Role of Patient-Reported Outcomes in Clinical Trials in Metastatic Colorectal Cancer: A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
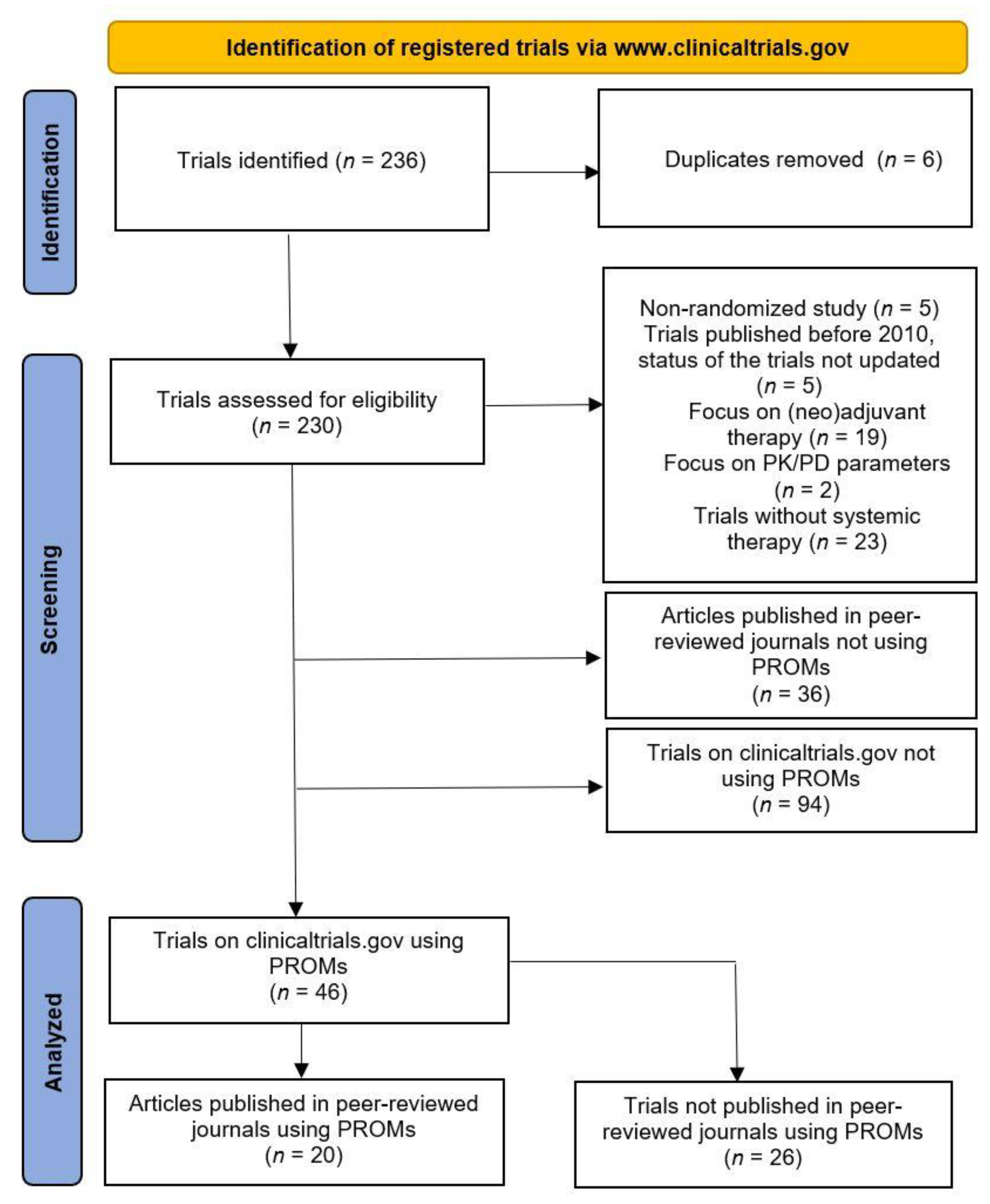
3.1. Data Search
3.2. Quality of Reporting on Patient-Reported Outcomes
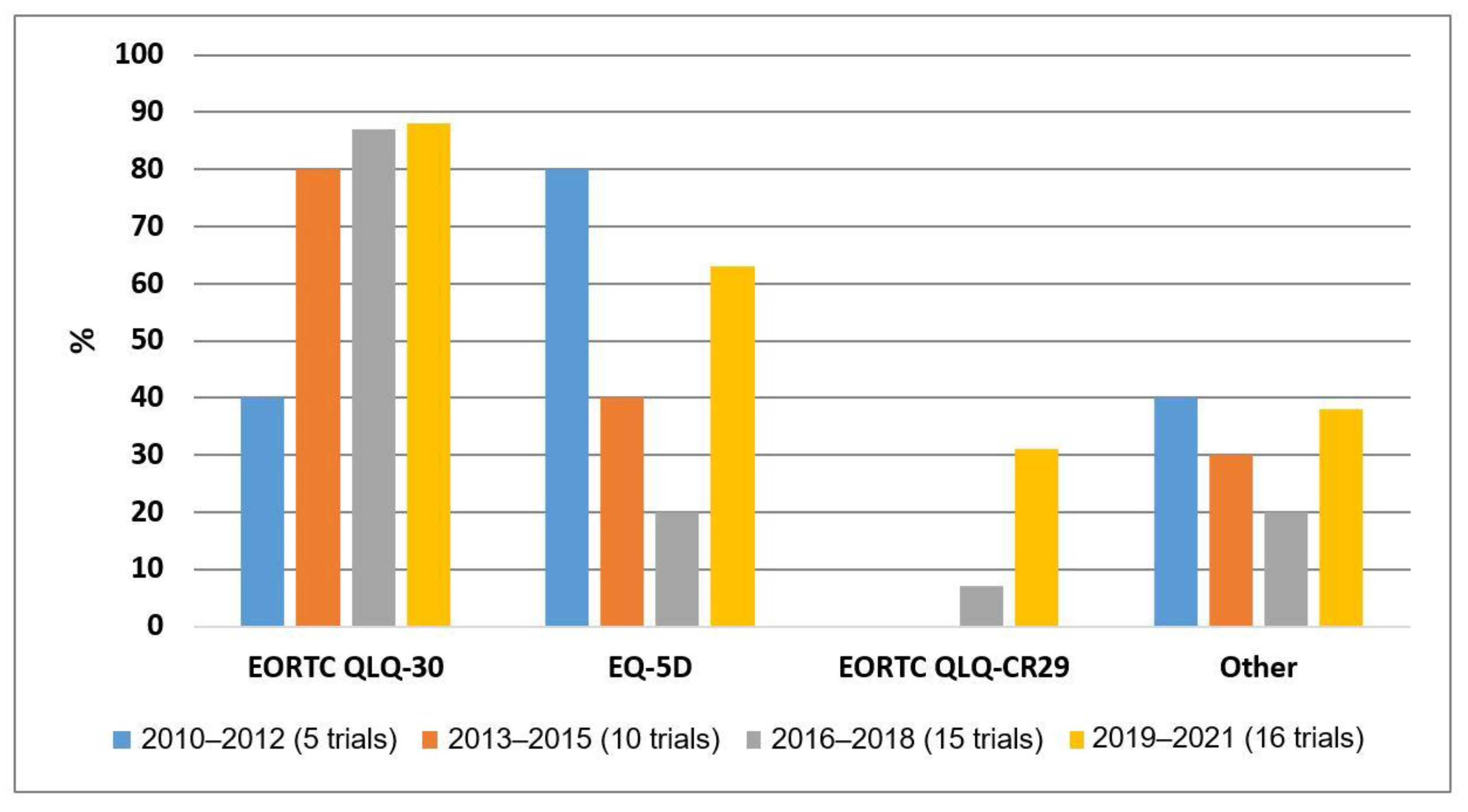
3.3. The Use of PROMs over Time in Clinical Trials
3.4. The Role of PROMs in the Determination of the Value of the New Treatment Modalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Americal Cancer Society. Survival Rates for Colorectal Cancer. 2021. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 October 2022).
- Board, R.E.; Valle, J.W. Metastatic Colorectal Cancer. Drugs 2007, 67, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, À.; Medina-Hayas, M. Colorectal cancer: From prevention to personalized medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef] [PubMed]
- Anatchkova, M.; Donelson, S.M.; Skalicky, A.M.; McHorney, C.A.; Jagun, D.; Whiteley, J. Exploring the implementation of patient-reported outcome measures in cancer care: Need for more real-world evidence results in the peer reviewed literature. J. Patient-Rep. Outcomes 2018, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Van de Glind, I.; Bakker-Jacobs, A.; Triemstra, M.; De Boer, D.; Van der Wees, P. Literature review on the use of PROMs: Current knowledge and scientific evidence for the use of Patient-Reported Outcome Measures. Available online: https://www.htx-h2020.eu/wp-content/uploads/2021/11/Lit_review_Use-PROMs-1.pdf (accessed on 21 December 2022).
- Hughes, S.; Aiyegbusi, O.L.; Lasserson, D.; Collis, P.; Glasby, J.; Calvert, M. Patient-reported outcome measurement: A bridge between health and social care? J. R Soc. Med. 2021, 114, 381–388. [Google Scholar] [CrossRef]
- Deshpande, P.; Sudeepthi, B.L.; Rajan, S.; Abdul Nazir, C.P. Patient-reported outcomes: A new era in clinical research. Perspect. Clin. Res. 2011, 2, 137–144. [Google Scholar] [CrossRef]
- Efficace, F.; Fayers, P.; Pusic, A.; Cemal, Y.; Yanagawa, J.; Jacobs, M.; La Sala, A.; Cafaro, V.; Whale, K.; Rees, J.; et al. Quality of Patient-Reported Outcome (PRO) Reporting Across Cancer Randomized Controlled Trials According to the CONSORT PRO Extension: A Pooled Analysis of 557 Trials. Cancer 2015, 121, 3335–3342. [Google Scholar] [CrossRef]
- Rees, J.R.; Whale, K.; Fish, D.; Fayers, P.; Cafaro, V.; Pusic, A.; Blazeby, J.M.; Efficace, F. Patient-reported outcomes in randomised controlled trials of colorectal cancer: An analysis determining the availability of robust data to inform clinical decision-making. J. Cancer. Res. Clin. Oncol. 2015, 141, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Bennett, L.; Zhao, Z.; Barber, B.; Zhou, X.; Peeters, M.; Zhang, J.; Xu, F.; Wiezorek, J.; Douillard, J-Y. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first-or second-line treatment. Br. J. Cancer 2011, 105, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Odom, D.; Barber, B.; Bennett, L.; Peeters, M.; Zhao, Z.; Kaye, J.; Wolf, M.; Wiezorek, J. Health-related quality of life and colorectal cancer-specific symptoms in patients with chemotherapy-refractory metastatic disease treated with panitumumab. Int. J. Colorectal Dis. 2011, 26, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Cunningham, D.; Sobrero, A.; Karapetis, C.S.; Rougier, P.; Koski, S.L.; Kocakova, I.; Bondarenko, I.; Bodoky, G.; Mainwaring, P.; et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: A double-blind, randomized phase III study (HORIZON III). J. Clin. Oncol. 2012, 30, 3588–3595. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Láng, I.; Köhne, C.H.; Folprecht, G.; Rougier, P.; Curran, D.; Hitre, E.; Sartorius, U.; Griebsch, I.; Van Cutsem, E. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur. J. Cancer 2013, 49, 439–448. [Google Scholar] [CrossRef]
- Carrato, A.; Swieboda-Sadlej, A.; Stazewska-Skurczynska, M.; Lim, R.; Roman, L.; Shparyk, Y.; Bondarenko, I.; Jonker, D.J.; Sun, Y.; De la Cruz, J.A.; et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J. Clin. Oncol. 2013, 31, 1341–1347. [Google Scholar] [CrossRef]
- Ringash, J.; Au, H.J.; Siu, L.L.; Shapiro, J.D.; Jonker, D.J.; Zalcberg, J.R.; Moore, M.J.; Strickland, A.; Kotb, R.; Jeffery, M.; et al. Quality of life in patients with K-RAS wild-type colorectal cancer: The CO.20 phase 3 randomized trial. Cancer 2014, 120, 181–189. [Google Scholar] [CrossRef]
- Siena, S.; Tabernero, J.; Bodoky, G.; Cunningham, D.; Rivera, F.; Ruff, P.; Canon, J.C.; Koukakis, R.; Demonty, G.; Hechmati, G.; et al. Quality of life during first-line FOLFOX4±panitumumab in RAS wild-type metastatic colorectal carcinoma: Results from a randomised controlled trial. ESMO Open 2016, 1, e000041. [Google Scholar] [CrossRef]
- Hamidou, Z.; Chibaudel, B.; Hebbar, M.; Hug de Larauze, M.; André, T.; Louvet, C.; Brusquant, D.; Garcia-Larnicol, M.-L.; De Gramont, A.; Bonnetain, F. Time to definitive health-related quality of life score deterioration in patients with resectable metastatic colorectal cancer treated with folfox4 versus sequential dose-dense folfox7 followed by folfiri: The mirox randomized phase III trial. PLoS ONE 2016, 11, e0157067. [Google Scholar] [CrossRef]
- Quidde, J.; Hegewisch-Becker, S.; Graeven, U.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Steffens, C.-C.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Quality of life assessment in patients with metastatic colorectal cancer receiving maintenance therapy after first-line induction treatment: A preplanned analysis of the phase III AIO KRK 0207 trial. Ann. Oncol. 2016, 27, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Hickish, T.; Andre, T.; Wyrwicz, L.; Saunders, M.; Sarosiek, T.; Kocsis, J.; Nemecek, R.; Rogowski, W.; Lesniewski-Kmak, K.; Petruzelka, L.; et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017, 18, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y.; et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Ghiringhelli, F.; Boige, V.; Le Malicot, K.; Taieb, J.; Bouché, O.; Phelip, J.-M.; François, E.; Borel, C.; Faroux, R.; et al. Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: A randomized phase III trial (PRODIGE 9). J. Clin. Oncol. 2018, 36, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lacas, B.; Bouché, O.; Etienne, P.L.; Gasmi, M.; Texereau, P.; Gargot, D.; Lombard-Bohas, C.; Azzedine, A.; Denis, B.; Geoffroy, P.; et al. Quality of life and cost of strategies of two chemotherapy lines in metastatic colorectal cancer: Results of the FFCD 2000-05 trial. Expert Rev. Pharm. Outcomes Res. 2019, 19, 601–608. [Google Scholar] [CrossRef]
- Lenz, H.J.; Argiles, G.; Yoshino, T.; Lonardi, S.; Falcone, A.; Limón, M.L.; Sobrero, A.; Hastedt, C.; Peil, B.; Voss, F.; et al. Health-related Quality of Life in the Phase III LUME-Colon 1 Study: Comparison and Interpretation of Results From EORTC QLQ-C30 Analyses. Clin. Colorectal. Cancer 2019, 18, 269–279. [Google Scholar] [CrossRef]
- Raimondi, A.; Di Maio, M.; Morano, F.; Corallo, S.; Lonardi, S.; Antoniotti, C.; Rimassa, L.; Sartore-Bianchi, A.; Tampellini, M.; Ritorto, G.; et al. Health-related quality of life in patients with RAS wild-type metastatic colorectal cancer treated with panitumumab-based first-line treatment strategy: A pre-specified secondary analysis of the Valentino study. Eur. J. Cancer 2020, 135, 230–239. [Google Scholar] [CrossRef]
- Wolstenholme, J.; Fusco, F.; Gray, A.M.; Moschandreas, J.; Virdee, P.S.; Love, S.; Van Hazel, G.; Gibbs, P.; Wasan, H.S.; Sharma, R.A. Quality of life in the FOXFIRE, SIRFLOX and FOXFIRE-global randomised trials of selective internal radiotherapy for metastatic colorectal cancer. Int. J. Cancer 2020, 147, 1078–1085. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Bruix, J.; Demetri, G.D.; Grothey, A.; Marian, M.; Bartsch, J.; Odom, D. Effect of Regorafenib in Delaying Definitive Deterioration in Health-Related Quality of Life in Patients with Advanced Cancer of Three Different Tumor Types. Cancer Manag. Res. 2021, 13, 5523–5533. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yohino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Quality of life with encorafenib plus cetuximab with or without binimetinib treatment in patients with BRAF V600E-mutant metastatic colorectal cancer: Patient-reported outcomes from BEACON CRC. ESMO Open 2022, 7, 100477. [Google Scholar] [CrossRef]
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardello, F.; De Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Del Mar, C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Intern. Med. 2015, 175, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, C.; Patel, S. Patient-reported outcome measures and patient-reported experience measures. BJA Education 2017, 17, 137–144. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Efficace, F.; Aaronson, N.; Calvert, M.; Kyte, D.; Cottone, F.; Cella, D.; Gamper, E.-M. Past and Current Practice of Patient-Reported Outcome Measurement in Randomized Cancer Clinical Trials: A Systematic Review. Value Health 2021, 24, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Davda, J.; Kibet, H.; Achieng, E.; Atundo, L.; Komen, T. Assessing the acceptability, reliability, and validity of the EORTC Quality of Life Questionnaire (QLQ-C30) in Kenyan cancer patients: A cross-sectional study. J. Patient-Rep. Outcomes 2021, 5, 4. [Google Scholar] [CrossRef]
- Schwenkglenks, M.; Matter-Walstra, K. Is the EQ-5D suitable for use in oncology? An overview of the literature and recent developments. Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 207–219. [Google Scholar] [CrossRef]
- Moher, D.; Pham, B.; Klassen, T.P.; Schulz, K.F.; Berlin, J.A.; Jadad, A.R.; Liberati, A. What contributions do languages other than English make on the results of meta-analyses? J. Clin. Epidemiol. 2000, 53, 964–972. [Google Scholar] [CrossRef]
- Jüni, P.; Holenstein, F.; Sterne, J.; Bartlett, C.; Egger, M. Direction and impact of language bias in meta-analyses of controlled trials: Empirical study. Int. J. Epidemiol. 2002, 31, 115–123. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Lee Aiyegbusi, O.; King, M.T.; Brundage, M.; Snyder, C.; Calvert, M. Knowledge translation concerns for the CONSORT-PRO extension reporting guidance: A review of reviews. Qual. Life Res. 2022, 31, 2939–2957. [Google Scholar] [CrossRef]
| Publication | n | Treatment Line | Primary Endpoint | %PROM Evaluable * | PROMs Used | Separate PRO Publication | Adjusted CONSORT PRO Checklist Score |
|---|---|---|---|---|---|---|---|
| Bennett [13] | 530 | 2 | PFS | 89 | EQ-5D + VAS | No | 5/6 = 0.83 |
| Odom [14] | 363 | 3 | PFS | 96 | EQ-5D, NFCSI -19 | No | 5/6 = 0.83 |
| Schmoll [15] | 1614 | 1 | PFS | 84 | FACT-C | Yes | 3/6 = 0.50 |
| Grothey [16] | 753 | 3 | OS | unknown | EORTC QLQ-C30, EQ-5D | No | 3/6 = 0.50 |
| Láng [17] | 666 | 1 | PFS | 94 | EORTC QLQ-C30 | Yes | 6/6 = 1.00 |
| Carrato [18] | 768 | 1 | PFS | 90 | EQ-5D, MDASI-GI | Yes | 3/6 = 0.50 |
| Ringash [19] | 750 | 3 | OS | 96 | EORTC QLQ-C30 | No | 6/6 = 1.00 |
| Siena [20] | 505 | 1 | PFS | 88 | EQ-5D + VAS | Yes | 4/6 = 0.67 |
| Hamidou [21] | 284 | 1 | DFS | 60 | EORTC QLQ-C30 | Yes | 5/6 = 0.83 |
| Quidde [22] | 472 | 1 | PFS | 88 | EORTC QLQ-C30, QLQ-CR29 | Yes | 6/6 = 1.00 |
| Hickish [23] | 333 | 3 | OS | 93 | EORTC QLQ-C30 | No | 3/6 = 0.50 |
| Jonker [24] | 282 | 3 | OS | unknown | EORTC QLQ-C30 | No | 2/6 = 0.33 |
| Aparicio [25] | 494 | 1 | DCD | 68 | EORTC QLQ-C30 | Yes | 2/6 = 0.33 |
| Lacas [26] | 410 | 1 | PFS | 99 | EORTC QLQ-C30 | Yes | 6/6 = 1.00 |
| Lenz [27] | 768 | >3 | OS | 88 | EORTC QLQ-C30, EQ-5D | No | 6/6 = 1.00 |
| Raimondi [28] | 229 | 1 | PFS | 92 | EORTC QLQ-C30, EORTC QLQ-CR29, EQ-5D | Yes | 6/6 = 1.00 |
| Wolstenholme [29] | 1103 | 1 | OS | 92 | EORTC QLQ-C30, EQ-5D, EORTC QLQ-LMC21 | Yes | 5/6 = 0.83 |
| Hofheinz [30] | 760 | 3 | OS | unknown | EQ-5D, EORTC QLQ-C30 | No | 5/6 = 0.83 |
| Andre [31] | 307 | 1 | OS, PFS | 96 | EORTC QLQ-C30, EORTC QLQ-CR29, EQ-5D | Yes | 6/6 = 1.00 |
| Kopetz [32] | 665 | 2, 3 | ORR, OS | >83 | EORTC QLQ-C30, FACT-C | No | 4/6 = 0.67 |
| CONSORT PRO Extension | Brief Explanatory Text | Present Review (n = 20) (%) | Rees et al. (n = 66) (%) |
|---|---|---|---|
| (P1b) The PRO should be identified in the abstract as a primary or secondary outcome | Explicitly identifying PROs in the trial abstract will facilitate indexing and identification of studies to inform clinical care and evidence synthesis | 15 (75) | 55 (83) |
| (P2b) The PRO hypothesis should be stated and relevant domains identified, if applicable * | PRO measures may be multi-dimensional and may assess patient status at several time points during a trial. A pre-specified hypothesis reduces the risk of multiple statistical testing and selective reporting of PROs based on statistically significant results | 7 (35) | 21 (32) |
| (P6a) Evidence of PRO instrument validity and reliability should be provided or cited if available † | This information will allow readers to assess the validity, reliability and appropriateness of the PRO being used | 20 (100) | 46 (70) |
| (P6aa) Mode of administration, including the person completing the PRO and methods of data collection (paper telephone electronic other). | Different methods of data collection could lead to potential bias when interpreting outcomes | 18 (90) | 20 (30) |
| (P12a) Statistical approaches for dealing with missing data are explicitly stated. | Missing PRO data is a potential source of bias. A number of methods for dealing with missing data are available with different strengths and limitations which should be described to facilitate interpretation | 12 (60) | 37 (56) |
| (P20/21) PRO-specific limitations and implications for generalizability and clinical practice should be discussed | PRO specific limitations may influence the generalizability of results and use in clinical practice | 17 (85) | 32 (48) |
| Clinical Outcome | ||||
|---|---|---|---|---|
| Favoring the Experimental Treatment | Equivalent | Favoring the Standard Treatment | ||
| Patient-reported outcome | Broadly favoring the experimental treatment | 5 | 0 | 0 |
| Neutral | 5 | 7 | 1 | |
| Broadly favoring the standard treatment | 0 | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maring, J.G.; Eijsink, J.F.H.; Tichelaar, F.D.; Veluwenkamp-Worawutputtapong, P.; Postma, M.J.; Touw, D.J.; de Groot, J.W.B. Role of Patient-Reported Outcomes in Clinical Trials in Metastatic Colorectal Cancer: A Scoping Review. Cancers 2023, 15, 1135. https://doi.org/10.3390/cancers15041135
Maring JG, Eijsink JFH, Tichelaar FD, Veluwenkamp-Worawutputtapong P, Postma MJ, Touw DJ, de Groot JWB. Role of Patient-Reported Outcomes in Clinical Trials in Metastatic Colorectal Cancer: A Scoping Review. Cancers. 2023; 15(4):1135. https://doi.org/10.3390/cancers15041135
Chicago/Turabian StyleMaring, Jan Gerard, Job F. H. Eijsink, Friso D. Tichelaar, Pawida Veluwenkamp-Worawutputtapong, Maarten J. Postma, Daan J. Touw, and Jan Willem B. de Groot. 2023. "Role of Patient-Reported Outcomes in Clinical Trials in Metastatic Colorectal Cancer: A Scoping Review" Cancers 15, no. 4: 1135. https://doi.org/10.3390/cancers15041135
APA StyleMaring, J. G., Eijsink, J. F. H., Tichelaar, F. D., Veluwenkamp-Worawutputtapong, P., Postma, M. J., Touw, D. J., & de Groot, J. W. B. (2023). Role of Patient-Reported Outcomes in Clinical Trials in Metastatic Colorectal Cancer: A Scoping Review. Cancers, 15(4), 1135. https://doi.org/10.3390/cancers15041135








