Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
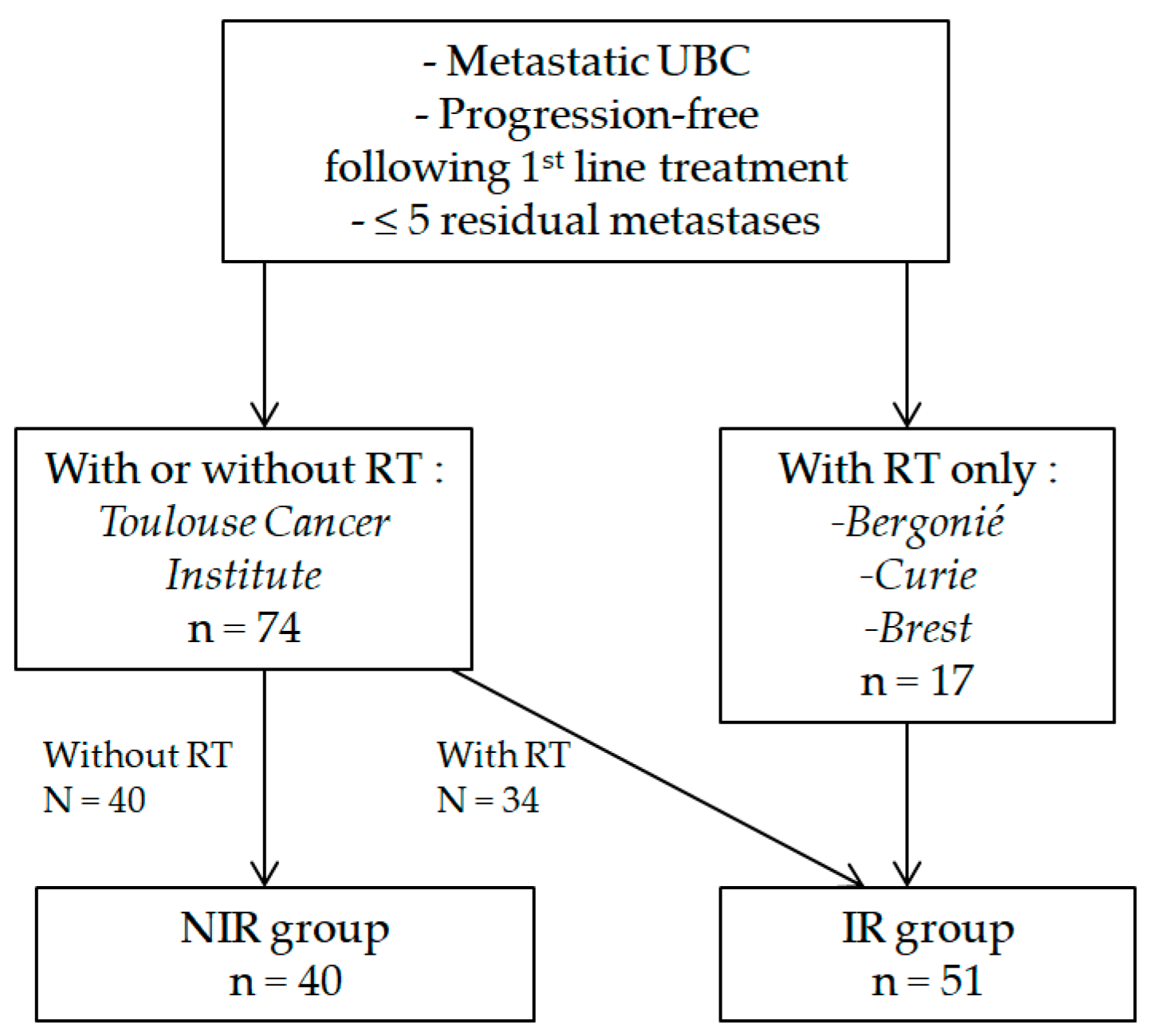
2.1. Patients
2.2. Counting Metastatic Lesions
2.3. Local Consolidative Radiotherapy
2.4. Follow Up
2.5. Toxicity Following Radiotherapy
2.6. Statistical Analysis
3. Results
3.1. Patients
3.2. Local Consolidative Radiotherapy
3.3. Treatment Response Analysis
3.4. PFS
3.4.1. Whole Population
3.4.2. Landmark Population Analysis
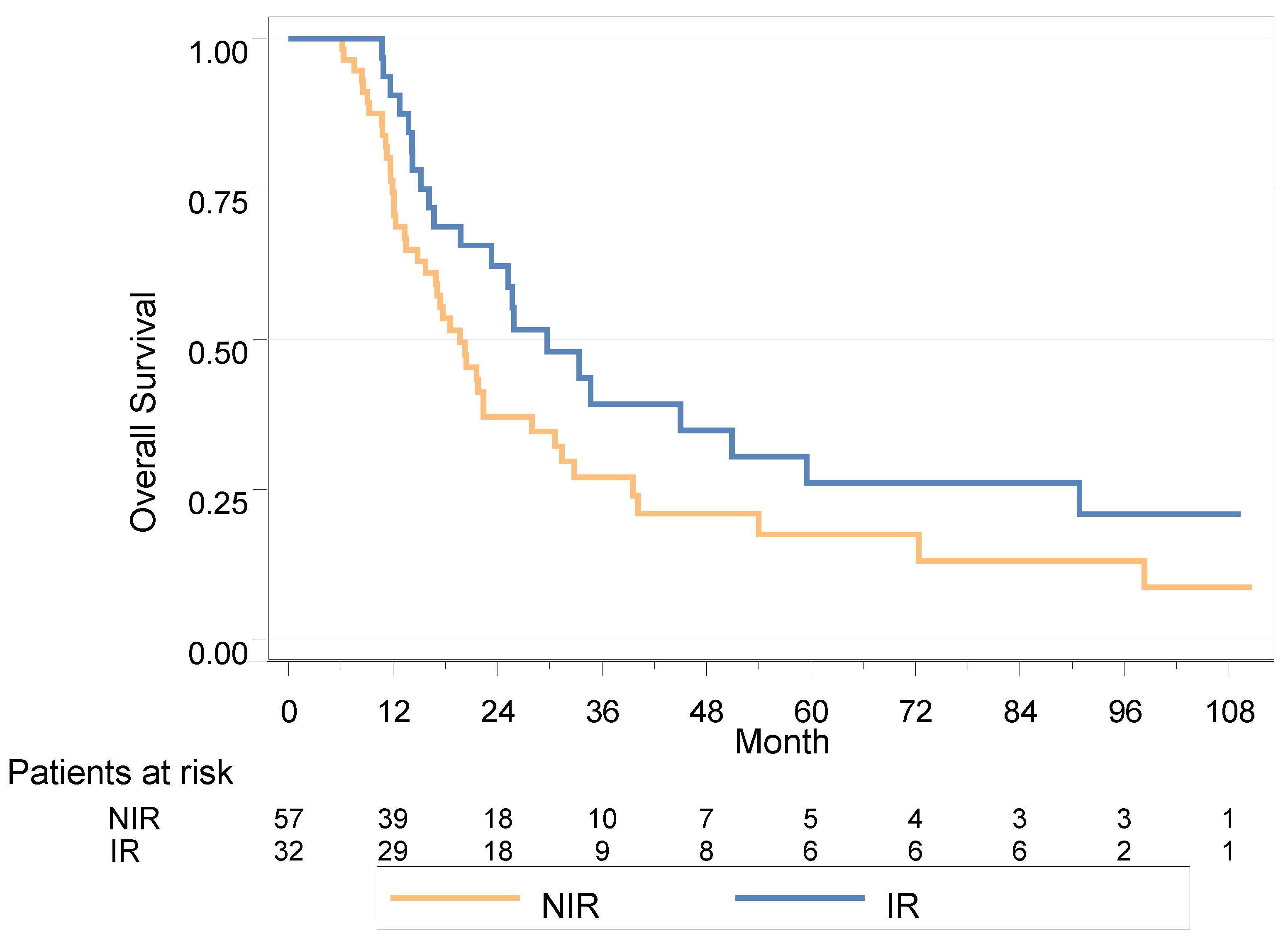
3.5. OS
3.5.1. Whole Population
3.5.2. Landmark Analysis
3.6. Toxicity in the IR Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Sargos, P.; Baumann, B.C.; Eapen, L.; Christodouleas, J.; Bahl, A.; Murthy, V.; Efstathiou, J.; Fonteyne, V.; Ballas, L.; Zaghloul, M.; et al. Risk Factors for Loco-Regional Recurrence after Radical Cystectomy of Muscle-Invasive Bladder Cancer: A Systematic-Review and Framework for Adjuvant Radiotherapy. Cancer Treat. Rev. 2018, 70, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hegarty, P.K.; Gee, J.R.; Clark, P.E.; Svatek, R.S.; Hegarty, N.; Shariat, S.F.; Xylinas, E.; Schmitz-Dräger, B.J.; Lotan, Y.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, Diagnosis, and Molecular Markers. Eur. Urol. 2013, 63, 4–15. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, J.A.; Spina, M.; van Groeningen, C.J.; Duclos, B.; et al. Seven Year Update of an EORTC Phase III Trial of High-Dose Intensity M-VAC Chemotherapy and G-CSF versus Classic M-VAC in Advanced Urothelial Tract Tumours. Eur. J. Cancer 2006, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.H.M.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized Phase III Trial of High–Dose-Intensity Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) Chemotherapy and Recombinant Human Granulocyte Colony-Stimulating Factor Versus Classic MVAC in Advanced Urothelial Tract Tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Maintenance Avelumab + Best Supportive Care (BSC) versus BSC Alone after Platinum-Based First-Line (1L) Chemotherapy in Advanced Urothelial Carcinoma (UC): JAVELIN Bladder 100 Phase III Interim Analysis. J. Clin. Oncol. 2020, 38, LBA1. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, K.; Kikuchi, E.; Watanabe, K.; Kufukihara, R.; Yanai, Y.; Takamatsu, K.; Matsumoto, K.; Hara, S.; Oyama, M.; Monma, T.; et al. Can Urologists Introduce the Concept of “Oligometastasis” for Metastatic Bladder Cancer after Total Cystectomy? Oncotarget 2017, 8, 111819–111835. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Sun, M.; Leow, J.J.; Preston, M.A.; Cole, A.P.; Gelpi-Hammerschmidt, F.; Hanna, N.; Meyer, C.P.; Kibel, A.S.; Lipsitz, S.R.; et al. Efficacy of High-Intensity Local Treatment for Metastatic Urothelial Carcinoma of the Bladder: A Propensity Score-Weighted Analysis From the National Cancer Data Base. J. Clin. Oncol. 2016, 34, 3529–3536. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Patel, S.A.; Brenneman, R.J.; Christodouleas, J.; Sargos, P.; Kim, E.; Weiss, A.; Hershatter, B.; Rao, Y.J.; Picus, J.; et al. Association Between Local Radiation Therapy to the Primary Bladder Tumor and Overall Survival for Patients with Metastatic Urothelial Cancer Receiving Systemic Chemotherapy. Eur. Urol. Oncol. 2022, 5, 246–250. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.R.; Nieuwenhuijzen, J.A.; Meinhardt, W.; Bais, E.M.; Horenblas, S. Long-Term Survival after Combined Modality Treatment in Metastatic Bladder Cancer Patients Presenting with Supra-Regional Tumor Positive Lymph Nodes Only. Eur. J. Surg. Oncol. 2009, 35, 352–355. [Google Scholar] [CrossRef]
- Necchi, A.; Giannatempo, P.; Lo Vullo, S.; Farè, E.; Raggi, D.; Nicolai, N.; Piva, L.; Biasoni, D.; Torelli, T.; Catanzaro, M.; et al. Postchemotherapy Lymphadenectomy in Patients with Metastatic Urothelial Carcinoma: Long-Term Efficacy and Implications for Trial Design. Clin. Genitourin. Cancer 2015, 13, 80–86.e1. [Google Scholar] [CrossRef]
- Sweeney, P.; Millikan, R.; Donat, M.; Wood, C.G.; Radtke, A.S.; Pettaway, C.A.; Grossman, H.B.; Dinney, C.P.N.; Swanson, D.A.; Pisters, L.L. Is There a Therapeutic Role for Post-Chemotherapy Retroperitoneal Lymph Node Dissection in Metastatic Transitional Cell Carcinoma of the Bladder? J. Urol. 2003, 169, 2113–2117. [Google Scholar] [CrossRef] [PubMed]
- Han, W.S.; Kim, K.; Park, J.S. Result of Surgical Resection for Pulmonary Metastasis from Urothelial Carcinoma. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 242–245. [Google Scholar] [CrossRef]
- Kanzaki, R.; Higashiyama, M.; Fujiwara, A.; Tokunaga, T.; Maeda, J.; Okami, J.; Nishimura, K.; Kodama, K. Outcome of Surgical Resection of Pulmonary Metastasis from Urinary Tract Transitional Cell Carcinoma. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 60–64. [Google Scholar] [CrossRef]
- Matsuguma, H.; Yoshino, I.; Ito, H.; Goya, T.; Matsui, Y.; Nakajima, J.; Ikeda, N.; Okumura, S.; Shiono, S.; Nomori, H.; et al. Is There a Role for Pulmonary Metastasectomy with a Curative Intent in Patients with Metastatic Urinary Transitional Cell Carcinoma? Ann. Thorac. Surg. 2011, 92, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, L.; Marulli, G.; Solli, P.; Cardillo, G.; Ghisalberti, M.; Mammana, M.; Carleo, F.; Spaggiari, L.; Rea, F. Long-Term Results and Prognostic Factors of Pulmonary Metastasectomy in Patients with Metastatic Transitional Cell Carcinoma. Thorac. Cardiovasc. Surg. 2017, 65, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Krege, S.; Suhr, J.; Rübben, H. Impact of Surgical Resection of Bladder Cancer Metastases Refractory to Systemic Therapy on Performance Score: A Phase II Trial. Urology 2001, 57, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kitamura, H.; Obara, W.; Matsumura, N.; Tsukamoto, T.; Fujioka, T.; Hara, I.; Murai, S.; Shinohara, N.; Nonomura, K. Outcome of Metastasectomy for Urothelial Carcinoma: A Multi-Institutional Retrospective Study in Japan. J. Urol. 2014, 191, 932–936. [Google Scholar] [CrossRef]
- Bekku, K.; Saika, T.; Kobayashi, Y.; Kioshimoto, R.; Kanbara, T.; Nasu, Y.; Kumon, H. Could Salvage Surgery after Chemotherapy Have Clinical Impact on Cancer Survival of Patients with Metastatic Urothelial Carcinoma? Int. J. Clin. Oncol. 2013, 18, 110–115. [Google Scholar] [CrossRef]
- Dodd, P.M.; McCaffrey, J.A.; Herr, H.; Mazumdar, M.; Bacik, J.; Higgins, G.; Boyle, M.G.; Scher, H.I.; Bajorin, D.F. Outcome of Postchemotherapy Surgery after Treatment with Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Patients with Unresectable or Metastatic Transitional Cell Carcinoma. J. Clin. Oncol. 1999, 17, 2546–2552. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Walsh, G.L.; Pisters, L.L.; Shen, Y.; Swanson, D.A.; Logothetis, C.J.; Millikan, R.E. Is There a Role for Surgery in the Management of Metastatic Urothelial Cancer? The M. D. Anderson Experience. J. Urol. 2004, 171, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Suttmann, H.; Albers, P.; Volkmer, B.; Gschwend, J.E.; Fechner, G.; Spahn, M.; Heidenreich, A.; Odenthal, A.; Seif, C.; et al. Surgery for Metastatic Urothelial Carcinoma with Curative Intent: The German Experience (AUO AB 30/05). Eur. Urol. 2009, 55, 1293–1299. [Google Scholar] [CrossRef]
- Kim, T.; Ahn, J.-H.; You, D.; Jeong, I.-G.; Hong, B.; Hong, J.H.; Ahn, H.; Lee, J.L. Pulmonary Metastasectomy Could Prolong Overall Survival in Select Cases of Metastatic Urinary Tract Cancer. Clin. Genitourin. Cancer 2015, 13, e297–e304. [Google Scholar] [CrossRef]
- Patel, V.; Collazo Lorduy, A.; Stern, A.; Fahmy, O.; Pinotti, R.; Galsky, M.D.; Gakis, G. Survival after Metastasectomy for Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Bladder Cancer 2017, 3, 121–132. [Google Scholar] [CrossRef]
- Shah, S.; Zhang, C.A.; Hancock, S.; Fan, A.; Skinner, E.; Srinivas, S. Consolidative Radiotherapy in Metastatic Urothelial Cancer. Clin. Genitourin. Cancer 2017, 15, 685–688. [Google Scholar] [CrossRef]
- Abe, T.; Minami, K.; Harabayashi, T.; Sazawa, A.; Chiba, H.; Kikuchi, H.; Miyata, H.; Frumido, J.; Matsumoto, R.; Osawa, T.; et al. Prognostic Impact of Local Radiotherapy on Metastatic Urothelial Carcinoma Patients Receiving Systemic Chemotherapy. Jpn. J. Clin. Oncol. 2020, 50, 206–213. [Google Scholar] [CrossRef]
- Leonetti, A.; D’Abbiero, N.; Baldari, G.; Andreani, S.; Ruffini, L.; Viansone, A.A.; Buti, S. Radiotherapy for the Treatment of Distant Nodes Metastases from Oligometastatic Urothelial Cancer: A Retrospective Case Series. Int. J. Urol. 2018, 25, 879–886. [Google Scholar] [CrossRef]
- Longo, N.; Celentano, G.; Napolitano, L.; La Rocca, R.; Capece, M.; Califano, G.; Ruvolo, C.C.; Mangiapia, F.; Fusco, F.; Morra, S.; et al. Metastasis-Directed Radiation Therapy with Consolidative Intent for Oligometastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2373. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Howard, J.M.; McLaughlin, M.; Meng, X.; Clinton, T.; Şanli, Ö.; Garant, A.; Bagrodia, A.; Margulis, V.; Lotan, Y.; et al. Metastasis-Directed Radiation Therapy after Radical Cystectomy for Bladder Cancer. Urol. Oncol. 2021, 39, 790.e1–790.e7. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Francolini, G.; Nicosia, L.; Alongi, F.; Livi, L.; Scorsetti, M. Stereotactic Body Radiation Therapy in the Management of Oligometastatic and Oligoprogressive Bladder Cancer and Other Urothelial Malignancies. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, 50–56. [Google Scholar] [CrossRef]
- Augugliaro, M.; Marvaso, G.; Ciardo, D.; Zerini, D.; Riva, G.; Rondi, E.; Vigorito, S.; Comi, S.; de Cobelli, O.; Orecchia, R.; et al. Recurrent Oligometastatic Transitional Cell Bladder Carcinoma: Is There Room for Radiotherapy? Neoplasma 2019, 66, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and Classification of Oligometastatic Disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus Recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Pos, F.J.; Hart, G.; Schneider, C.; Sminia, P. Radical Radiotherapy for Invasive Bladder Cancer: What Dose and Fractionation Schedule to Choose? Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1168–1173. [Google Scholar] [CrossRef]
- Park, H.S.; Gross, C.P.; Makarov, D.V.; Yu, J.B. Immortal Time Bias: A Frequently Unrecognized Threat to Validity in the Evaluation of Postoperative Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1365–1373. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors Involved in Cancer Metastasis: A Better Understanding to “Seed and Soil” Hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; Vlaskou Badra, E.; Adilovic, S.; Ahmadsei, M.; Christ, S.M.; van Timmeren, J.E.; Kroeze, S.G.C.; Mayinger, M.; Guckenberger, M.; Andratschke, N. Evaluation of the Prognostic Value of the ESTRO EORTC Classification of Oligometastatic Disease in Patients Treated with Stereotactic Body Radiotherapy: A Retrospective Single Center Study. Radiother. Oncol. 2022, 168, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Nevens, D.; Jongen, A.; Kindts, I.; Billiet, C.; Deseyne, P.; Joye, I.; Lievens, Y.; Guckenberger, M. Completeness of Reporting Oligometastatic Disease Characteristics in the Literature and Influence on Oligometastatic Disease Classification Using the ESTRO/EORTC Nomenclature. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 587–595. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non–Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation Effects on Antitumor Immune Responses: Current Perspectives and Challenges. Ther. Adv. Med. Oncol. 2018, 10, 1758834017742575. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and Anti–PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Ronet, C.; de Olza, M.O.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation Modulates the Peptide Repertoire, Enhances MHC Class I Expression, and Induces Successful Antitumor Immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, J.; Mazieres, J.; Gomez-Roca, C.; Ayyoub, M.; Moyal, E.C.J. Radiotherapy in the Era of Immunotherapy With a Focus on Non-Small-Cell Lung Cancer: Time to Revisit Ancient Dogmas? Front. Oncol. 2021, 11, 662236. [Google Scholar] [CrossRef] [PubMed]

| TOTAL N = 91 | NIR N = 40 | IR N = 51 | p Value | |
|---|---|---|---|---|
| Center | ||||
| Toulouse Cancer Institute | 74 (81.3%) | 40 (100.0%) | 34 (66.7%) | |
| Institut Bergonié | 10 (11.0%) | 0 | 10 (19.6%) | |
| CHU Brest | 3 (3.3%) | 0 | 3 (5.9%) | |
| Institut Curie | 4 (4.4%) | 0 | 4 (7.8%) | |
| Sex | 0.176 | |||
| Male | 81 (89.0%) | 38 (95.0%) | 43 (84.3%) | |
| Female | 10 (11.0%) | 2 (5.0%) | 8 (15.7%) | |
| Age | 0.665 | |||
| Median | 62 | 62 | 63 | |
| (Range) | (37.0–83.0) | (45.0–83.0) | (37.0–80.0) | |
| ECOG performance status | 0.715 | |||
| 0 | 60 (65.9%) | 27 (67.5%) | 33 (64.7%) | |
| 1 | 26 (28.6%) | 10 (25.0%) | 16 (31.4%) | |
| 2 | 4 (4.4%) | 2 (5.0%) | 2 (3.9%) | |
| 3 | 1 (1.1%) | 1 (2.5%) | 0 (0.0%) | |
| Histological variant | 0.526 | |||
| No | 69 (83.1%) | 31 (86.1%) | 38 (80.9%) | |
| Yes | 14 (16.9%) | 5 (13.9%) | 9 (19.1%) | |
| Missing | 8 | 4 | 4 | |
| Type of variant (n = 14) | 0.550 | |||
| Epidermoid | 1 (7.1%) | 1 (20.0%) | 0 (0.0%) | |
| Glandular | 1 (7.1%) | 0 (0.0%) | 1 (11.1%) | |
| Nested | 2 (14.3%) | 1 (20.0%) | 1 (11.1%) | |
| Micropapillary | 5 (35.7%) | 1 (20.0%) | 4 (44.4%) | |
| Plasmocytoid | 3 (21.4%) | 2 (40.0%) | 1 (11.1%) | |
| Sarcomatoid | 2 (14.3%) | 0 (0.0%) | 2 (22.2%) | |
| Presence of CIS | 0.018 | |||
| No | 47 (75.8%) | 18 (62.1%) | 29 (87.9%) | |
| Yes | 15 (24.2%) | 11 (37.9%) | 4 (12.1%) | |
| Missing | 29 | 11 | 18 | |
| T clinical status at diagnosis | 0.019 | |||
| T1 | 16 (18.4%) | 9 (22.5%) | 7 (14.9%) | |
| T2 | 52 (59.8%) | 22 (55.0%) | 30 (63.8%) | |
| T3 | 8 (9.2%) | 7 (17.5%) | 1 (2.1%) | |
| T4 | 11 (12.6%) | 2 (5.0%) | 9 (19.1%) | |
| Missing | 4 | 0 | 4 | |
| N clinical status at diagnosis | 0.396 | |||
| N0 | 46 (51.1%) | 23 (57.5%) | 23 (46.0%) | |
| N1 | 10 (11.1%) | 3 (7.5%) | 7 (14.0%) | |
| N2 | 15 (16.7%) | 8 (20.0%) | 7 (14.0%) | |
| N3 | 19 (21.1%) | 6 (15.0%) | 13 (26.0%) | |
| Missing | 1 | 0 | 1 | |
| Metastatic presentation | 0.001 | |||
| Metachronous | 46 (50.5%) | 28 (70.0%) | 18 (35.3%) | |
| Synchronous | 45 (49.5%) | 12 (30.0%) | 33 (64.7%) | |
| Time from diagnosis to metastasis (months) | 0.001 | |||
| Median | 0.5 | 13.1 | 0.0 | |
| (Range) | (0.0–50.7) | (0.0–250.7) | (0.0–92.6) | |
| Number of metastatic lesions at metastatic presentation | 0.040 | |||
| Median | 2 | 3 | 2 | |
| (Range) | (1–9) | (1–5) | (1–9) | |
| Topography of metastatic lesions at metastatic presentation | ||||
| Bone | 0.428 | |||
| No | 62 (68.1%) | 29 (72.5%) | 33 (64.7%) | |
| Yes | 29 (31.9%) | 11 (27.5%) | 18 (35.3%) | |
| Lung | 0.062 | |||
| No | 73 (81.1%) | 29 (72.5%) | 44 (88.0%) | |
| Yes | 17 (18.9%) | 11 (27.5%) | 6 (12.0%) | |
| Missing | 1 | 0 | 1 | |
| Liver | 0.293 | |||
| No | 83 (91.2%) | 35 (87.5%) | 48 (94.1%) | |
| Yes | 8 (8.8%) | 5 (12.5%) | 3 (5.9%) | |
| Central Nervous System | 0.440 | |||
| No | 90 (98.9%) | 39 (97.5%) | 51 (100.0%) | |
| Yes | 61 (67.0%) | 29 (72.5%) | 32 (62.7%) | |
| Extra Pelvic Node | 0.326 | |||
| No | 30 (33.0%) | 11 (27.5%) | 19 (37.3%) | |
| Yes | 61 (67.0%) | 29 (72.5%) | 32 (62.7%) | |
| Duration of first-line systemic therapy | 0.248 | |||
| Median | 3.8 | 3.9 | 3.8 | |
| Range | 0.7–7.4 | 1.6–4.9 | 0.7–7.4 | |
| Number of residual metastatic lesions following first-line therapy | 0.179 | |||
| Median | 1 | 2 | 1 | |
| (range) | (0–5) | (0–5) | (0–5) |
| Irradiated Volume | N (%) | Total Dose Median (Range) | Dose per Fraction (Gy) Median (Range) | Number of Fractions Median (Range) | Total Dose EQD2 Gy |
|---|---|---|---|---|---|
| Bladder | 36 (70.6%) | 64.0 (45.0–66.0) | 2.0 (1.7–3.0) | 33 (15–36) | 64.0 (48.0–66.0) |
| Pelvic Nodes | 39 * (76.5%) | 50.0 (24.0–63.0) | 1.85 (1.6–3.0) | 25 (10–34) | 48.5 (24.0–63.5) |
| Metastasis | 38 (74.5%) (56 lesions) | 54.0 (30.0–67.5) | 1.9 (1.7–18.0) | 30 (3–34) | 53.0 (45.0–132) |
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Sex | Male | 1 | |||
| Female | 0.52 (0.22–1.21) | 0.123 | |||
| Presence of histological variant | No | 1 | |||
| Yes | 1.05 (0.55–2.00) | 0.881 | |||
| Presence of CIS | No | 1 | 1 | ||
| Yes | 1.59 (0.86–2.96) | 0.137 | 1.48 (0.79–2.80) | 0.224 | |
| T clinical status at diagnosis | T1–T2 | 1 | |||
| T3–T4 | 1.08 (0.62–1.88) | 0.787 | |||
| N clinical status at diagnosis | N0 | 1 | |||
| N+ | 0.97 (0.61–1.53) | 0.891 | |||
| Metastatic presentation | Synchronous | 1 | |||
| Metachronous | 1.29 (0.82–2.02) | 0.271 | |||
| Time from diagnosis to metastasis | <24 months | 1 | 1 | ||
| ≥24 months | 1.89 (1.07–3.36) | 0.026 | 2.01 (0.90–4.50) | 0.089 | |
| Number of metastatic lesions at metastatic presentation | ≤3 | 1 | |||
| >3 | 1.11 (0.69–1.79) | 0.674 | |||
| Lung metastatic lesion | No | 1 | |||
| Yes | 0.98 (0.55–1.72) | 0.937 | |||
| Liver metastatic lesion | No | 1 | 1 | ||
| Yes | 2.76 (1.31–5.81) | 0.005 | 1.60 (0.63–4.07) | 0.322 | |
| Extra-pelvic node only * | No | 1 | 1 | ||
| Yes | 0.65 (0.40–1.07) | 0.113 | 0.56 (0.29–1.11) | 0.097 | |
| Number of residual metastatic lesions following first-line therapy | 0 | 1 | |||
| 1 | 1.06 (0.56–1.98) | ||||
| ≥2 | 1.38 (0.80–2.36) | 0.438 | |||
| Local consolidative radiotherapy | No (NIR) | 1 | 1 | ||
| Yes (IR) | 0.52 (0.34–0.84) | 0.006 | 0.57 (0.31–1.07) | 0.082 | |
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Sex | Male | 1 | |||
| Female | 0.55 (0.22–1.36) | 0.188 | |||
| Presence of histological variant | No | 1 | |||
| Yes | 1.06 (0.52–2.16) | 0.881 | |||
| Presence of CIS | No | 1 | 1 | ||
| Yes | 1.80 (0.93–3.47) | 0.076 | 1.49 (0.76–2.91) | 0.244 | |
| T clinical status at diagnosis | T1–T2 | 1 | |||
| T3–T4 | 1.12 (0.59–2.01) | 0.709 | |||
| N clinical status at diagnosis | N0 | 1 | |||
| N+ | 1.27 (0.77–2.08) | 0.348 | |||
| Metastatic presentation | Synchronous | 1 | |||
| Metachronous | 1.06 (0.65–1.74) | 0.805 | |||
| Time from diagnosis to metastasis | <24 months | 1 | |||
| ≥24 months | 1.37 (0.76–2.49) | 0.293 | |||
| Number of metastatic lesions at metastatic presentation | ≤3 | 1 | |||
| >3 | 1.13 (0.68–1.88) | 0.635 | |||
| Lung metastatic lesion | No | 1 | |||
| Yes | 0.94 (0.51–1.74) | 0.844 | |||
| Liver metastatic lesion | No | 1 | |||
| Yes | 1.69 (0.76–3.74) | 0.192 | |||
| Extra-pelvic node only * | No | 1 | |||
| Yes | 0.73 (0.42–1.26) | 0.254 | |||
| Number of residual metastatic lesions following first-line therapy | 0 | 1 | 1 | ||
| 1 | 0.61 (0.29–1.29) | 0.68 (0.26–1.80) | |||
| ≥2 | 1.24 (0.70–2.18) | 0.120 | 1.36 (0.69–2.68) | 0.367 | |
| Local consolidative radiotherapy | No (NIR) | 1 | 1 | ||
| Yes (IR) | 0.63 (0.37–1.05) | 0.074 | 0.48 (0.25–0.92) | 0.026 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboudaram, A.; Chaltiel, L.; Pouessel, D.; Graff-Cailleaud, P.; Benziane-Ouaritini, N.; Sargos, P.; Schick, U.; Créhange, G.; Cohen-Jonathan Moyal, E.; Chevreau, C.; et al. Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis. Cancers 2023, 15, 1161. https://doi.org/10.3390/cancers15041161
Aboudaram A, Chaltiel L, Pouessel D, Graff-Cailleaud P, Benziane-Ouaritini N, Sargos P, Schick U, Créhange G, Cohen-Jonathan Moyal E, Chevreau C, et al. Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis. Cancers. 2023; 15(4):1161. https://doi.org/10.3390/cancers15041161
Chicago/Turabian StyleAboudaram, Amélie, Léonor Chaltiel, Damien Pouessel, Pierre Graff-Cailleaud, Nicolas Benziane-Ouaritini, Paul Sargos, Ulrike Schick, Gilles Créhange, Elizabeth Cohen-Jonathan Moyal, Christine Chevreau, and et al. 2023. "Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis" Cancers 15, no. 4: 1161. https://doi.org/10.3390/cancers15041161
APA StyleAboudaram, A., Chaltiel, L., Pouessel, D., Graff-Cailleaud, P., Benziane-Ouaritini, N., Sargos, P., Schick, U., Créhange, G., Cohen-Jonathan Moyal, E., Chevreau, C., & Khalifa, J. (2023). Consolidative Radiotherapy for Metastatic Urothelial Bladder Cancer Patients with No Progression and with No More than Five Residual Metastatic Lesions Following First-Line Systemic Therapy: A Retrospective Analysis. Cancers, 15(4), 1161. https://doi.org/10.3390/cancers15041161





