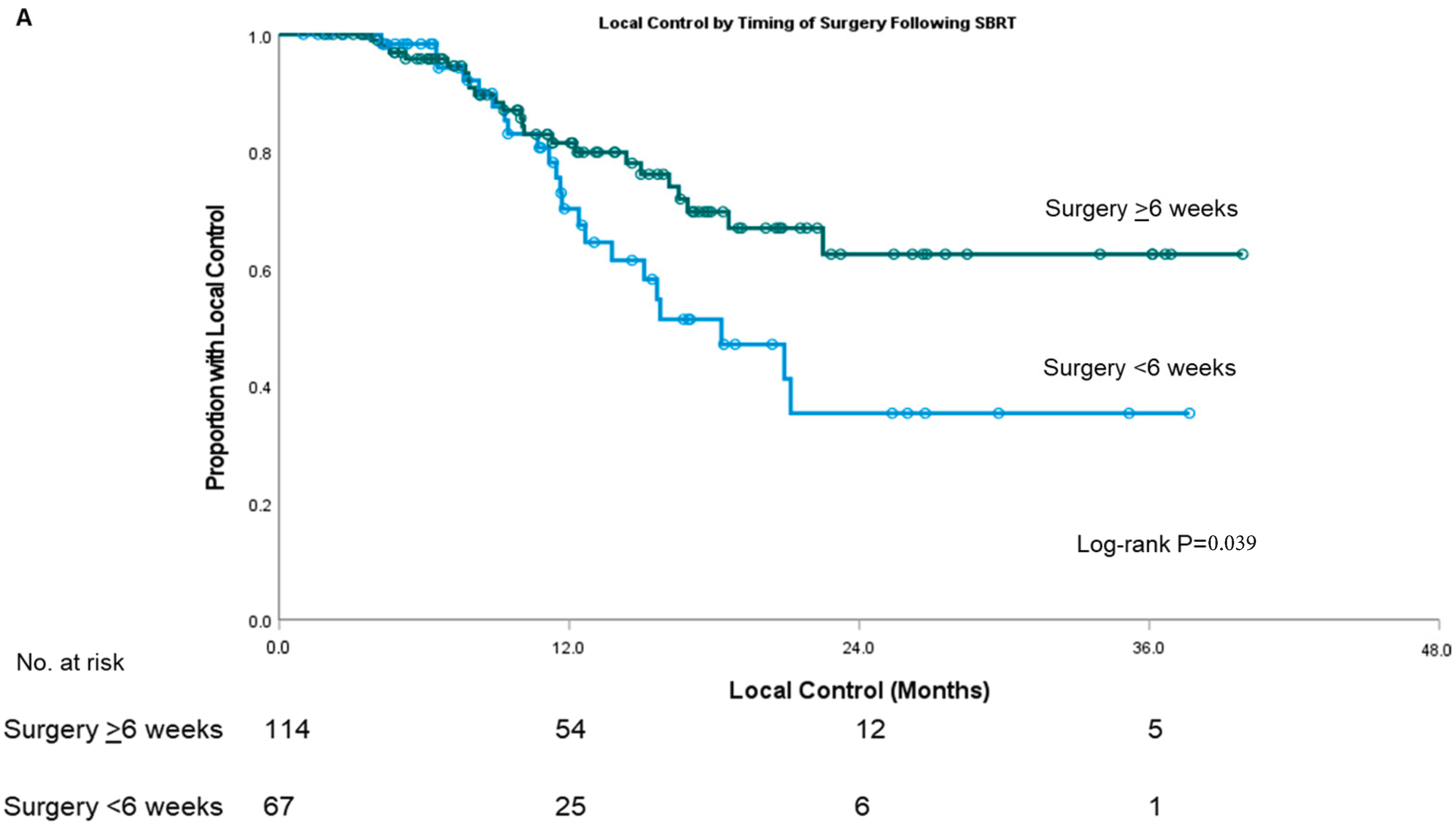
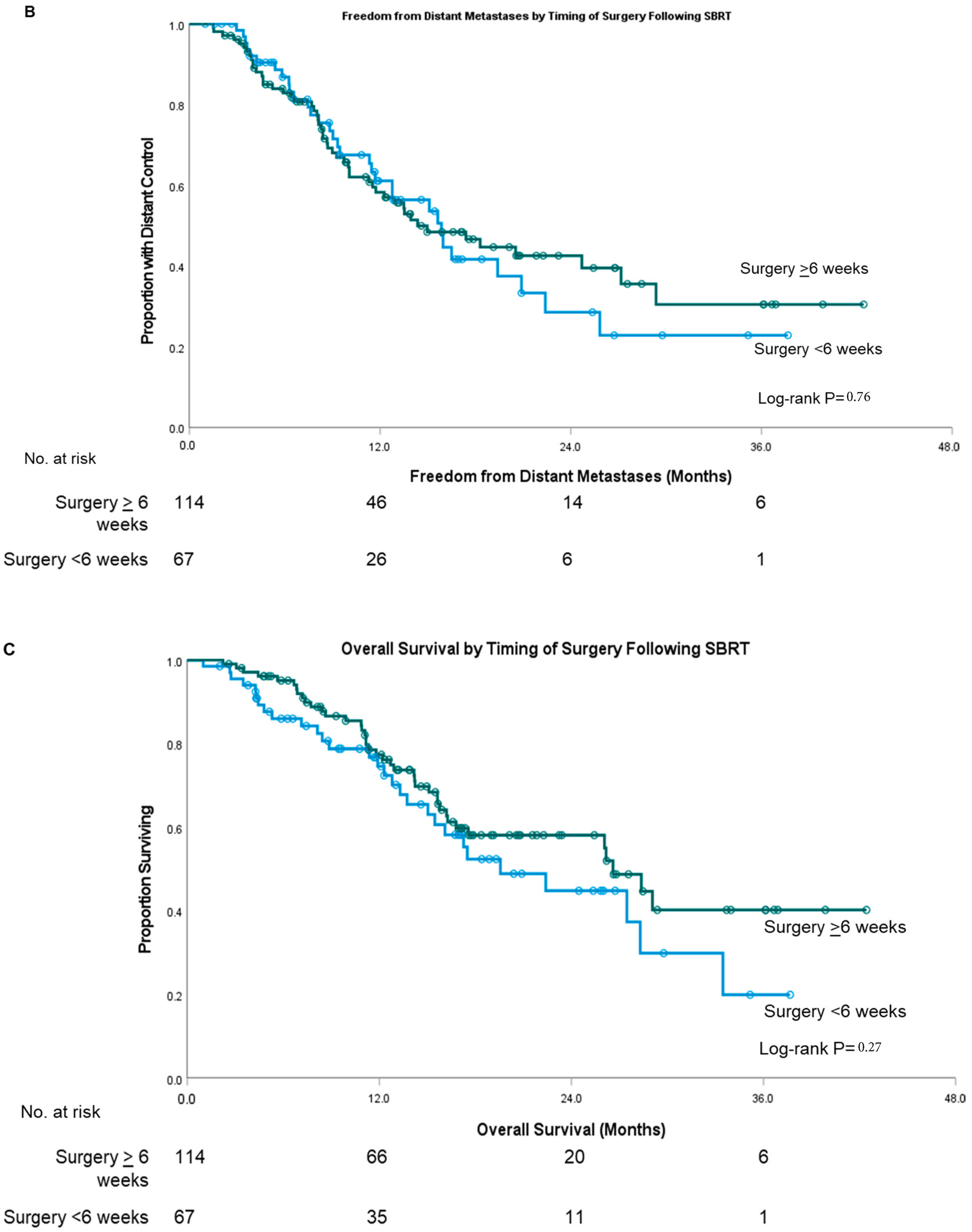
| Covariate | Surgery <6 Weeks from SBRT | Surgery ≥6 Weeks from SBRT | p-Value | |
|---|---|---|---|---|
| Number of Patients | 67 | 104 | -- | |
| Age Median (IQR) | 66.3 | 65.5 | ||
| Sex | Female | 31 (46%) | 57 (55%) | 0.28 |
| Male | 36 (54%) | 47 (45%) | ||
| Race | White | 53 (79%) | 90 (87%) | |
| Other a | 14 (21%) | 14 (13%) | ||
| Tumor Location | Pancreas Head | 40 (60%) | 66 (63%) | 0.62 |
| Other b | 27 (40%) | 38 (37%) | ||
| Disease Stage at Diagnosis | Borderline Resectable | 34 (51%) | 46 (44%) | 0.40 |
| Locally Advanced | 33 (49%) | 58 (56%) | ||
| Surgery | Whipple | 46 (69%) | 70 (67%) | 0.51 |
| Total Pancreatectomy | 1 (1%) | 5 (5%) | ||
| Distal Pancreatectomy | 20 (30%) | 29 (28%) | ||
| Pathologic Complete Response | No | 63 (94%) | 99 (95%) | 0.74 |
| Yes | 4 (6%) | 5 (5%) | ||
| Surgical Margin | R0 | 60 (90%) | 95 (91%) | 0.69 |
| R1 or Higher | 7 (10%) | 9 (9%) | ||
| Pathologic Nodal Status | Negative | 33 (49%) | 60 (58%) | 0.28 |
| Positive | 34 (51%) | 44 (42%) | ||
| Baseline ECOG | 0–1 | 65 (98%) | 97 (96%) | 0.37 |
| 2 or Higher | 1 (2%) | 4 (4%) | ||
| Neoadjuvant Chemotherapy Duration | ≥4 Months | 10 (15%) | 10 (10%) | 0.97 |
| <4 Months | 57 (85%) | 94 (90%) | ||
| Baseline CA19-9 | <200 | 35 (54%) | 48 (48%) | 0.43 |
| ≥200 | 30 (46%) | 53 (52%) | ||
| Adjuvant Chemotherapy | No | 39 (42%) | 28 (36%) | 0.53 |
| Yes | 55 (58%) | 49 (64%) |
| Covariate | Univariable Cox p | Univariable Cox HR (95% CI) | Multivariable Cox p | Multivariable Cox HR (95% CI) | |
|---|---|---|---|---|---|
| Timing of Surgery Post-SBRT | ≥6 weeks | 0.042 | 0.55 (0.30–0.98) | 0.013 | 0.46 (0.25–0.85) |
| <6 weeks | Reference | ||||
| Pathologic Node Status | Positive | 0.021 | 2.01 (1.11–3.63) | 0.019 | 2.09 (1.13–3.88) |
| Negative | Reference | ||||
| Baseline CA19-9, U/mL | ≥200 | 0.018 | 2.09 (1.14–3.84) | 0.002 | 2.73 (1.44–5.18) |
| <200 | Reference | ||||
| Age | 0.43 | 0.99 (0.95–1.02) | |||
| Sex | Male | 0.026 | 2.02 (1.09–3.75) | 0.06 | 1.83 (0.97–3.43) |
| Female | -- | Reference | |||
| ECOG PS | 2 or Higher | 0.77 | 1.35 (0.19–9.80) | ||
| 0–1 | Reference | ||||
| Tumor Location | Other a | 0.44 | 0.78 (0.42–1.46) | ||
| Head | Reference | ||||
| Neoadjuvant Chemotherapy Regimen | FOLFIRINOX | 0.86 | 1.07 (0.50–2.31) | ||
| Gemcitabine/Nab-paclitaxel | Reference | ||||
| Neoadjuvant Chemotherapy Duration | ≥4 Months | 0.26 | 0.63 (0.28–1.40) | ||
| <4 Months | |||||
| Pathologic Complete Response | Present | 0.18 | 0.26 (0.04–1.86) | ||
| Absent | Reference | ||||
| Surgical Margin | R0 | 0.40 | 0.45 (0.61–3.43) | ||
| R1 or Higher | Reference | ||||
| Adjuvant Chemotherapy | Yes | 0.38 | 0.81 (0.51–1.30) | ||
| No | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Reddy, A.; Hill, C.; Sehgal, S.; He, J.; Zheng, L.; Herman, J.; Meyer, J.; Narang, A. The Timing of Surgery Following Stereotactic Body Radiation Therapy Impacts Local Control for Borderline Resectable or Locally Advanced Pancreatic Cancer. Cancers 2023, 15, 1252. https://doi.org/10.3390/cancers15041252
Lin T, Reddy A, Hill C, Sehgal S, He J, Zheng L, Herman J, Meyer J, Narang A. The Timing of Surgery Following Stereotactic Body Radiation Therapy Impacts Local Control for Borderline Resectable or Locally Advanced Pancreatic Cancer. Cancers. 2023; 15(4):1252. https://doi.org/10.3390/cancers15041252
Chicago/Turabian StyleLin, Timothy, Abhinav Reddy, Colin Hill, Shuchi Sehgal, Jin He, Lei Zheng, Joseph Herman, Jeffrey Meyer, and Amol Narang. 2023. "The Timing of Surgery Following Stereotactic Body Radiation Therapy Impacts Local Control for Borderline Resectable or Locally Advanced Pancreatic Cancer" Cancers 15, no. 4: 1252. https://doi.org/10.3390/cancers15041252
APA StyleLin, T., Reddy, A., Hill, C., Sehgal, S., He, J., Zheng, L., Herman, J., Meyer, J., & Narang, A. (2023). The Timing of Surgery Following Stereotactic Body Radiation Therapy Impacts Local Control for Borderline Resectable or Locally Advanced Pancreatic Cancer. Cancers, 15(4), 1252. https://doi.org/10.3390/cancers15041252







