Alpha-Fetoprotein Combined with Radiographic Tumor Burden Score to Predict Overall Survival after Liver Resection in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Outcome Measurement
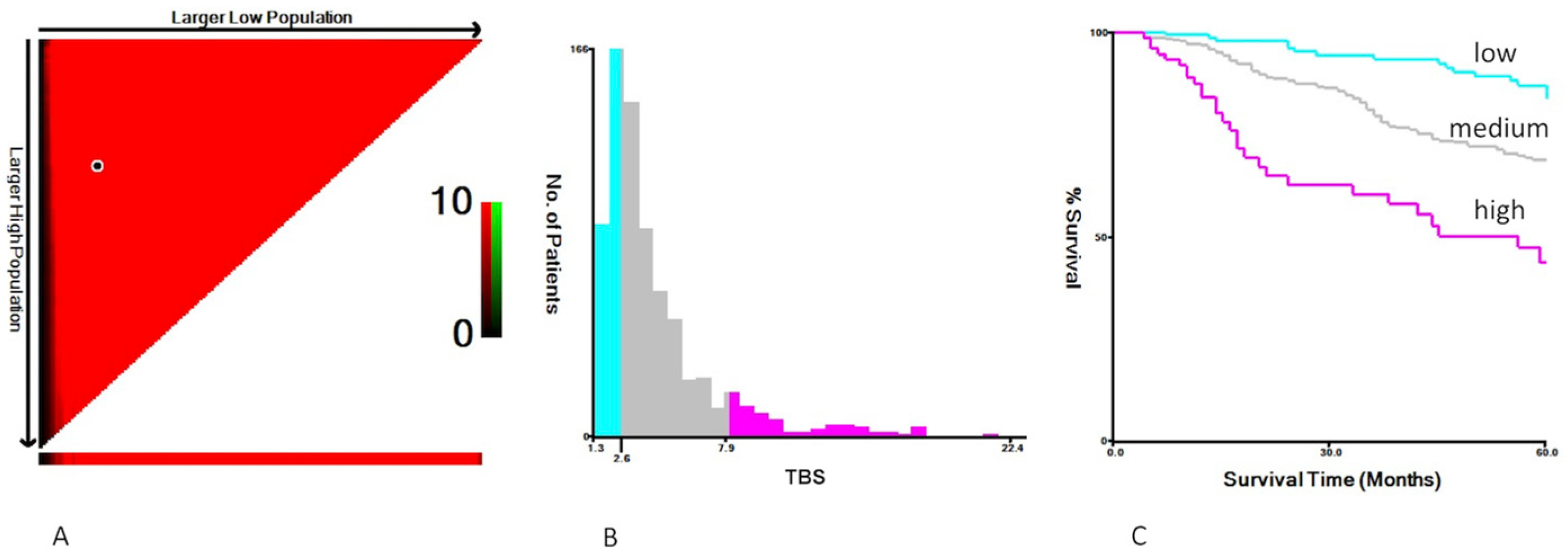
4. Definitions
5. Statistical Analysis
6. Results
6.1. Clinical and Pathological Characteristics of Patients
6.2. The Association of TBS and AFP with OS
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Chen, K.-Y.; Tseng, J.-H.; Ho, M.-C.; Lee, R.-C.; Liang, P.-C.; Liao, L.-Y.; Huang, K.-W.; et al. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2020, 120, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: A multicentre study. J. Hepatol. 2014, 62, 617–624. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef]
- Tsilimigras, D.; Hyer, J.; Diaz, A.; Bagante, F.; Ratti, F.; Marques, H.; Soubrane, O.; Lam, V.; Poultsides, G.; Popescu, I.; et al. Synergistic Impact of Alpha-Fetoprotein and Tumor Burden on Long-Term Outcomes Following Curative-Intent Resection of Hepatocellular Carcinoma. Cancers 2021, 13, 747. [Google Scholar] [CrossRef]
- Edmonson, H.; Steiner, P. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Everhart, J.E.; Wright, E.C.; Goodman, Z.D.; Dienstag, J.L.; Hoefs, J.C.; Kleiner, D.E.; Ghany, M.G.; Mills, A.S.; Nash, S.R.; Govindarajan, S.; et al. Prognostic value of Ishak fibrosis stage: Findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2009, 51, 585–594. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual, 7th ed.; Edge, S.B., Byrd, D.R., Compton, C.C., Eds.; Springer: New York, NY, USA, 2010; p. 175. [Google Scholar]
- Abou-Alfa, G.K.; Pawlik, T.M.; Shindoh, J. Liver. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Ed.; AJCC: Chicago, IL, USA, 2017; p. 287. [Google Scholar]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]
- Lang, H.; Sotiropoulos, G.C.; Dömland, M.; Frühauf, N.R.; Paul, A.; Hüsing, J.; Malagó, M.; Broelsch, C.E. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br. J. Surg. 2004, 92, 198–202. [Google Scholar] [CrossRef]
- Ding, H.-F.; Zhang, X.-F.; Bagante, F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; Alexandrescu, S.; et al. Prediction of tumor recurrence by α-fetoprotein model after curative resection for hepatocellular carcinoma. Eur. J. Surg. Oncol. (EJSO) 2020, 47, 660–666. [Google Scholar] [CrossRef]
- Hyun, M.H.; Lee, Y.-S.; Kim, J.H.; Lee, C.U.; Jung, Y.K.; Seo, Y.S.; Yim, H.J.; Yeon, J.E.; Byun, K.S. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology 2018, 68, 977–993. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Ms, J.M.H.; Sahara, K.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; Soubrane, O.; et al. Recurrence Patterns and Outcomes after Resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann. Surg. Oncol. 2020, 27, 2321–2331. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, C.; Lin, C.; Wang, C.; Chen, C.; Cheng, Y.; Wang, J.; Yen, Y.; Chen, C. Liver resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guideline recommendations: Results from a high-volume liver surgery center in East Asia. J. Surg. Oncol. 2020, 122, 1587–1594. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.-N.; Nagorney, D.M.; Ng, I.O.-L.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplant. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Cho, Y.; Sinn, D.H.; Yu, S.J.; Gwak, G.Y.; Kim, J.H.; Yoo, Y.J.; Jun, D.W.; Kim, T.Y.; Lee, H.Y.; Cho, E.J.; et al. Survival Analysis of Single Large (>5 cm) Hepatocellular Carcinoma Patients: BCLC A versus B. PLoS ONE 2016, 11, e0165722. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Bagante, F.; Sahara, K.; Moris, D.; Hyer, J.M.; Wu, L.; Ratti, F.; Marques, H.P.; Soubrane, O.; Paredes, A.Z.; et al. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current BCLC Classification. Ann. Surg. Oncol. 2019, 26, 3693–3700. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Elfadaly, A.N.; Tsilimigras, D.I.; Hyer, J.M.; Paro, A.; Bagante, F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; et al. Impact of Tumor Burden Score on Conditional Survival after Curative-Intent Resection for Hepatocellular Carcinoma: A Multi-Institutional Analysis. World J. Surg. 2021, 45, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Mehta, R.; Guglielmi, A.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; Alexandrescu, S.; et al. Recurrence beyond the Milan criteria after curative-intent resection of hepatocellular carcinoma: A novel tumor-burden based prediction model. J. Surg. Oncol. 2020, 122, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, Z.; Lima, H.A.; Alaimo, L.; Endo, Y.; Shaikh, C.F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Poultsides, G.A.; et al. Impact of tumor burden score on timing and patterns of recurrence after curative-intent resection of hepatocellular carcinoma. Surgery 2022, 172, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.A.; Alaimo, L.; Brown, Z.J.; Endo, Y.; Moazzam, Z.; Tsilimigras, D.I.; Shaikh, C.; Resende, V.; Guglielmi, A.; Ratti, F.; et al. Application of hazard functions to investigate recurrence after curative-intent resection for hepatocellular carcinoma. HPB 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Mehta, R.M.; Paredes, A.Z.M.; Moris, D.M.; Sahara, K.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; et al. Overall Tumor Burden Dictates Outcomes for Patients Undergoing Resection of Multinodular Hepatocellular Carcinoma Beyond the Milan Criteria. Ann. Surg. 2020, 272, 574–581. [Google Scholar] [CrossRef]
- Lima, H.A.; Endo, Y.; Alaimo, L.; Moazzam, Z.; Munir, M.M.; Shaikh, C.; Resende, V.; Guglielmi, A.; Marques, H.P.; Cauchy, F.; et al. Tumor Burden Score and Serum Alpha-fetoprotein Subclassify Intermediate-Stage Hepatocellular Carcinoma. J. Gastrointest. Surg. 2022, 26, 2512–2521. [Google Scholar] [CrossRef]
- Endo, Y.; Alaimo, L.; Lima, H.A.; Moazzam, Z.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; Kitago, M.; Poultsides, G.A.; et al. A Novel Online Calculator to Predict Risk of Microvascular Invasion in the Preoperative Setting for Hepatocellular Carcinoma Patients Undergoing Curative-Intent Surgery. Ann. Surg. Oncol. 2022, 30, 725–733. [Google Scholar] [CrossRef]
- Fukami, Y.; Saito, T.; Osawa, T.; Arikawa, T.; Matsumura, T.; Kurahashi, S.; Komatsu, S.; Kaneko, K.; Sano, T. Preoperative Controlling Nutritional Status plus Tumor Burden Score for the Assessment of Prognosis after Curative Liver Resection for Hepatocellular Carcinoma. Med. Princ. Pract. 2020, 30, 131–137. [Google Scholar] [CrossRef]
- Endo, Y.; Lima, H.A.; Alaimo, L.; Moazzam, Z.; Brown, Z.; Shaikh, C.F.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; et al. Preoperative risk score (PreopScore) to predict overall survival after resection for hepatocellular carcinoma. HPB, 2023; in press. [Google Scholar] [CrossRef]
- Lima, H.A.; Endo, Y.; Moazzam, Z.; Alaimo, L.; Shaikh, C.; Munir, M.M.; Resende, V.; Guglielmi, A.; Marques, H.P.; Cauchy, F.; et al. TAC score better predicts survival than the BCLC following resection of hepatocellular carcinoma. J. Surg. Oncol. 2022, 127, 374–384. [Google Scholar] [CrossRef]
- Beumer, B.R.; Buettner, S.; Galjart, B.; van Vugt, J.L.; de Man, R.A.; Ijzermans, J.N.; Koerkamp, B.G. Systematic review and meta-analysis of validated prognostic models for resected hepatocellular carcinoma patients. Eur. J. Surg. Oncol. (EJSO) 2021, 48, 492–499. [Google Scholar] [CrossRef] [PubMed]

| Total, n = 743 | |
|---|---|
| Age (year) | |
| ≤65 | 414 (55.7%) |
| >65 | 329 (44.3%) |
| Sex | |
| Male | 572 (77.0%) |
| Female | 171 (23.0%) |
| Cirrhosis | |
| Presence | 287 (38.6%) |
| Absence | 420 (56.5%) |
| Unknown | 36 (4.8%) |
| HBsAg | |
| Positive | 390 (52.5%) |
| Negative | 353 (47.5%) |
| Anti-HCV | |
| Positive | 251 (33.8%) |
| Negative | 492 (66.2%) |
| AFP (ng/mL) | |
| <400 | 623 (83.8%) |
| ≥400 | 120 (16.2%) |
| Child–Pugh class | |
| A | 731 (98.4%) |
| B | 11 (1.5%) |
| Unknown | 1 (0.1%) |
| Type of resection | |
| Major | 352 (47.4%) |
| Minor | 394 (53.0) |
| Tumor differentiation | |
| Poor | 29 (3.9%) |
| Moderate | 658 (88.6%) |
| Well | 56 (7.5%) |
| Pathology T-stage | |
| 1–2 | 680 (91.5%) |
| 3–4 | 63 (8.5%) |
| Tumor burden score | |
| Low < 2.6 | 193 (26.0%) |
| Medium: 2.6–7.9 | 474 (63.8%) |
| High > 7.9 | 75 (10.1%) |
| BCLC stage | |
| 0 | 136 (18.3) |
| A | 538 (72.4%) |
| B | 69 (9.3%) |
| Radiographic tumor size (cm) | |
| ≤5 | 573 (77.1%) |
| >5 | 170 (22.9%) |
| Radiographic tumor number | |
| 1 | 637 (85.7%) |
| 2 | 78 (10.5%) |
| 3 | 16 (2.2%) |
| 4 | 11 (1.5%) |
| 5 | 1 (0.1%) |
| TBS ≤ 7.9 and AFP < 400 ng/mL, n = 577 | TBS ≤ 7.9 and AFP ≥ 400 ng/mL, n = 90 | TBS > 7.9 and AFP < 400 ng/mL, n = 46 | TBS > 7.9 and AFP ≥ 400 ng/mL, n = 30 | p | |
|---|---|---|---|---|---|
| Age (year) | 0.283 | ||||
| ≤65 | 381 (66%) | 58 (64.4%) | 27 (58.7%) | 24 (80%) | |
| >65 | 196 (34%) | 32 (35.6%) | 19 (41.3%) | 6 (20%) | |
| Sex | 0.014 | ||||
| Male | 488 (77.6%) | 59 (65.6%) | 41 (89.1%) | 23 (76.7%) | |
| Female | 129 (22.4%) | 31 (34.4%) | 5 (10.9%) | 7 (23.3%) | |
| Cirrhosis | <0.001 | ||||
| Yes | 231 (40%) | 43 (47.8%) | 7 (15.2%) | 6 (20%) | |
| No | 323 (56%) | 43 (47.8%) | 33 (71.7%) | 21 (70%) | |
| Unknown | 23 (4.0%) | 4 (4.4%) | 6 (13.0%) | 3 (10.0%) | |
| HBsAg | 0.076 | ||||
| Positive | 302 (52.3%) | 52 (57.8%) | 17 (37%) | 19 (36.7%) | |
| Negative | 275 (47.7%) | 38 (42.2%) | 29 (63.0%) | 11 (63.3%) | |
| Anti-HCV | 0.023 | ||||
| Positive | 210 (36.4%) | 26 (28.9%) | 12 (26.1%) | 4 (13.3%) | |
| Negative | 367 (63.6%) | 64 (71.1%) | 34 (73.9%) | 26 (86.7%) | |
| Child–Pugh class | 0.168 | ||||
| A | 568 (98.4%) | 90 (100%) | 43 (93.5%) | 30 (100%) | |
| B | 8 (1.4%) | 0 | 3 (6.5%) | 0 | |
| Unknown | 1 (0.2%) | 0 | 0 | 0 | |
| Type of resection | <0.001 | ||||
| Major | 245 (42.5%) | 41 (42.6%) | 37 (80.4%) | 26 (86.7%) | |
| Minor | 332 (57.5%) | 49 (54.4%) | 9 (19.6%) | 4 (13.3%) | |
| Tumor differentiation | <0.001 | ||||
| Poor | 14 (2.4%) | 8 (8.9%) | 3 (6.5%) | 4 (13.3%) | |
| Moderate | 510 (88.4%) | 81 (90.0%) | 42 (91.3%) | 24 (83.3%) | |
| Well | 53 (9.2%) | 1 (1.1%) | 1 (2.2%) | 1 (3.3%) | |
| Pathology T-stage | <0.001 | ||||
| 1–2 | 551 (95.5%) | 84 (93.3%) | 33 (71.7%) | 12 (40%) | |
| 3–4 | 26 (4.5%) | 6 (6.7%) | 13 (28.3%) | 18 (60%) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (year) | ||||
| ≤65 | 1.00 (reference) | 1.00 (reference) | ||
| >65 | 2.083 (1.488–2.915) | <0.001 | 2.146 (1.523–3.024) | <0.001 |
| Cirrhosis | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.525 (1.081–2.153) | 0.016 | 1.692 (1.190–2.405) | 0.003 |
| Unknown | 1.382 (0.597–3.197) | 0.450 | 0.969 (0.414–2.268) | 0.942 |
| Tumor differentiation | ||||
| Well or moderate | 1.00 (reference) | 1.00 (reference) | ||
| Poor | 2.895 (1.514–5.535) | 0.001 | 2.027 (1.044–3.939) | 0.037 |
| Group | ||||
| TBS ≤ 7.9 and AFP < 400 ng/mL | 1.00 (reference) | 1.00 (reference) | ||
| TBS ≤ 7.9 and AFP ≥ 400 ng/mL | 1.560 (0.966–2.520) | 0.069 | 1.394 (0.858–2.266) | 0.180 |
| TBS > 7.9 and AFP < 400 ng/mL | 2.077 (1.193–3.617) | 0.010 | 2.063 (1.175–3.623) | 0.012 |
| TBS > 7.9 and AFP ≥ 400 ng/mL | 5.378 (3.094–9.349) | <0.001 | 6.57 (3.684–11.719) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-H.; Liu, Y.-W.; Li, W.-F.; Wang, C.-C.; Yong, C.-C.; Lin, C.-C.; Lin, C.-Y. Alpha-Fetoprotein Combined with Radiographic Tumor Burden Score to Predict Overall Survival after Liver Resection in Hepatocellular Carcinoma. Cancers 2023, 15, 1203. https://doi.org/10.3390/cancers15041203
Yen Y-H, Liu Y-W, Li W-F, Wang C-C, Yong C-C, Lin C-C, Lin C-Y. Alpha-Fetoprotein Combined with Radiographic Tumor Burden Score to Predict Overall Survival after Liver Resection in Hepatocellular Carcinoma. Cancers. 2023; 15(4):1203. https://doi.org/10.3390/cancers15041203
Chicago/Turabian StyleYen, Yi-Hao, Yueh-Wei Liu, Wei-Feng Li, Chih-Chi Wang, Chee-Chien Yong, Chih-Che Lin, and Chih-Yun Lin. 2023. "Alpha-Fetoprotein Combined with Radiographic Tumor Burden Score to Predict Overall Survival after Liver Resection in Hepatocellular Carcinoma" Cancers 15, no. 4: 1203. https://doi.org/10.3390/cancers15041203
APA StyleYen, Y.-H., Liu, Y.-W., Li, W.-F., Wang, C.-C., Yong, C.-C., Lin, C.-C., & Lin, C.-Y. (2023). Alpha-Fetoprotein Combined with Radiographic Tumor Burden Score to Predict Overall Survival after Liver Resection in Hepatocellular Carcinoma. Cancers, 15(4), 1203. https://doi.org/10.3390/cancers15041203





