Correlations between [68Ga]Ga-DOTA-TOC Uptake and Absorbed Dose from [177Lu]Lu-DOTA-TATE
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Image Acquisition
2.3. [68Ga]Ga-DOTA-TOC PET Measurements
2.4. [177Lu]Lu-DOTA-TATE SPECT Measurements and Tumour Dosimetry
2.5. Prediction Analyses
2.6. Group Analysis
2.7. Statistics
3. Results
3.1. Patient Characteristics and Tumour Parameters
3.2. Correlation between [68Ga]Ga-DOTA-TOC and [177Lu]Lu-DOTA-TATE Parameters for the Full Data Set
3.3. Absorbed Dose Prediction
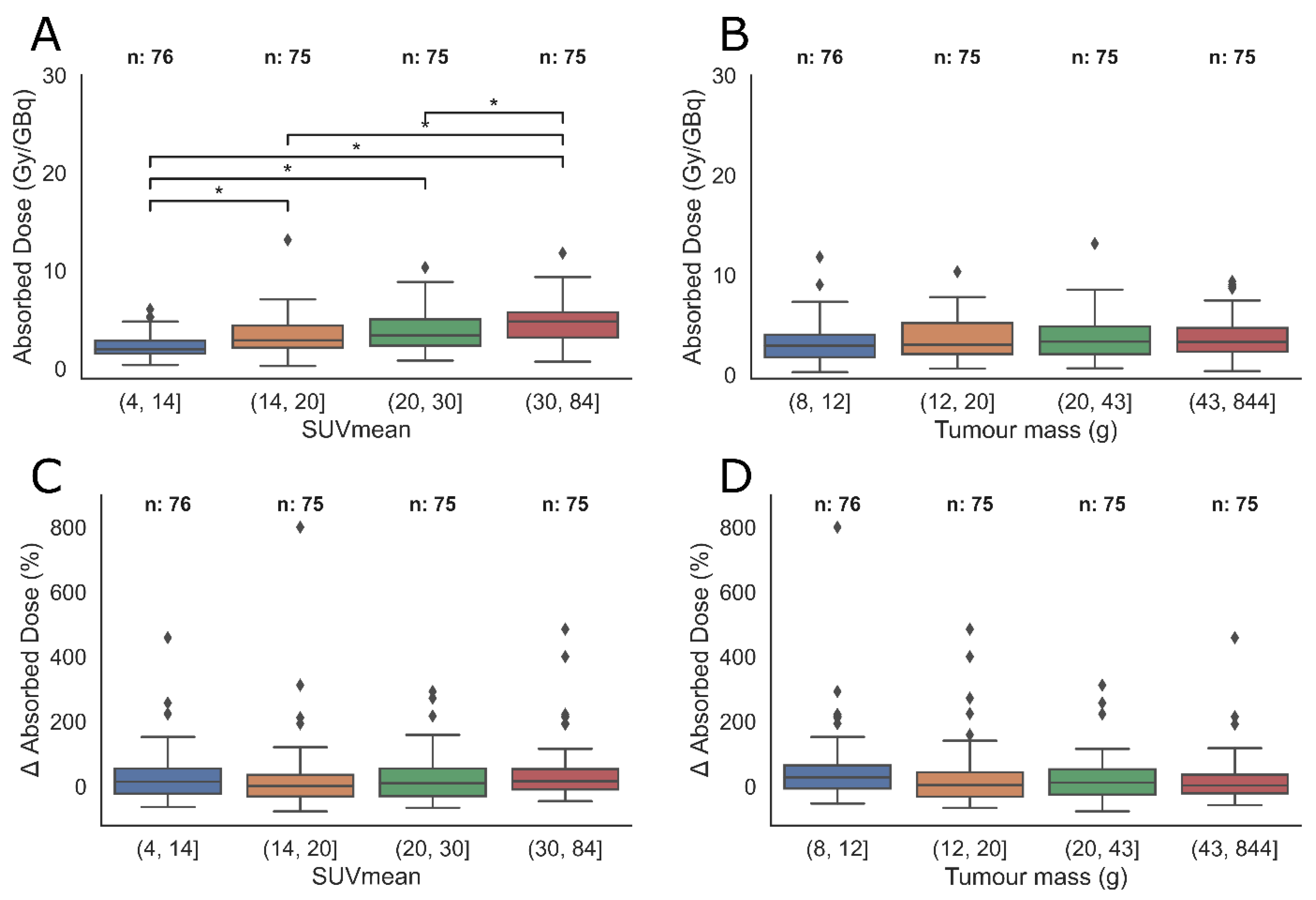
3.4. Tumour Sub-Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Meeker, A.; Heaphy, C. Gastroenteropancreatic endocrine tumors. Mol. Cell. Endocrinol. 2014, 386, 101–120. [Google Scholar] [CrossRef]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.L.; Bussolati, G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef]
- Baumann, T.; Rottenburger, C.; Nicolas, G.; Wild, D. Gastroenteropancreatic neuroendocrine tumours (GEP-NET)—Imaging and staging. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 45–57. [Google Scholar] [CrossRef]
- Camus, B.; Cottereau, A.S.; Palmieri, L.J.; Dermine, S.; Tenenbaum, F.; Brezault, C.; Coriat, R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Ginj, M.; Zhang, H.; Waser, B.; Baum, R.P.; Reubi, J.C.; Maecke, H. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.; van Eijck, C.H.; Valkema, R.; de Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2010, 17, R53–R73. [Google Scholar] [CrossRef] [PubMed]
- Soydal, Ç.; Peker, A.; Özkan, E.; Küçük, Ö.N.; Kir, M.K. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turk. J. Med. Sci. 2016, 46, 409–413. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. (177)Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Pak, K.; Koo, P.J.; Kwak, J.J.; Chang, S. The efficacy of (177)Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: A meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. New Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef]
- Sjogreen Gleisner, K.; Chouin, N.; Gabina, P.M.; Cicone, F.; Gnesin, S.; Stokke, C.; Konijnenberg, M.; Cremonesi, M.; Verburg, F.A.; Bernhardt, P.; et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1778–1809. [Google Scholar] [CrossRef]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske-Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef]
- Roth, D.; Gustafsson, J.R.; Warfvinge, C.F.; Sundlöv, A.; Åkesson, A.; Tennvall, J.; Sjögreen Gleisner, K. Dosimetric quantities of neuroendocrine tumors over treatment cycles with (177)Lu-DOTA-TATE. J. Nucl. Med. 2022, 63, 399–405. [Google Scholar] [CrossRef]
- Wehrmann, C.; Senftleben, S.; Zachert, C.; Müller, D.; Baum, R.P. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother. Radiopharm. 2007, 22, 406–416. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Sundin, A.; Sörensen, J.; Lubberink, M.; Sandström, M.; Garske-Román, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: Net uptake rate for accurate quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Esser, J.P.; Krenning, E.P.; Teunissen, J.J.; Kooij, P.P.; van Gameren, A.L.; Bakker, W.H.; Kwekkeboom, D.J. Comparison of [(177)Lu-DOTA(0),Tyr(3)]octreotate and [(177)Lu-DOTA(0),Tyr(3)]octreotide: Which peptide is preferable for PRRT? Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1346–1351. [Google Scholar] [CrossRef]
- Thuillier, P.; Maajem, M.; Schick, U.; Blanc-Beguin, F.; Hennebicq, S.; Metges, J.P.; Salaun, P.Y.; Kerlan, V.; Bourhis, D.; Abgral, R. Clinical Assessment of 177Lu-DOTATATE Quantification by Comparison of SUV-Based Parameters Measured on Both Post-PRRT SPECT/CT and 68Ga-DOTATOC PET/CT in Patients With Neuroendocrine Tumors: A Feasibility Study. Clin. Nucl. Med. 2021, 46, 111–118. [Google Scholar] [CrossRef]
- Ezziddin, S.; Lohmar, J.; Yong-Hing, C.J.; Sabet, A.; Ahmadzadehfar, H.; Kukuk, G.; Biersack, H.J.; Guhlke, S.; Reichmann, K. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin. Nucl. Med. 2012, 37, e141–e147. [Google Scholar] [CrossRef] [PubMed]
- Eckerman, K.; Endo, A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. SUV of [68Ga]DOTATOC-PET/CT Predicts Response Probability of PRRT in Neuroendocrine Tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef]
- Thuillier, P.; Bourhis, D.; Metges, J.P.; Le Pennec, R.; Amrane, K.; Schick, U.; Blanc-Beguin, F.; Hennebicq, S.; Salaun, P.Y.; Kerlan, V.; et al. Prospective study of dynamic whole-body 68Ga-DOTATOC-PET/CT acquisition in patients with well-differentiated neuroendocrine tumors. Sci. Rep. 2021, 11, 4727. [Google Scholar] [CrossRef]
- Jahn, U.; Ilan, E.; Sandström, M.; Garske-Román, U.; Lubberink, M.; Sundin, A. 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy: Dose Response in Small Intestinal Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 662–670. [Google Scholar] [CrossRef]
- Jahn, U.; Ilan, E.; Sandström, M.; Lubberink, M.; Garske-Román, U.; Sundin, A. Peptide Receptor Radionuclide Therapy (PRRT) with (177)Lu-DOTATATE.; Differences in Tumor Dosimetry, Vascularity and Lesion Metrics in Pancreatic and Small Intestinal Neuroendocrine Neoplasms. Cancers 2021, 13, 962. [Google Scholar] [CrossRef]
- Kupitz, D.; Wetz, C.; Wissel, H.; Wedel, F.; Apostolova, I.; Wallbaum, T.; Ricke, J.; Amthauer, H.; Grosser, O.S. Software-assisted dosimetry in peptide receptor radionuclide therapy with 177Lutetium-DOTATATE for various imaging scenarios. PLoS ONE 2017, 12, e0187570. [Google Scholar] [CrossRef] [PubMed]
- Hanscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Dose Mapping After Endoradiotherapy with (177)Lu-DOTATATE/DOTATOC by a Single Measurement after 4 Days. J. Nucl. Med. 2018, 59, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chicheportiche, A.; Sason, M.; Godefroy, J.; Krausz, Y.; Zidan, M.; Oleinikov, K.; Meirovitz, A.; Gross, D.J.; Grozinsky-Glasberg, S.; Ben-Haim, S. Simple model for estimation of absorbed dose by organs and tumors after PRRT from a single SPECT/CT study. EJNMMI Phys. 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
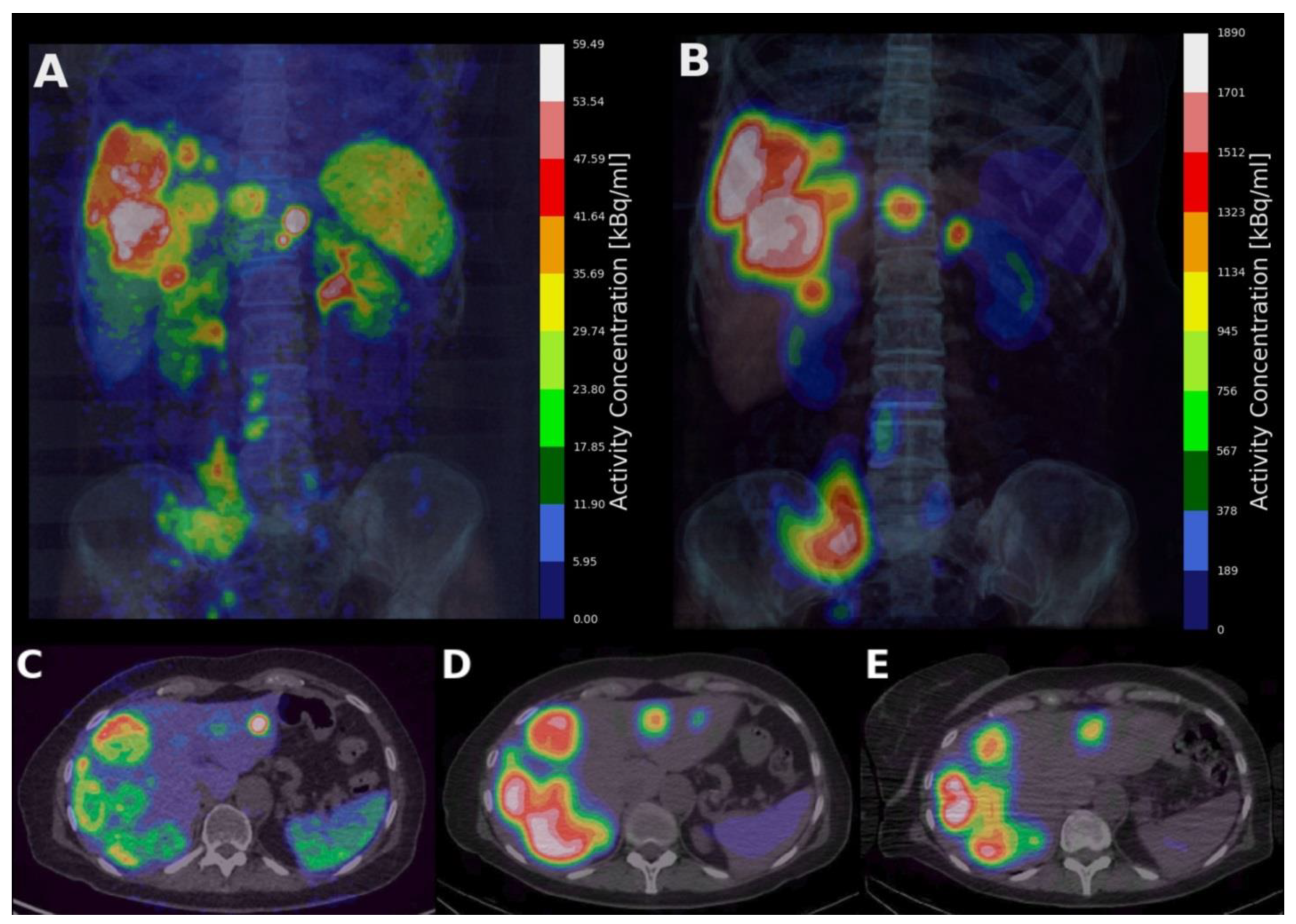
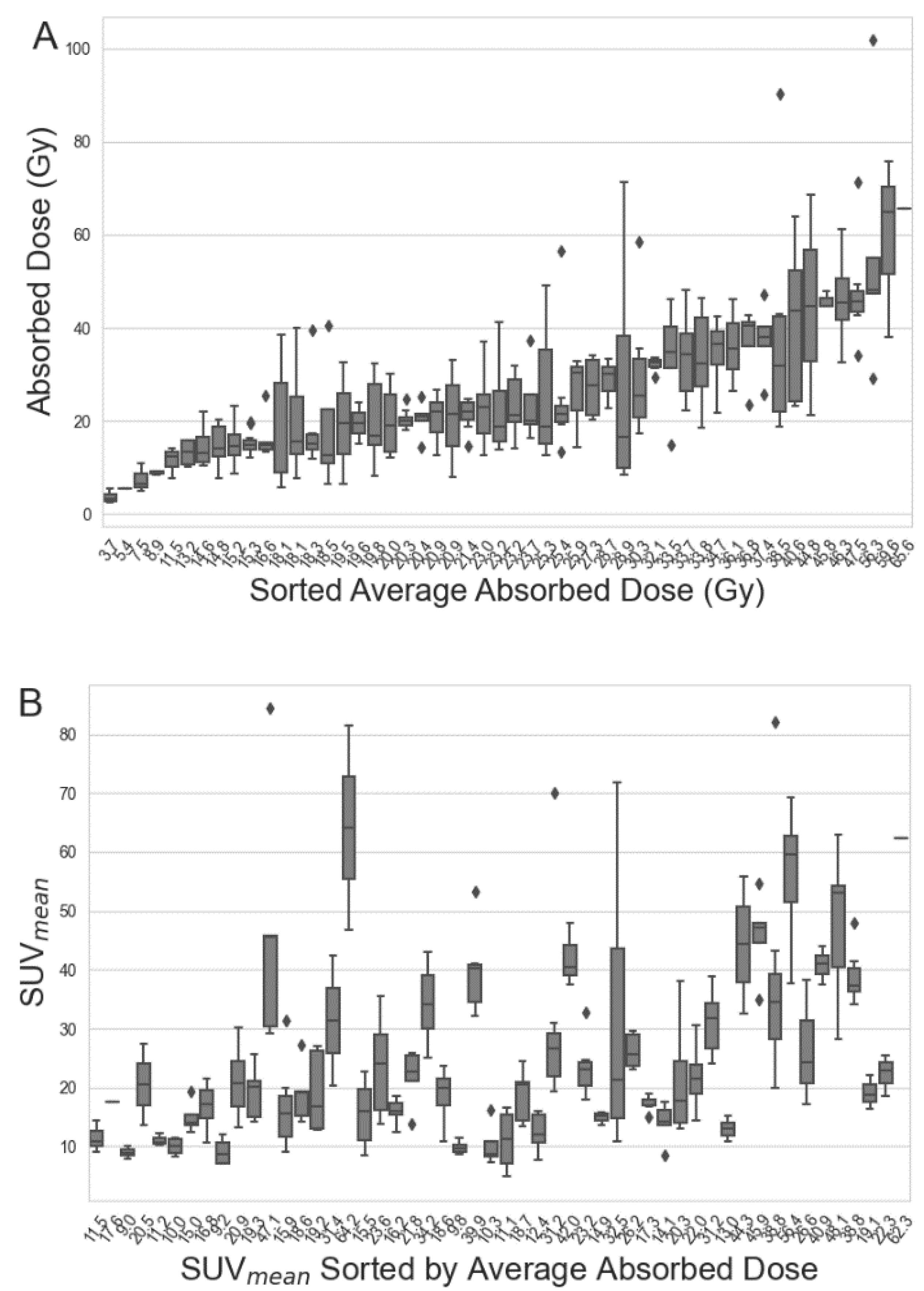
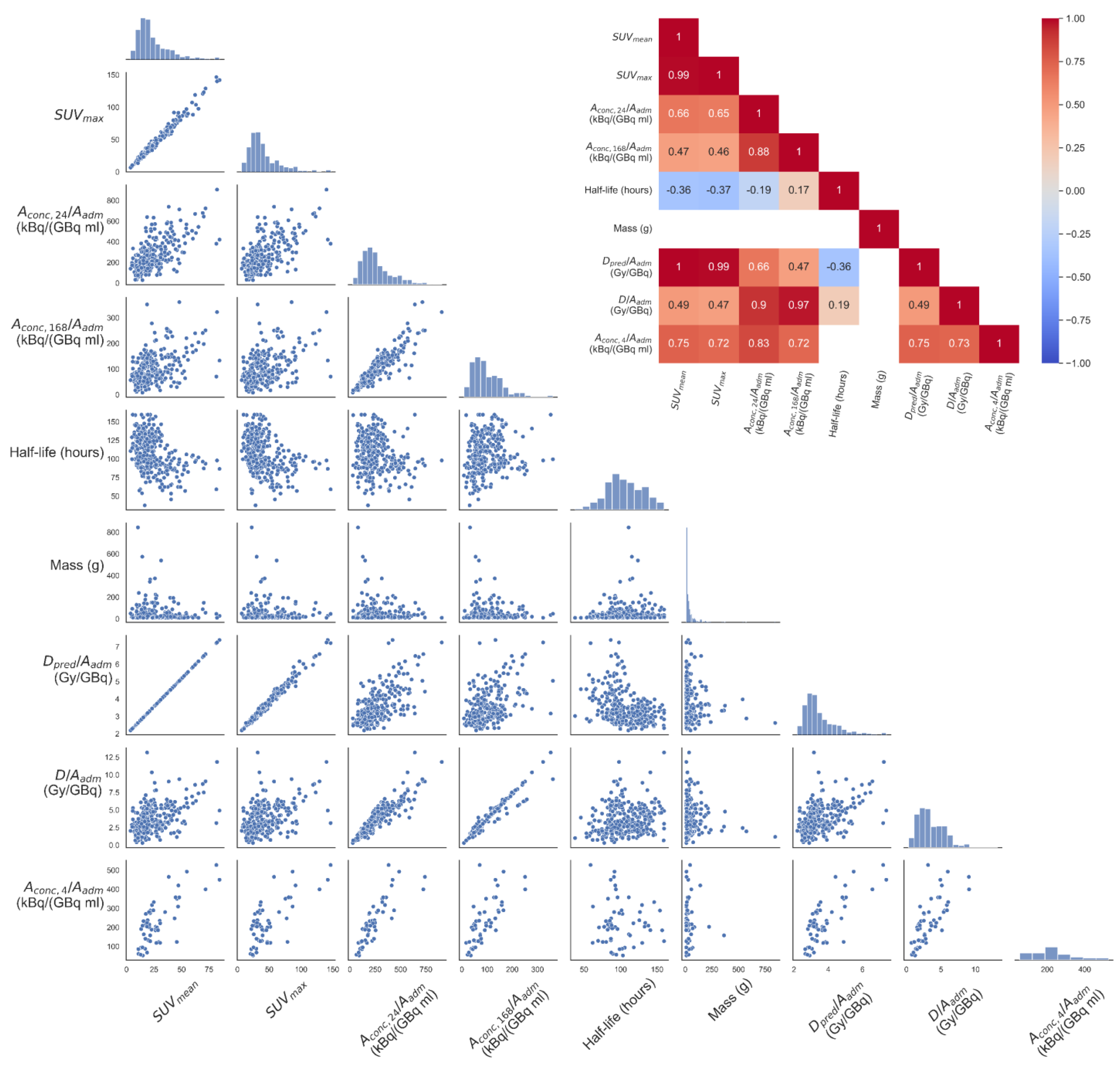
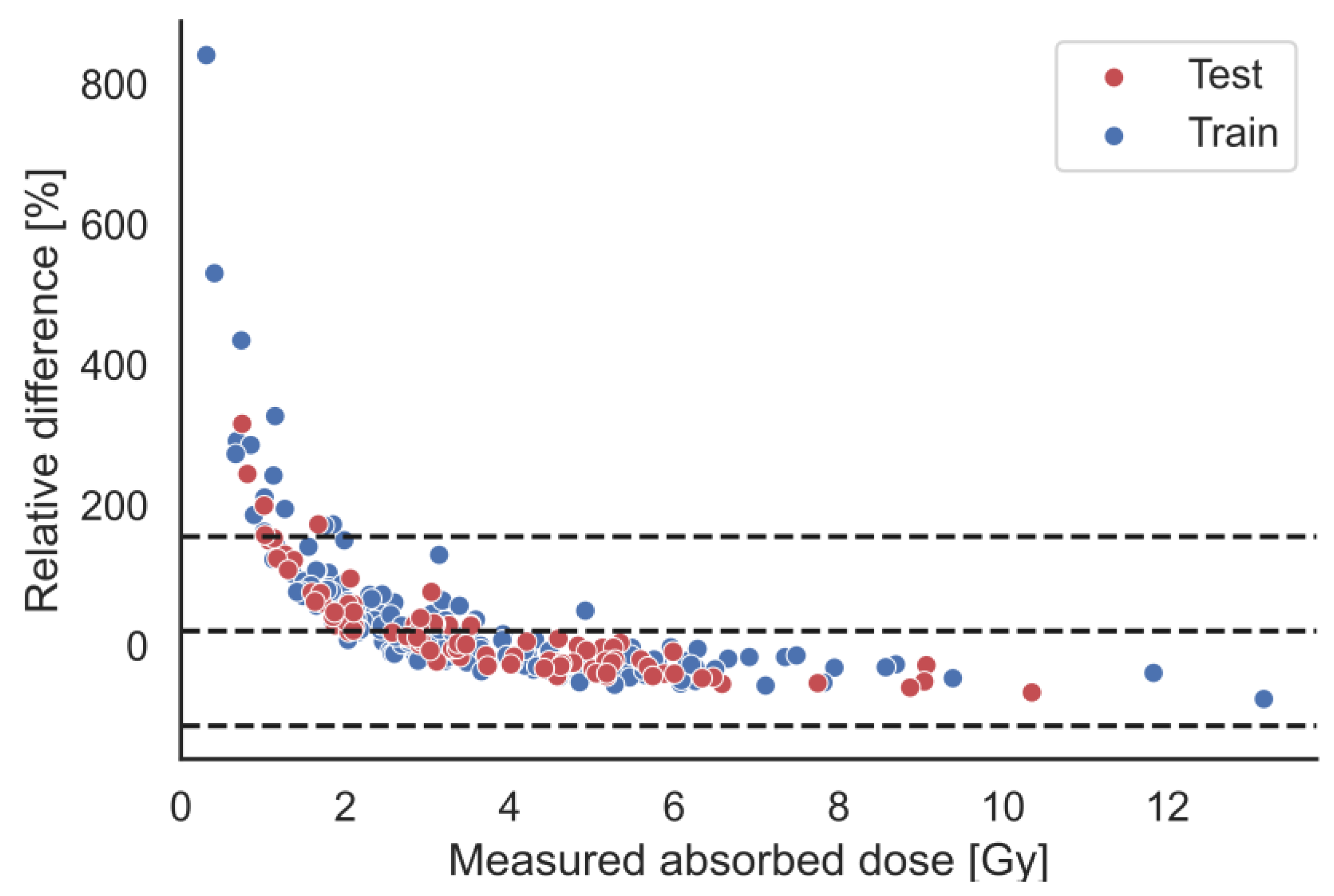

| Total | Training Dataset | Test Dataset | |
|---|---|---|---|
| Characteristic | |||
| Number of patients | 54 | 37 | 17 |
| Number of tumours | 301 | 210 | 91 |
| PET parameters | |||
| Mean ± Standard deviation | |||
| SUVmean | 24.0 ± 14.5 | 24.1 ± 15.1 | 23.7 ± 12.9 |
| SUVmax | 41.0 ± 24.9 | 41.2 ± 26.0 | 40.7 ± 22.2 |
| SRETV [mL] | 29.4 ± 45.9 | 31.1 ± 49.8 | 25.5 ± 35.5 |
| SPECT parameters | |||
| Mean ± Standard deviation | |||
| Absorbed dose [Gy] | 26.9 ± 15.1 | 26.6 ± 15.1 | 27.6 ± 15.2 |
| Absorbed dose/Aadm [Gy/GBq] | 3.6 ± 2.0 | 3.5 ± 2.0 | 3.8 ± 2.0 |
| [(kBq/mL)/GBq] * | 224.8 ± 113.1 | 220.0 ± 92.3 | 235.1 ± 151.3 |
| [(kBq/mL)/GBq] | 262.3 ± 148.4 | 256.2 ± 148.2 | 276.3 ± 148.7 |
| [(kBq/mL)/GBq] | 99.0 ± 58.7 | 96.7 ± 60.4 | 104.2 ± 54.4 |
| Effective half-life after t24 [hours] | 105.7 ± 24.5 | 106.3 ± 23.7 | 104.3 ± 26.3 |
| Tumour mass [g] | 45.8 ± 81.8 | 45.3 ± 73.6 | 47.1 ± 98.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruvoll, R.; Blakkisrud, J.; Mikalsen, L.T.; Connelly, J.; Stokke, C. Correlations between [68Ga]Ga-DOTA-TOC Uptake and Absorbed Dose from [177Lu]Lu-DOTA-TATE. Cancers 2023, 15, 1134. https://doi.org/10.3390/cancers15041134
Bruvoll R, Blakkisrud J, Mikalsen LT, Connelly J, Stokke C. Correlations between [68Ga]Ga-DOTA-TOC Uptake and Absorbed Dose from [177Lu]Lu-DOTA-TATE. Cancers. 2023; 15(4):1134. https://doi.org/10.3390/cancers15041134
Chicago/Turabian StyleBruvoll, Ragnar, Johan Blakkisrud, Lars Tore Mikalsen, James Connelly, and Caroline Stokke. 2023. "Correlations between [68Ga]Ga-DOTA-TOC Uptake and Absorbed Dose from [177Lu]Lu-DOTA-TATE" Cancers 15, no. 4: 1134. https://doi.org/10.3390/cancers15041134
APA StyleBruvoll, R., Blakkisrud, J., Mikalsen, L. T., Connelly, J., & Stokke, C. (2023). Correlations between [68Ga]Ga-DOTA-TOC Uptake and Absorbed Dose from [177Lu]Lu-DOTA-TATE. Cancers, 15(4), 1134. https://doi.org/10.3390/cancers15041134






