Association of Telomere Length with Colorectal Cancer Risk and Prognosis: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Source and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics and Findings
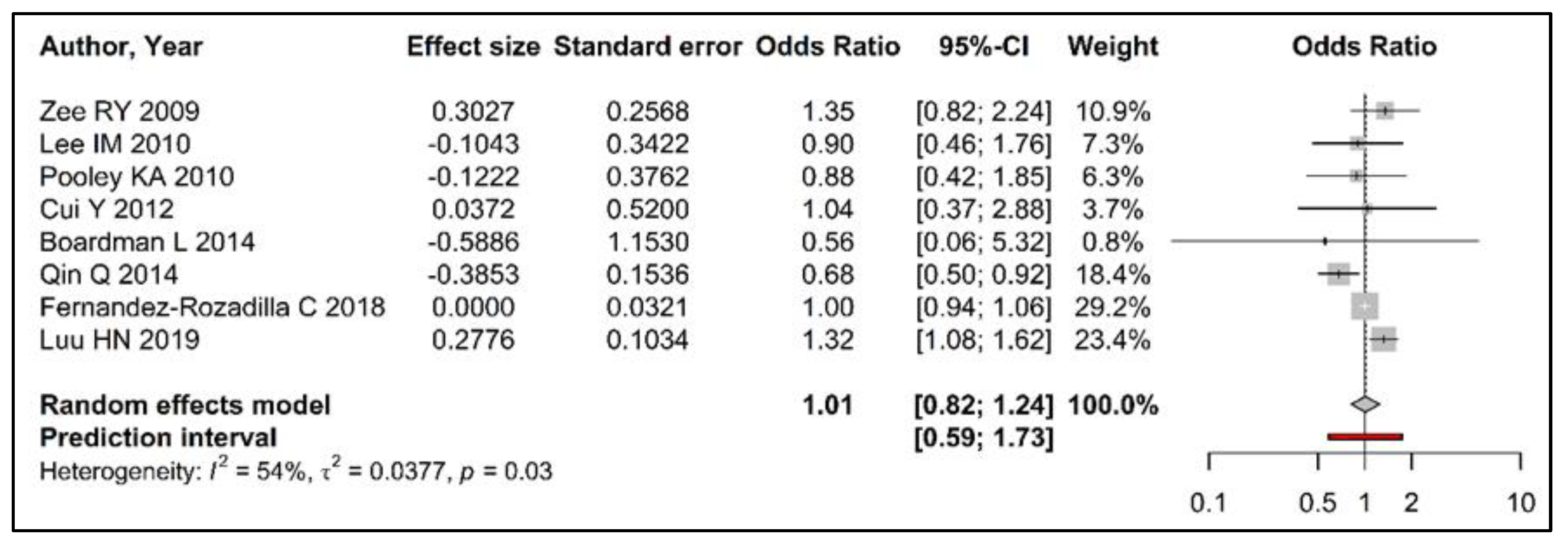
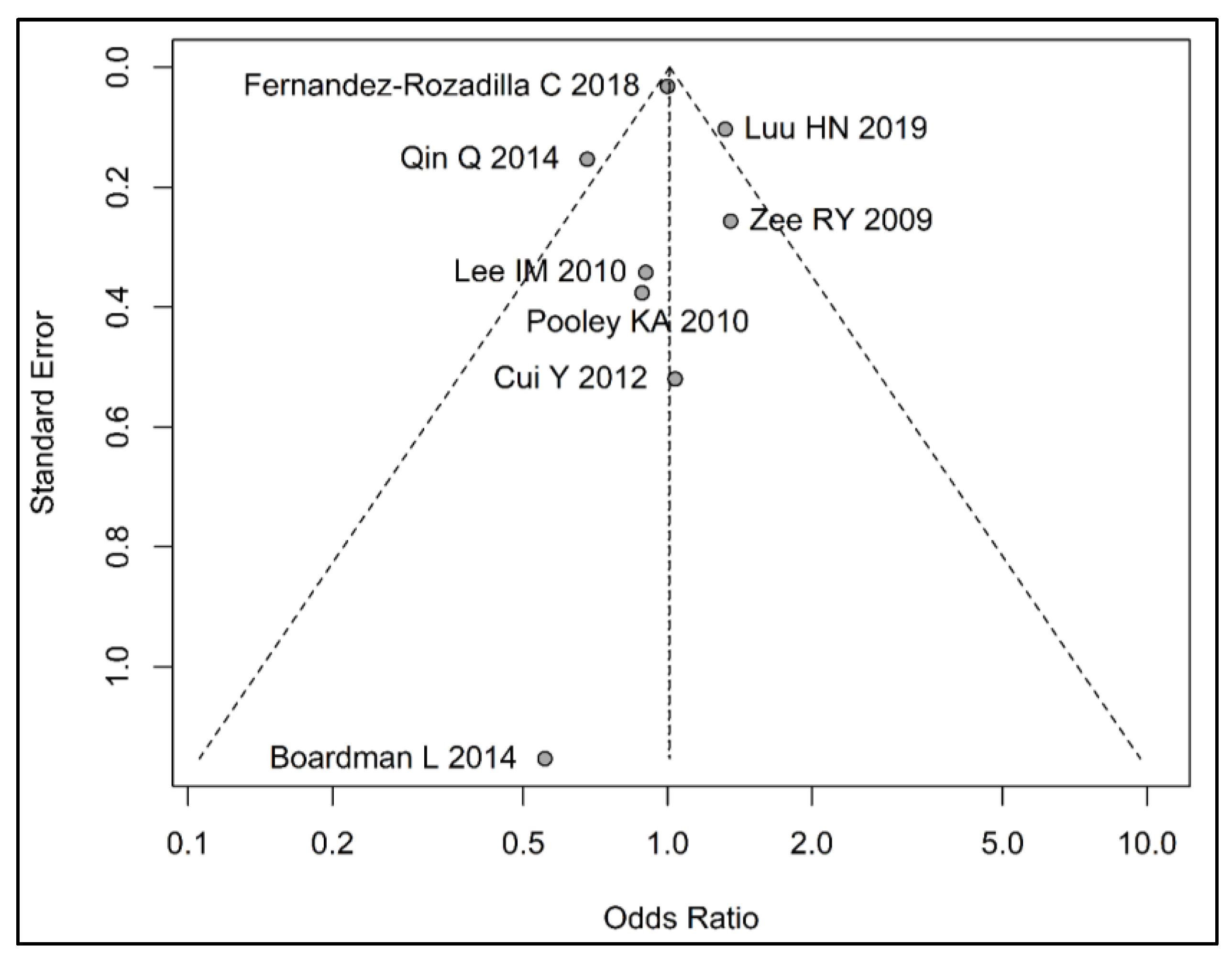
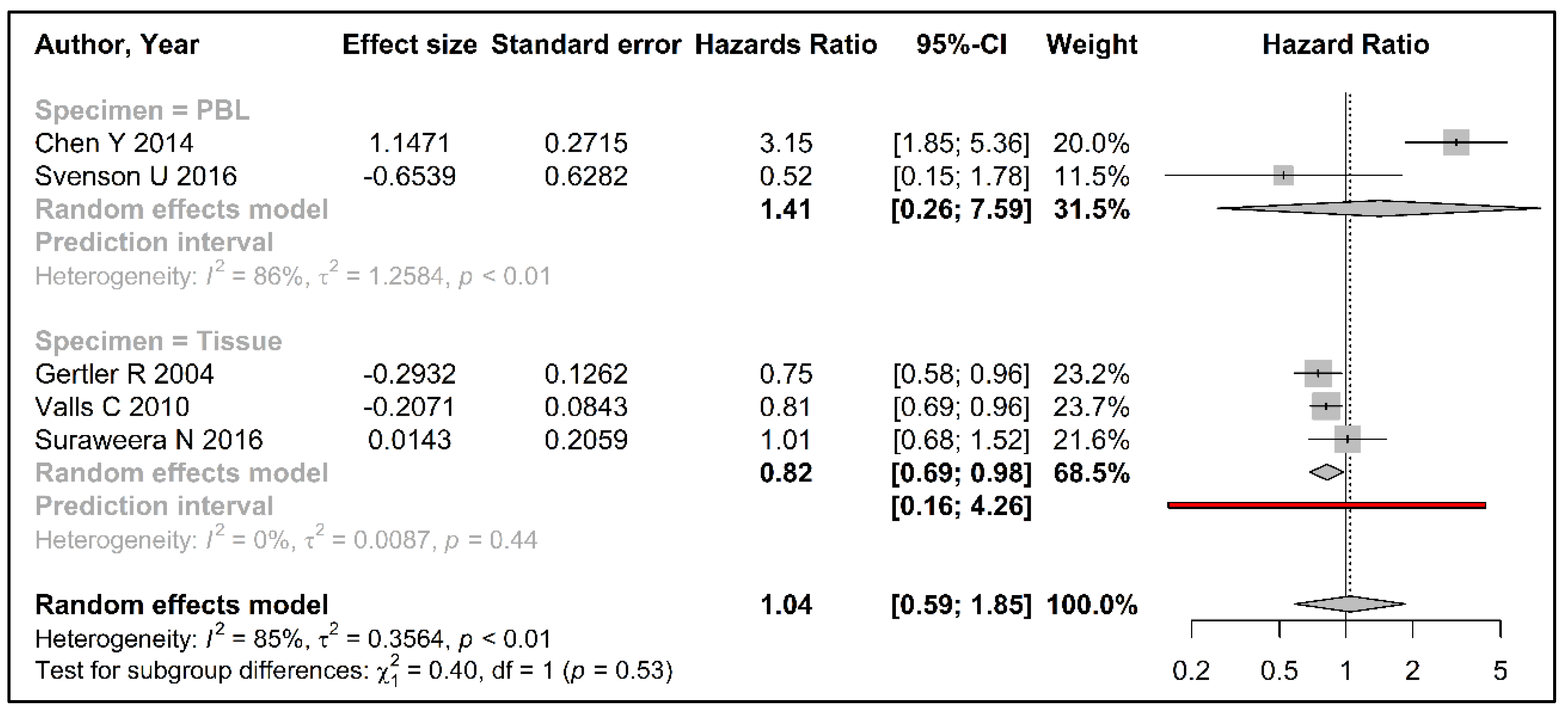
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Karlseder, J.; Smogorzewska, A.; de Lange, T. Senescence induced by altered telomere state, not telomere loss. Science 2002, 295, 2446–2449. [Google Scholar] [CrossRef]
- Bernal, A.; Tusell, L. Telomeres: Implications for Cancer Development. Int. J. Mol. Sci. 2018, 19, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Rehkopf, D.H.; Dow, W.H.; Rosero-Bixby, L.; Lin, J.; Epel, E.S.; Blackburn, E.H. Longer leukocyte telomere length in Costa Rica’s Nicoya Peninsula: A population-based study. Exp. Gerontol. 2013, 48, 1266–1273. [Google Scholar] [CrossRef]
- Garcia-Cao, M.; O’Sullivan, R.; Peters, A.H.; Jenuwein, T.; Blasco, M.A. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat. Genet. 2004, 36, 94–99. [Google Scholar] [CrossRef]
- Astuti, Y.; Wardhana, A.; Watkins, J.; Wulaningsih, W. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 2017, 158, 480–489. [Google Scholar] [CrossRef]
- Jones, R.E.; Oh, S.; Grimstead, J.W.; Zimbric, J.; Roger, L.; Heppel, N.H.; Ashelford, K.E.; Liddiard, K.; Hendrickson, E.A.; Baird, D.M. Escape from telomere-driven crisis is DNA ligase III dependent. Cell Rep. 2014, 8, 1063–1076. [Google Scholar] [CrossRef]
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Neumann, A.A.; Yeager, T.R.; Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598–610. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef]
- Mandal, P. Recent advances of Blood telomere length (BTL) shortening: A potential biomarker for development of cancer. Pathol. Oncol. Res. POR. 2019, 25, 1263–1265. [Google Scholar] [CrossRef]
- Niewisch, M.R.; Savage, S.A. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev. Hematol. 2019, 12, 1037–1052. [Google Scholar] [CrossRef]
- Naing, C.; Aung, K.; Lai, P.K.; Mak, J.W. Association between telomere length and the risk of colorectal cancer: A meta-analysis of observational studies. BMC Cancer 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, W.; Xue, W.; Zou, Y.; Xie, C.; Du, J.; Jin, G. The association between telomere length and cancer risk in population studies. Sci. Rep. 2016, 6, 22243. [Google Scholar] [CrossRef]
- Qin, Q.; Sun, J.; Yin, J.; Liu, L.; Chen, J.; Zhang, Y.; Li, T.; Shi, Y.; Wei, S.; Nie, S. Telomere length in peripheral blood leukocytes is associated with risk of colorectal cancer in Chinese population. PLoS ONE 2014, 9, e88135. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.N.; Qi, M.Y.Z.; Wang, R.W.; Adams-Haduch, J.; Miljkovic, I.; Opresko, P.L.; Jin, A.Z.; Koh, W.P.; Yuan, J.M. Association Between Leukocyte Telomere Length and Colorectal Cancer Risk in the Singapore Chinese Health Study. Clin. Transl. Gastroenterol. 2019, 10, e00043. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, L.; Zhou, N.; Li, N.; Bulibu, G.; Xu, C.; Zhang, Y.; Tang, Y. Meta-analysis of associations between telomere length and colorectal cancer survival from observational studies. Oncotarget 2017, 8, 62500–62507. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Z. Telomere Length as a Prognostic Factor for Overall Survival in Colorectal Cancer Patients. Cell. Physiol. Biochem. 2016, 38, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Bmj 2009, 339, b2535. [Google Scholar] [CrossRef]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167–171. [Google Scholar]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis in R: A Hand-on Guide. 2019. Available online: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/ (accessed on 10 January 2020).
- Balduzzi, S.; Ruecker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health. 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Boardman, L.A.; Litzelman, K.; Seo, S.; Johnson, R.A.; Vanderboom, R.J.; Kimmel, G.W.; Cunningham, J.M.; Gangnon, R.E.; Engelman, C.D.; Riegert-Johnson, D.L.; et al. The Association of Telomere Length with Colorectal Cancer Differs by the Age of Cancer Onset. Clin. Transl. Gastroenterol. 2014, 5, e52. [Google Scholar] [CrossRef]
- Fernandez-Rozadilla, C.; Kartsonaki, C.; Woolley, C.; McClellan, M.; Whittington, D.; Horgan, G.; Leedham, S.; Kriaucionis, S.; East, J.; Tomlinson, I. Telomere length and genetics are independent colorectal tumour risk factors in an evaluation of biomarkers in normal bowel. Br. J. Cancer 2018, 118, 727–732. [Google Scholar] [CrossRef]
- Lee, I.M.; Lin, J.; Castonguay, A.J.; Barton, N.S.; Buring, J.E.; Zee, R.Y. Mean leukocyte telomere length and risk of incident colorectal carcinoma in women: A prospective, nested case-control study. Clin. Chem. Lab. Med. 2010, 48, 259–262. [Google Scholar] [CrossRef]
- Zee, R.Y.; Castonguay, A.J.; Barton, N.S.; Buring, J.E. Mean telomere length and risk of incident colorectal carcinoma: A prospective, nested case-control approach. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2280–2282. [Google Scholar] [CrossRef]
- Pooley, K.A.; Sandhu, M.S.; Tyrer, J.; Shah, M.; Driver, K.E.; Luben, R.N.; Bingham, S.A.; Ponder, B.A.; Pharoah, P.D.; Khaw, K.T.; et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010, 70, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cai, Q.; Qu, S.; Chow, W.H.; Wen, W.; Xiang, Y.B.; Wu, J.; Rothman, N.; Yang, G.; Shu, X.O.; et al. Association of leukocyte telomere length with colorectal cancer risk: Nested case-control findings from the Shanghai Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1807–1813. [Google Scholar] [CrossRef]
- Svenson, U.; Oberg, A.; Stenling, R.; Palmqvist, R.; Roos, G. Telomere length in peripheral leukocytes is associated with immune cell tumor infiltration and prognosis in colorectal cancer patients. Tumor Biol. 2016, 37, 10877–10882. [Google Scholar] [CrossRef]
- Gertler, R.; Rosenberg, R.; Stricker, D.; Friederichs, J.; Hoos, A.; Werner, M.; Ulm, K.; Holzmann, B.; Nekarda, H.; Siewert, J.R. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J. Clin. Oncol. 2004, 22, 1807–1814. [Google Scholar] [CrossRef]
- Valls, C.; Pinol, C.; Rene, J.M.; Buenestado, J.; Vinas, J. Telomere length is a prognostic factor for overall survival in colorectal cancer. Color. Dis. 2011, 13, 1265–1272. [Google Scholar] [CrossRef]
- Suraweera, N.; Mouradov, D.; Li, S.; Jorissen, R.N.; Hampson, D.; Ghosh, A.; Sengupta, N.; Thaha, M.; Ahmed, S.; Kirwan, M.; et al. Relative telomere lengths in tumor and normal mucosa are related to disease progression and chromosome instability profiles in colorectal cancer. Oncotarget 2016, 7, 36474–36488. [Google Scholar] [CrossRef]
- Kroupa, M.; Rachakonda, S.K.; Liska, V.; Srinivas, N.; Urbanova, M.; Jiraskova, K.; Schneiderova, M.; Vycital, O.; Vymetalkova, V.; Vodickova, L.; et al. Relationship of telomere length in colorectal cancer patients with cancer phenotype and patient prognosis. Br. J. Cancer 2019, 121, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, F.; He, X.; Bao, G.; Liu, X.; Wan, S.; Xing, J. Short leukocyte telomere length predicts poor prognosis and indicates altered immune functions in colorectal cancer patients. Ann. Oncol. 2014, 25, 869–876. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Zhu, W.; Liu, T.; Xie, S.H.; Zhong, L.X.; Cai, Y.Y.; Li, X.N.; Liang, M.; Chen, W.; et al. The Association of Telomere Length in Peripheral Blood Cells with Cancer Risk: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Druliner, B.R.; Ruan, X.; Johnson, R.; Grill, D.; O’Brien, D.; Lai, T.P.; Rashtak, S.; Felmlee-Devine, D.; Washechek-Aletto, J.; Malykh, A.; et al. Time Lapse to Colorectal Cancer: Telomere Dynamics Define the Malignant Potential of Polyps. Clin. Transl. Gastroenterol. 2016, 7, e188. [Google Scholar] [CrossRef] [PubMed]
- Mehrez, F.; Bougatef, K.; Monache, E.D.; Arisi, I.; Proietti-De-Santis, L.; Prantera, G.; Zouiten, L.; Caputo, M.; Ben Ammar Elgaaied, A.; Bongiorni, S. Telomere length measurement in tumor and non-tumor cells as a valuable prognostic for tumor progression. Cancer Genet. 2019, 238, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Bertorelle, R.; Rampazzo, E.; Pucciarelli, S.; Nitti, D.; De Rossi, A. Telomeres, telomerase and colorectal cancer. World J. Gastroenterol. 2014, 20, 1940–1950. [Google Scholar] [CrossRef]
- Kibriya, M.G.; Raza, M.; Kamal, M.; Haq, Z.; Paul, R.; Mareczko, A.; Pierce, B.L.; Ahsan, H.; Jasmine, F. Relative Telomere Length Change in Colorectal Carcinoma and Its Association with Tumor Characteristics, Gene Expression and Microsatellite Instability. Cancers 2022, 14, 2250. [Google Scholar] [CrossRef]
- Hou, L.; Joyce, B.T.; Gao, T.; Liu, L.; Zheng, Y.; Penedo, F.J.; Liu, S.; Zhang, W.; Bergan, R.; Dai, Q.; et al. Blood Telomere Length Attrition and Cancer Development in the Normative Aging Study Cohort. EBioMedicine 2015, 2, 591–596. [Google Scholar] [CrossRef]
- Cunningham, J.M.; Johnson, R.A.; Litzelman, K.; Skinner, H.G.; Seo, S.; Engelman, C.D.; Vanderboom, R.J.; Kimmel, G.W.; Gangnon, R.E.; Riegert-Johnson, D.L.; et al. Telomere length varies by DNA extraction method: Implications for epidemiologic research. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Lindrose, A.R.; McLester-Davis, L.W.Y.; Tristano, R.I.; Kataria, L.; Gadalla, S.M.; Eisenberg, D.T.A.; Verhulst, S.; Drury, S. Method comparison studies of telomere length measurement using qPCR approaches: A critical appraisal of the literature. PLoS ONE 2021, 16, e0245582. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR-Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 2019, 99, 271–278. [Google Scholar] [CrossRef]
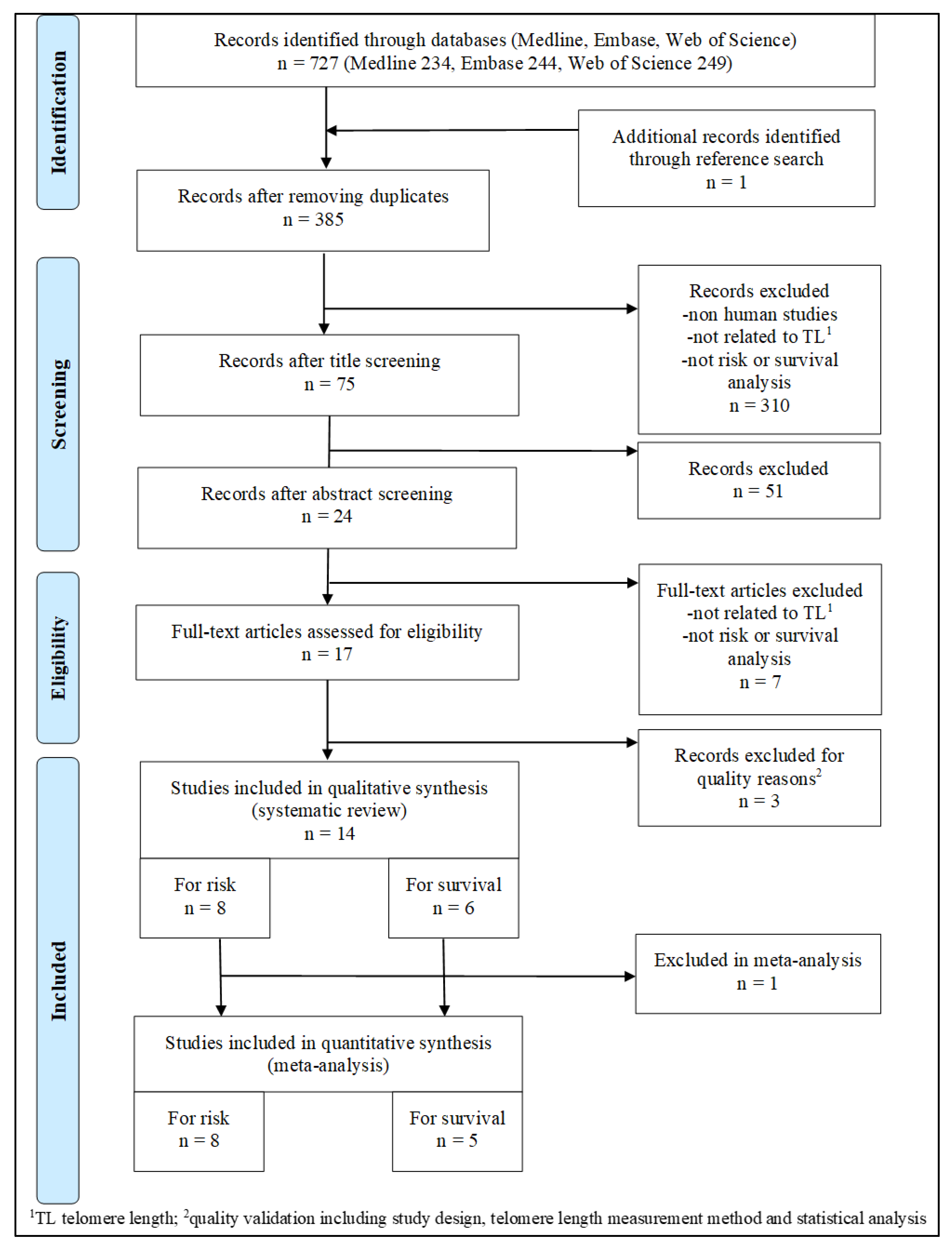
| Author Year | Country | Study Design | Participants’ Characteristics (Cases/Controls) | ||||
|---|---|---|---|---|---|---|---|
| n | Age | % Male | BMI | % Ever Smokers | |||
| (a) Risk analyses | |||||||
| Zee RY 2009 [32] | USA | Prospective Case control | 191/306 | 58.1 (±8.0)/60.5 (±8.7) | 100 | 24.8 (±2.6)/25.2 (±2.9) | 60.8/64.4 |
| Lee IM 2010 [31] | USA | Prospective Case control | 134/357 | 60.1 (±8.7)/60.7 (±8.6) | 0 | 26.2 (±5.6)/25.9 (±4.9) | 53.0/47.1 |
| Pooley KA 2010 [33] | UK | Prospective Case control | 185/406 | 64 (40–80)/64 (41–80) | nr 1 | 26.8 (±4.2)/26.3 (±4.0) | 50/50 |
| Cui Y 2012 [34] | China | Prospective Case control | 441/549 | 58.5 (±8.7)/58.6 (±8.6) | 0 | 24.6 (±3.3)/24.8 (±3.5) | 2.5/3.8 |
| Boardman L 2014 [29] | USA | Retrospective Case control | 598/2212 | 48.3 (±8.3)/56.8 (±12.1) | 50/52 | 27.6 (±6.1)/28.0 (±5.7) | 52/49 |
| Qin Q 2014 [20] | China | Retrospective Case control | 628/1256 | 58.8 (±11.8)/58.8 (±11.4) | 54.1/54.9 | 23.2 (±3.3)/23.0 (±3.2) | 38.2/28.7 |
| Fernandez-Rozadilla C 2018 [30] | UK | Retrospective Case control | 211/106 | 66 (±8.8)/53 (±16.9) | 53.08/48.11 | nr 1 | nr 1 |
| Luu HN 2019 [21] | China | Prospective Case control | 776/25,764 | 65.9 (±7.9)/62.72 (±7.6) | 55.15/45.82 | 23.3 (±3.4)/23.3(±3.5) | 39.6/31.8 |
| (b) Survival analyses | |||||||
| Gertler R 2004 [36] | Germany | Prospective overall survival | 57 | 64.6 (±13.6) | 52.6 | nr 1 | nr 1 |
| Valls C 2011 [37] | Spain | Prospective overall survival | 147 | age (≤70) 46% | 54.0 | nr 1 | nr 1 |
| Chen Y 2014 [40] | China | Prospective overall survival | 571 | 58.4 (±12.3) | 54.7 | nr 1 | nr 1 |
| Svenson U 2016 [35] | Sweden | Prospective CRC specific survival | 130 | 70 (26–93) | 52.31 | nr 1 | nr 1 |
| Suraweera N 2016 [38] | UK, Australia | Prospective overall survival | 281 | nr 1 | 52.0 | 27.6 (±5.0) | 47.8 |
| Kroupa M 2019 [39] | Czech Republic | Prospective overall survival | 661 | 68 (33–96) | 62.8 | nr 1 | 43.5 |
| Author Year | DNA Extraction Method | TL 1 Measurement Method | TL 1 Parametrization | Reported Risk Estimates (95%CI) | Calculated Risk Estimates (95% CI) |
|---|---|---|---|---|---|
| Zee RY 2009 [32] | QIAprep 2 | RTL 3 | Continuous | 1.25 (0.86–1.81) | 1.35 (0.82–2.24) |
| Lee IM 2010 [31] | QIAprep 2 | RTL 3 | Continuous | 0.94 (0.65–1.38) | 0.90 (0.46–1.76) |
| Pooley KA 2010 [33] | nr 4 | RTL 3 | TL 1 Q4 (shortest)/Q1 (longest) | 1.13 (0.54–2.36) | 0.89 (0.42–1.85) |
| Cui Y 2012 [34] | QIAamp 2 | RTL 3 | TL 1 Q1 (shortest)/Q3 TL 1 Q5 (longest)/Q3 | 1.56 (0.92–2.64) 1.61(0.94–2.75) | 1.04 (0.38–2.88) |
| Boardman L 2014 [29] | phenol/chloroform | RTL 3 | P10 (shorter)/P50 | 1.91 (1.07–3.41) | 0.56 (0.06–5.32) |
| Qin Q 2014 [20] | RelaxGene 5 | RTL 3 | TL 1 Q1 (shortest)/Q4 (longest) | 1.47 (1.09–1.99) | 0.68 (0.50–0.92) |
| Fernandez-Rozadilla C 2018 [30] | QIAamp 2 | RTL 3 | Continuous | 1.00 (0.88–1.14) | 1.00 (0.94–1.07) |
| Luu HN 2019 [21] | QIAamp 2 | RTL 3 | TL 1 Q4 (longest)/Q1 (shortest) | 1.32 (1.08–1.62) | 1.32 (1.08–1.62) |
| Author Year | Specimen | DNA Extraction Method | TL 1 Measurement Method | Follow-Up Time (months) | Survival Comparison Groups (%) | KM 5 yrs 2 Survival (%) | Reported Risk Estimates (95%CI) | Calculated Risk Estimates (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Gertler R 2004 [36] | Tissue | QIAamp 3 | TRF 4 | 75.5 (52–87) | TRF ratio > 0.9 (25) TRF ratio ≤ 0.9 (75) | 25.6 78.2 | 3.30 (1.20–9.00) | 0.75 (0.58–0.96) |
| Valls C 2011 [37] | Tissue | nr 5 | TRF 4 | 45.1 (1.6–59.8) | TRF ratio > 1 (23.2) TRF ratio ≤ 1 (76.8) | 55.2 64.6 | 2.44 (1.20–4.98) | 0.81 (0.69–0.96) |
| Chen Y 2014 [40] | PBL 6 | RelaxGene 7 | RTL 8 | 28 (6–60) | RTL ≤ 0.704 (59.2) RTL > 0.704 (40.8) | 52.6 70.3 | 2.43 (1.53–3.45) | 3.15 (1.85–5.36) |
| Svenson U 2016 [35] | PBL 6 | QIAamp 3 | RTL 8 | 202 | Q1 RTL (shortest) Q2–Q4 RTL | 96.0 74.0 | 0.52 (0.15–1.76) | 0.52 (0.15–1.76) |
| Suraweera N 2016 [38] | Tissue | DNAeasy 9 | RTL 8 | 45.2 | RTL continuous | nr 5 | 0.99 (0.75–1.32) | 1.01 (0.68–1.52) |
| Kroupa M 2019 [39] | Tissue | DNAeasy 9 | RTL 8 | nr 5 | RTL ratio < 0.9 RTL ratio ≥ 0.9 | 69.4 59.5 | nr 5 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauleck, S.; Sinnott, J.A.; Zheng, Y.-L.; Gadalla, S.M.; Viskochil, R.; Haaland, B.; Cawthon, R.M.; Hoffmeister, A.; Hardikar, S. Association of Telomere Length with Colorectal Cancer Risk and Prognosis: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1159. https://doi.org/10.3390/cancers15041159
Pauleck S, Sinnott JA, Zheng Y-L, Gadalla SM, Viskochil R, Haaland B, Cawthon RM, Hoffmeister A, Hardikar S. Association of Telomere Length with Colorectal Cancer Risk and Prognosis: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(4):1159. https://doi.org/10.3390/cancers15041159
Chicago/Turabian StylePauleck, Svenja, Jennifer A. Sinnott, Yun-Ling Zheng, Shahinaz M. Gadalla, Richard Viskochil, Benjamin Haaland, Richard M. Cawthon, Albrecht Hoffmeister, and Sheetal Hardikar. 2023. "Association of Telomere Length with Colorectal Cancer Risk and Prognosis: A Systematic Review and Meta-Analysis" Cancers 15, no. 4: 1159. https://doi.org/10.3390/cancers15041159
APA StylePauleck, S., Sinnott, J. A., Zheng, Y.-L., Gadalla, S. M., Viskochil, R., Haaland, B., Cawthon, R. M., Hoffmeister, A., & Hardikar, S. (2023). Association of Telomere Length with Colorectal Cancer Risk and Prognosis: A Systematic Review and Meta-Analysis. Cancers, 15(4), 1159. https://doi.org/10.3390/cancers15041159






