Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Variables of Interest
2.2. Statistical Analysis
3. Results
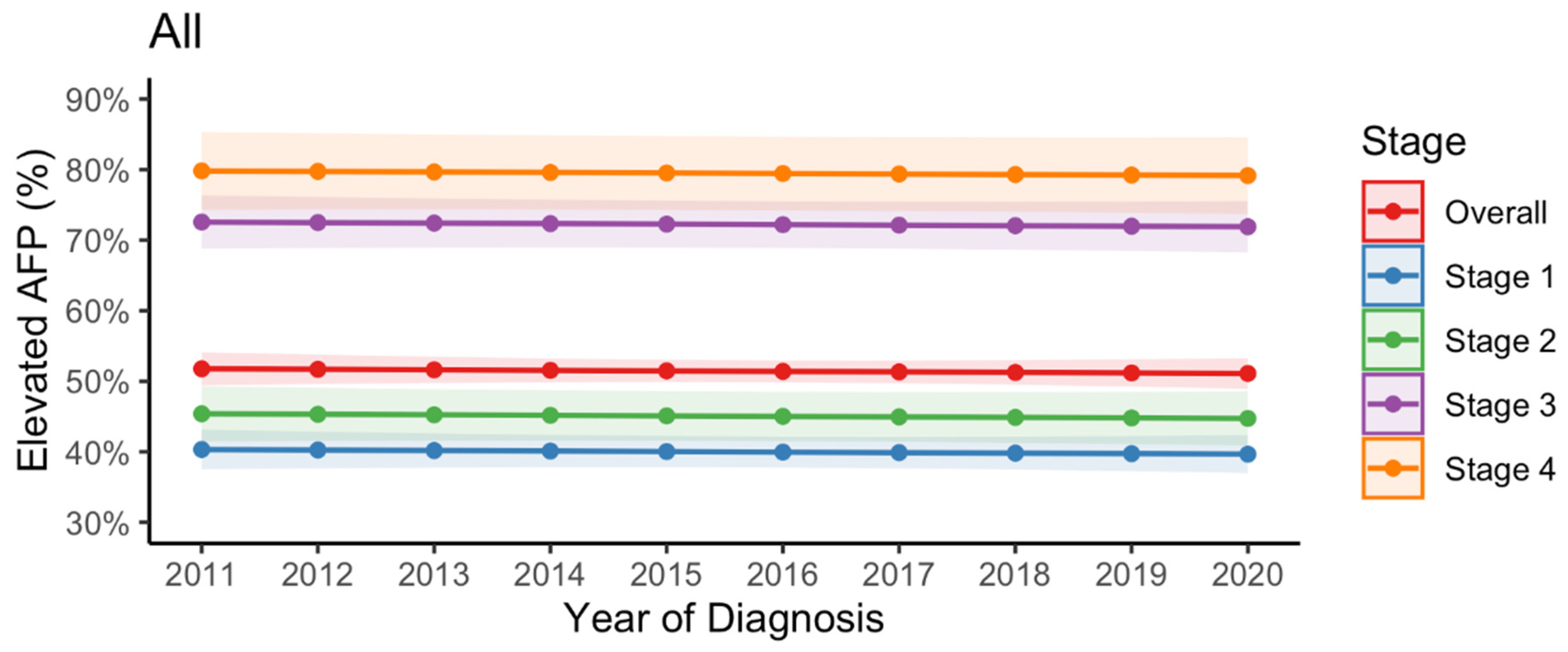
3.1. Trend in Elevated AFP Levels at the Time of HCC Diagnosis
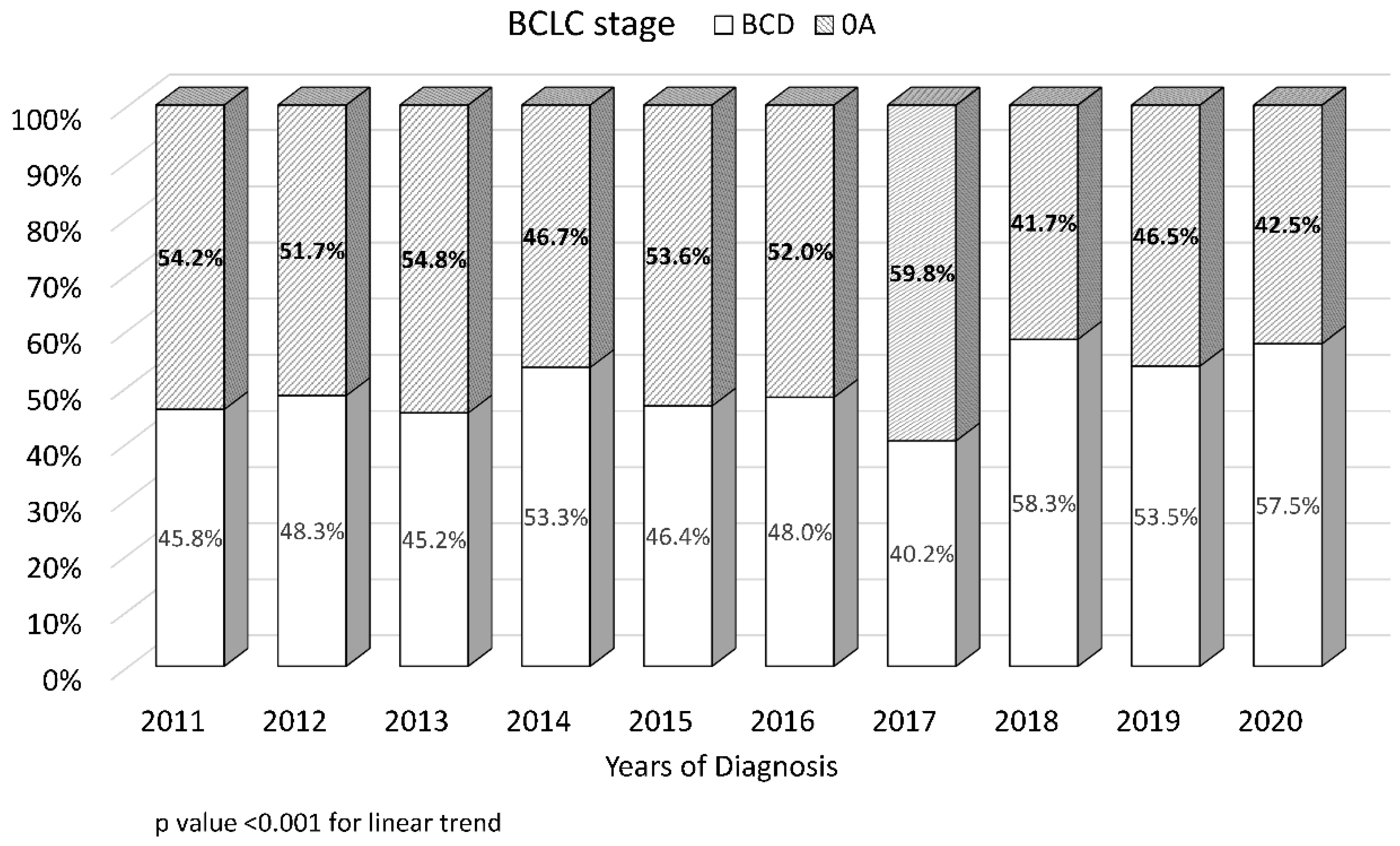
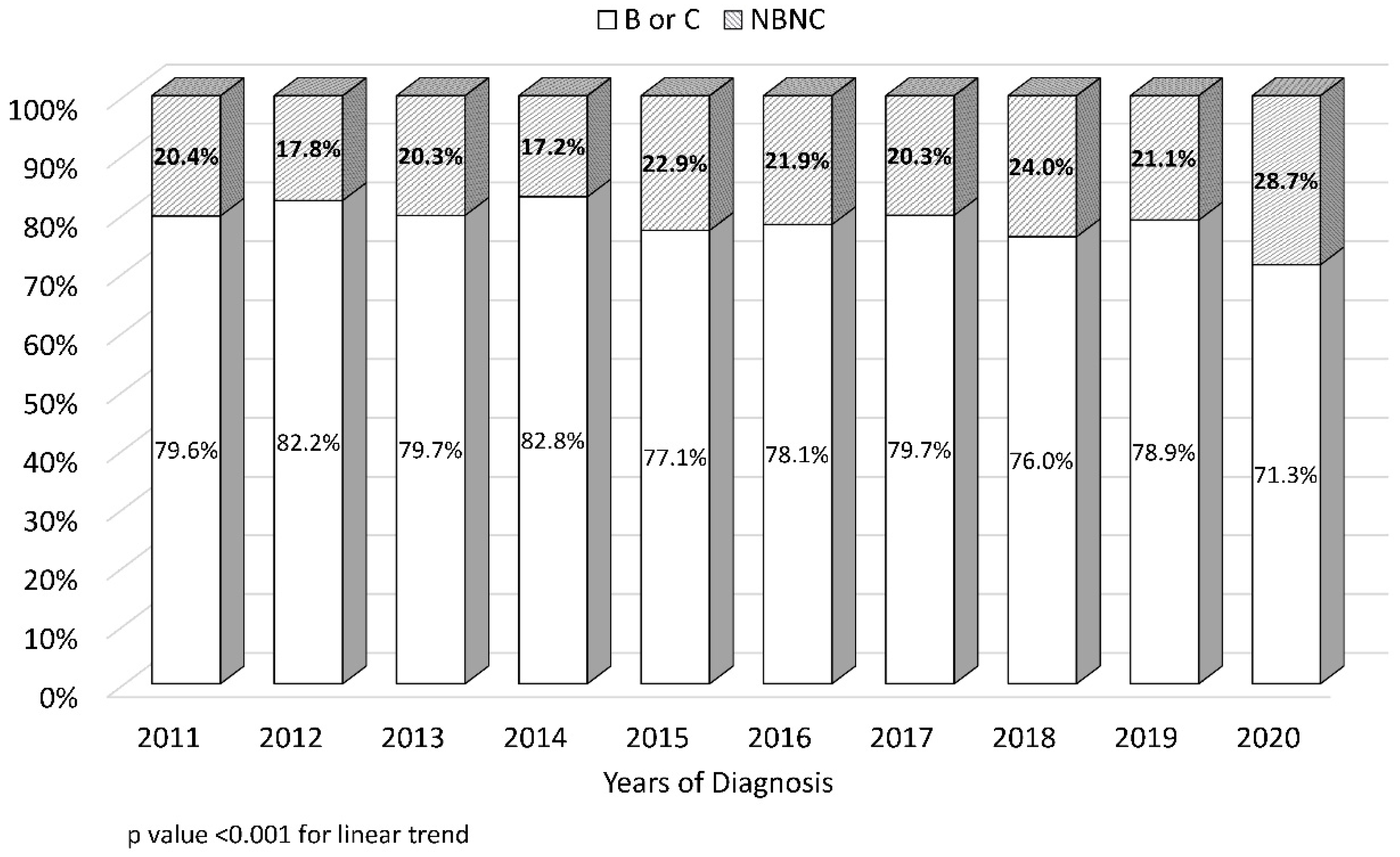
3.2. Trends in Early-Stage Tumor Prevalence and Non-Viral Etiology at the Time of HCC Diagnosis
3.3. Patients’ Characteristics Categorized by AFP Level
3.4. Variables Associated with Elevated AFP Level
3.5. Proportion of BCLC Stages 0–A Patients in NBNC-HCC vs. HBV- or HCV-Related HCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Giannini, E.G.; Sammito, G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Italian Liver Cancer (ITA.LI.CA) Group. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: Implications for its clinical use. Cancer 2014, 120, 2150–2157. [Google Scholar] [CrossRef]
- Vipani, A.; Lauzon, M.; Luu, M.; Roberts, L.R.; Singal, A.G.; Yang, J.D. Decreasing Trend of Serum α-Fetoprotein Level in Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2022, 20, 1177–1179.e4. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. American Joint Committee on Cancer Staging Manual, 7th ed.; Edge, S.B., Byrd, D.R., Compton, C.C., Fritz, A.G., Greene, F.L., Rotti, A., III, Eds.; Springer: New York, NY, USA, 2010; p. 175. [Google Scholar]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Wright, E.C.; Goodman, Z.D.; Dienstag, J.L.; Hoefs, J.C.; Kleiner, D.E.; Ghany, M.G.; Mills, A.S.; Nash, S.R.; Govindarajan, S.; et al. Prognostic value of Ishak fibrosis stage: Findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010, 51, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Ibrahim, A.; Helmy, A.; Sarin, S.K.; Omata, M.; Kumar, A.; Bernstien, D.; Maruyama, H.; Saraswat, V.; Chawla, Y.; et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: A 2016 update. Hepatol. Int. 2017, 11, 1–30. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Lüdecke, D. sjPlot: Da ta Visualization for Statistics in Social Science. R Package Version 2.8.8. 2021. Available online: https://CRAN.R-project.org/package¼sjPlot (accessed on 1 January 2020).
- Lin, S.H.; Lin, C.Y.; Hsu, N.T.; Yen, Y.H.; Kee, K.M.; Wang, J.H.; Hu, T.H.; Chen, C.H.; Hung, C.H.; Chen, C.H.; et al. Reappraisal of the roles of alpha-fetoprotein in hepatocellular carcinoma surveillance using large-scale nationwide database and hospital-based information. J. Formos. Med. Assoc. 2022, 121, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.W.; Yeh, M.L.; Yang, J.D.; Chen, V.L.; Nguyen, P.; Giama, N.H.; Huang, C.F.; Hsing, A.W.; Dai, C.Y.; Huang, J.F.; et al. More advanced disease and worse survival in cryptogenic compared to viral hepatocellular carcinoma. Liver Int. 2018, 38, 895–902. [Google Scholar] [CrossRef]
- Chen, V.L.; Yeh, M.L.; Yang, J.D.; Leong, J.; Huang, D.Q.; Toyoda, H.; Chen, Y.L.; Guy, J.; Maeda, M.; Tsai, P.C.; et al. Effects of Cirrhosis and Diagnosis Scenario in Metabolic-Associated Fatty Liver Disease-Related Hepatocellular Carcinoma. Hepatol. Commun. 2020, 5, 122–132. [Google Scholar] [CrossRef]
- Richardson, P.; Duan, Z.; Kramer, J.; Davila, J.A.; Tyson, G.L.; El-Serag, H.B. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin. Gastroenterol. Hepatol. 2012, 10, 428–433. [Google Scholar] [CrossRef]
- Gawrieh, S.; Dakhoul, L.; Miller, E.; Scanga, A.; deLemos, A.; Kettler, C.; Burney, H.; Liu, H.; Abu-Sbeih, H.; Chalasani, N.; et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: A United States multicentre study. Aliment. Pharmacol. Ther. 2019, 50, 809–821. [Google Scholar] [CrossRef]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lee, C.M.; Lin, S.M.; Chu, H.C.; Wu, T.C.; Yang, S.S.; Kuo, H.S.; et al. Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009, 101, 1348–1355. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Sung, J.J.; Chow, W.C.; Farrell, G.; Lee, C.Z.; Yuen, H.; Tanwandee, T.; Tao, Q.M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef]
- Beste, L.A.; Green, P.; Berry, K.; Belperio, P.; Ioannou, G.N. Hepatitis C-Related Hepatocellular Carcinoma Incidence in the Veterans Health Administration After Introduction of Direct-Acting Antivirals. JAMA 2020, 324, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.C.; Huang, S.F.; Chen, P.J.; Chen, C.L.; Chen, C.L.; Wu, C.C.; Tsai, C.C.; Lee, P.H.; Chen, M.F.; Lee, C.M.; et al. The hepatitis viral status in patients with hepatocellular carcinoma: A study of 3843 patients from Taiwan Liver Cancer Network. Medicine 2016, 95, e3284. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Hsu, C.T.; Yeh, M.L.; Huang, C.F.; Huang, C.I.; Liang, P.C.; Lin, Y.H.; Hsieh, M.Y.; Wei, Y.J.; Hsieh, M.H.; et al. Early Fibrosis but Late Tumor Stage and Worse Outcomes in Hepatocellular Carcinoma Patients without Hepatitis B or Hepatitis C. Dig. Dis. Sci. 2020, 65, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F.; Feng, Z.; Marrero, J.A.; Khaderi, S.; Singal, A.G. Risk factors for cirrhosis in contemporary hepatology practices-findings from the Texas hepatocellular carcinoma Consortium cohort. Gastroenterology 2020, 159, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamada, S.; Takano, N.; Okamura, Y.; Takami, H.; Inokawa, Y.; Sonohara, F.; Tanaka, N.; Shimizu, D.; Hattori, N.; et al. Different Characteristics of Serum Alfa Fetoprotein and Serum Des-gamma-carboxy Prothrombin in Resected Hepatocellular Carcinoma. In Vivo 2021, 35, 1749–1760. [Google Scholar]
- Taura, N.; Ichikawa, T.; Miyaaki, H.; Ozawa, E.; Tsutsumi, T.; Tsuruta, S.; Kato, Y.; Goto, T.; Kinoshita, N.; Fukushima, M.; et al. Frequency of elevated biomarkers in patients with cryptogenic hepatocellular carcinoma. Med. Sci. Monit. 2013, 19, 742–750. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Zhou, Q.; Dao, D.Y.; Lo, Y.M.D. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Gual, A.; Segura, L.; Contel, M.; Heather, N.; Colom, J. Audit-3 and audit-4: Effectiveness of two short forms of the alcohol use disorders identification test. Alcohol Alcohol. 2002, 37, 591–596. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef]
- Su, T.H.; Peng, C.Y.; Chang, S.H.; Tseng, T.C.; Liu, C.J.; Chen, C.L.; Liu, C.H.; Yang, H.C.; Chen, P.J.; Kao, J.H. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J. Formos. Med. Assoc. 2022, 121, 703–711. [Google Scholar] [CrossRef]
- Degasperi, E.; Perbellini, R.; D’Ambrosio, R.; Uceda Renteria, S.C.; Ceriotti, F.; Perego, A.; Orsini, C.; Borghi, M.; Iavarone, M.; Bruccoleri, M.; et al. Prothrombin induced by vitamin K absence or antagonist-II and alpha foetoprotein to predict development of hepatocellular carcinoma in Caucasian patients with hepatitis C-related cirrhosis treated with direct-acting antiviral agents. Aliment. Pharmacol. Ther. 2022, 55, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Elevated AFP (≥20 ng/mL), n = 2036 | Normal AFP (<20 ng/mL), n = 1941 | p |
|---|---|---|---|
| Age (years) | 63 (55–71) | 63 (56–71) | 0.286 |
| Male | 1437 (70.6%) | 1434 (73.9%) | 0.02 |
| BMI (kg/m2) | 24.2 (22.1–26.9) | 24.9 (22.5–27.8) | <0.001 |
| Diagnosis method | <0.001 | ||
| Clinical diagnosis | 896 (44.0%) | 593 (30.6%) | |
| Pathology diagnosis | 1140 (56.0%) | 1348 (69.4%) | |
| AUD | 0.460 | ||
| Yes | 260 (12.8%) | 225 (11.6%) | |
| No | 1762 (86.5%) | 1705 (87.8%) | |
| Not available | 11 (0.6%) | 14 (0.7%) | |
| HBsAg | 0.002 | ||
| Positive | 1006 (49.4%) | 866 (44.6%) | |
| Negative | 1030 (50.6%) | 1075 (55.4%) | |
| Anti-HCV | 0.327 | ||
| Positive | 741(36.4%) | 674 (34.7%) | |
| Negative | 1295 (63.6%) | 1266 (65.2%) | |
| Not available | 0 | 1 (0.1%) | |
| Cirrhosis | 0.001 | ||
| Yes | 1469 (72.2%) | 1294 (66.7%) | |
| No | 561 (27.6%) | 640 (33.0%) | |
| Not available | 7 (0.4%) | 6 (0.3%) | |
| Child–Pugh class | <0.001 | ||
| A | 1587 (77.9%) | 1627 (83.80%) | |
| B | 345 (16.9%) | 235 (12.1%) | |
| C | 70 (3.4%) | 51 (2.6%) | |
| Not available | 34 (1.7%) | 28 (1.4%) | |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 0.731 |
| Total bilirubin (mg/dL) | 1.1 (0.8–1.7) | 1.0 (0.7–1.4) | <0.001 |
| INR | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | <0.001 |
| Tumor size (mm) | 46 (26–98) | 28 (20–45) | <0.001 |
| Tumor number | <0.001 | ||
| Single | 1064 (52.3%) | 1360 (70.1%) | |
| Multiple | 972 (47.7%) | 581 (29.9%) | |
| 7th edition AJCC stage | <0.001 | ||
| 1 | 788 (38.7%) | 1168 (60.2%) | |
| 2 | 340 (16.7%) | 414 (21.3%) | |
| 3 | 617 (30.3%) | 233 (12.0%) | |
| 4 | 279 (13.7%) | 72 (3.7%) | |
| Not available | 12 (0.6%) | 54 (2.8%) | |
| BCLC stage | <0.001 | ||
| 0 | 206 (10.1%) | 293 (15.1%) | |
| A | 566 (27.3%) | 867 (44.7%) | |
| B | 446 (21.9%) | 362 (18.7%) | |
| C | 676 (33.2%) | 279 (14.4%) | |
| D | 124 (6.1%) | 81(4.2%) | |
| Not available | 28 (1.4%) | 59 (3.0%) |
| Variable | Univariate | p | Multivariate | p |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (per 10 years) | 0.955 (0.904–1.010) | 0.105 | ||
| Female vs. male | 1.20 (1.04–1.39) | 0.011 | 1.462 (1.256–1.701) | <0.001 |
| AUD | 1.077 (0.887–1.306) | 0.454 | ||
| HBsAg or anti-HCV-positive | 1.355 (1.160–1.583) | <0.001 | 1.720 (1.451–2.038) | <0.001 |
| Cirrhosis | 1.275 (1.110–1.465) | 0.001 | 1.288 (1.099–1.509) | 0.02 |
| Child–Pugh class B or C vs. A | 1.525(1.287–1.806) | <0.001 | 0.964 (0.770–1.205) | 0.745 |
| Creatinine >1.2 mg/dL | 0.983 (0.849–1.138) | 0.821 | ||
| Total bilirubin >1.4 mg/dL | 1.454 (1.262–1.676) | <0.001 | 1.218 (1.020–1.455) | 0.030 |
| INR >1.2 | 1.238 (1.002–1.530) | 0.048 | 0.897 (0.687–1.172) | 0.425 |
| Tumor size, per 10 mm increase | 1.167 (1.146–1.189) | <0.001 | 1.155 (1.127–1.183) | <0.001 |
| Multiple tumors | 2.076 (1.818–2.371) | <0.001 | 1.406 (1.205–1.641) | <0.001 |
| BCLC stage (O–A as reference) | ||||
| B–D | 2.640 (2.317–3.009) | <0.001 | 1.247 (1.037–1.500) | 0.019 |
| 7th edition AJCC Stage 1 as reference | ||||
| Stage 2 | 1.202 (1.013–1.426) | 0.035 | ||
| Stage 3 | 3.909 (3.270–4.674) | <0.001 | ||
| Stage 4 | 6.071 (4.553–8.096) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-H.; Kee, K.-M.; Li, W.-F.; Liu, Y.-W.; Wang, C.-C.; Hu, T.-H.; Tsai, M.-C.; Lin, C.-Y. Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients. Cancers 2023, 15, 1222. https://doi.org/10.3390/cancers15041222
Yen Y-H, Kee K-M, Li W-F, Liu Y-W, Wang C-C, Hu T-H, Tsai M-C, Lin C-Y. Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients. Cancers. 2023; 15(4):1222. https://doi.org/10.3390/cancers15041222
Chicago/Turabian StyleYen, Yi-Hao, Kwong-Ming Kee, Wei-Feng Li, Yueh-Wei Liu, Chih-Chi Wang, Tsung-Hui Hu, Ming-Chao Tsai, and Chih-Yun Lin. 2023. "Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients" Cancers 15, no. 4: 1222. https://doi.org/10.3390/cancers15041222
APA StyleYen, Y.-H., Kee, K.-M., Li, W.-F., Liu, Y.-W., Wang, C.-C., Hu, T.-H., Tsai, M.-C., & Lin, C.-Y. (2023). Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients. Cancers, 15(4), 1222. https://doi.org/10.3390/cancers15041222





