Evaluation of the EPR Effect in the CAM-Model by Molecular Imaging with MRI and PET Using 89Zr-Labeled HSA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis, Radiolabeling, and Stability
2.2. Preparation of Cell Culture
2.3. Internalisation of [89Zr]Zr-DFO-HSA
2.4. CAM Experiments
2.5. Animal Studies
2.6. MRI and PET Measurements
2.7. Ex Vivo Validation
2.8. Digital Autoradiography and Histopathological Analyses
2.9. Statistical Evaluation
3. Results
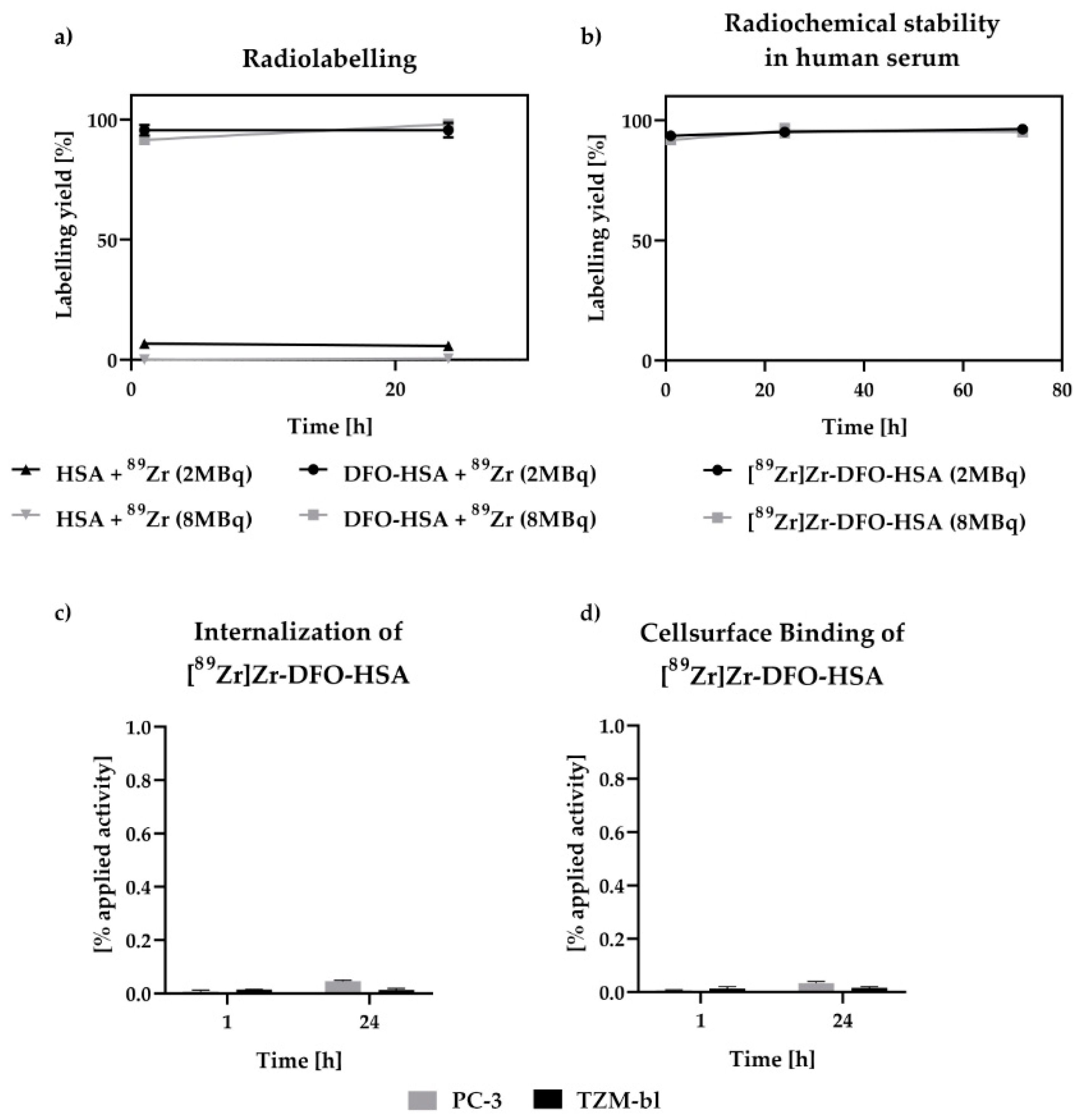
3.1. Radiochemical Stability and Internalization Studies of [89Zr]Zr-DFO-HSA
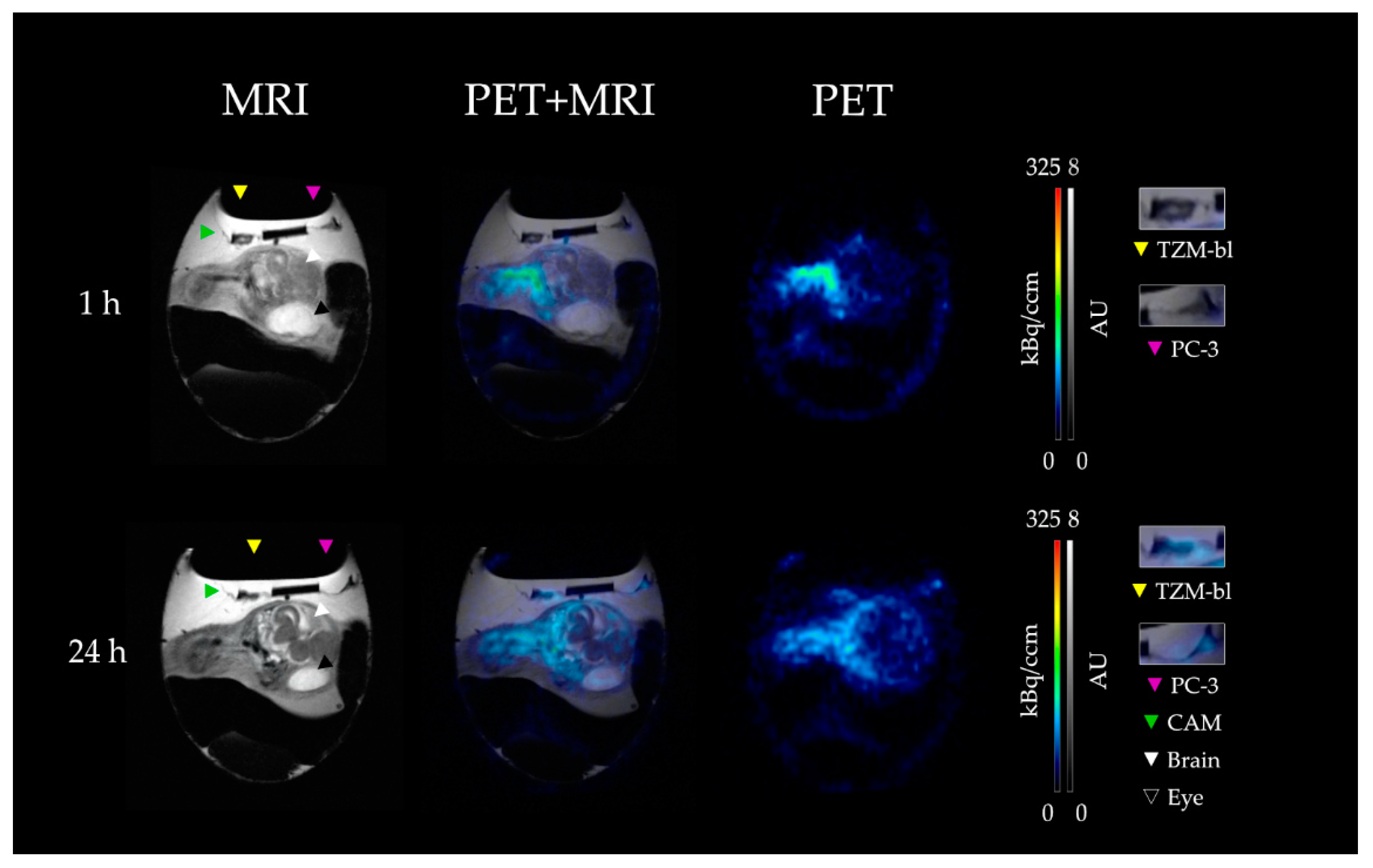
3.2. PET-MRI Analysis
3.3. Analysis of Tumor-to-Blood Ratios over 24 h
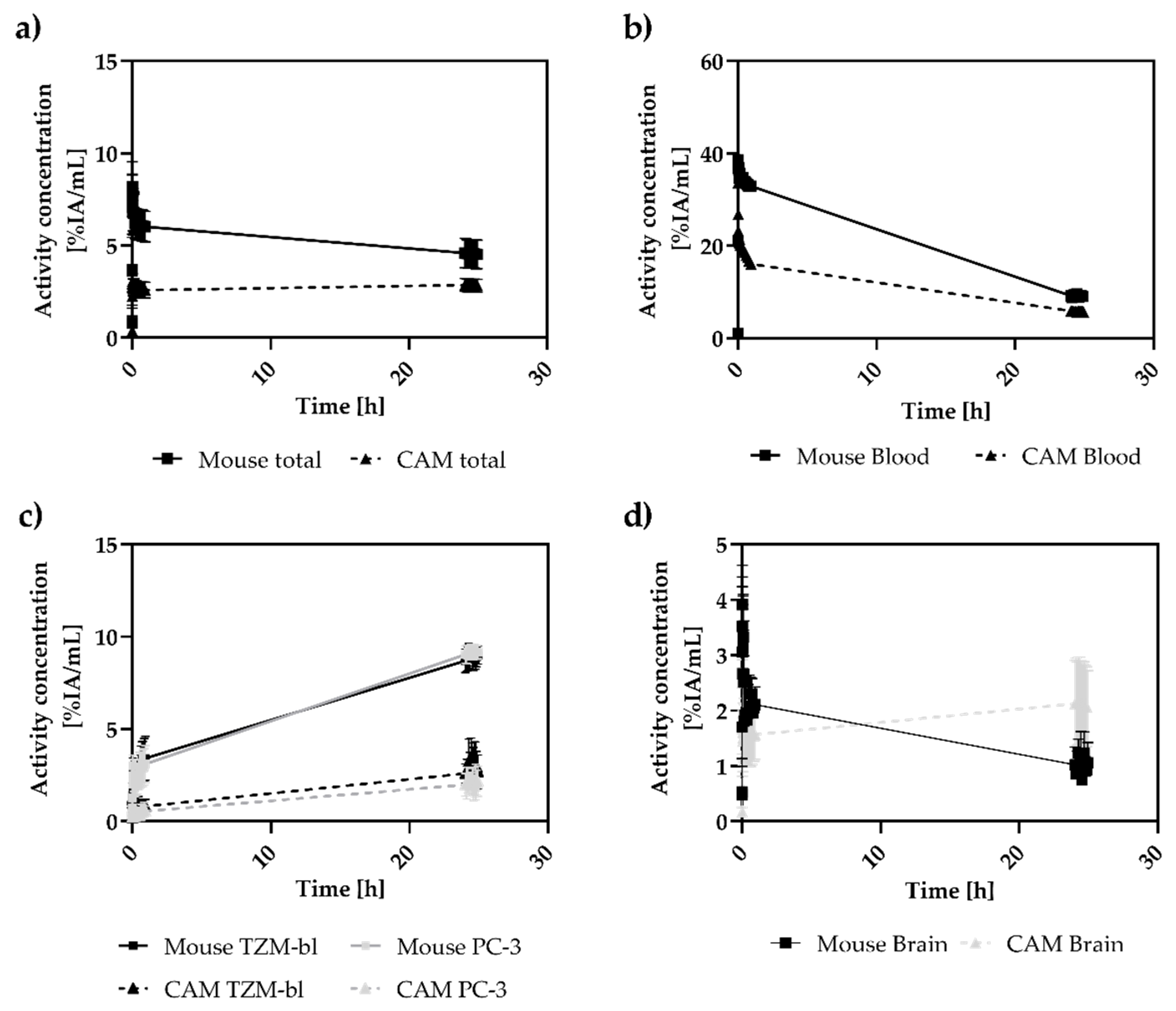
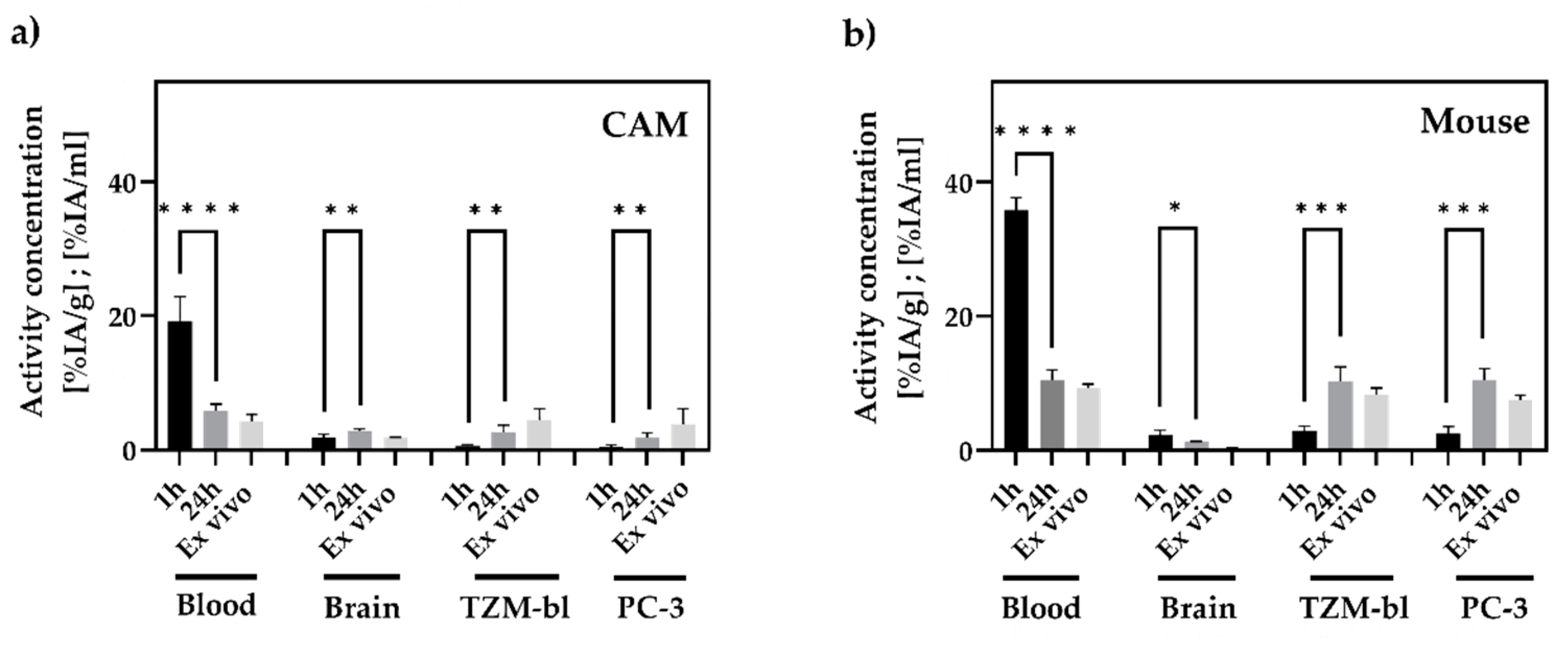
3.4. In Vivo and Ex Vivo Biodistribution Analysis
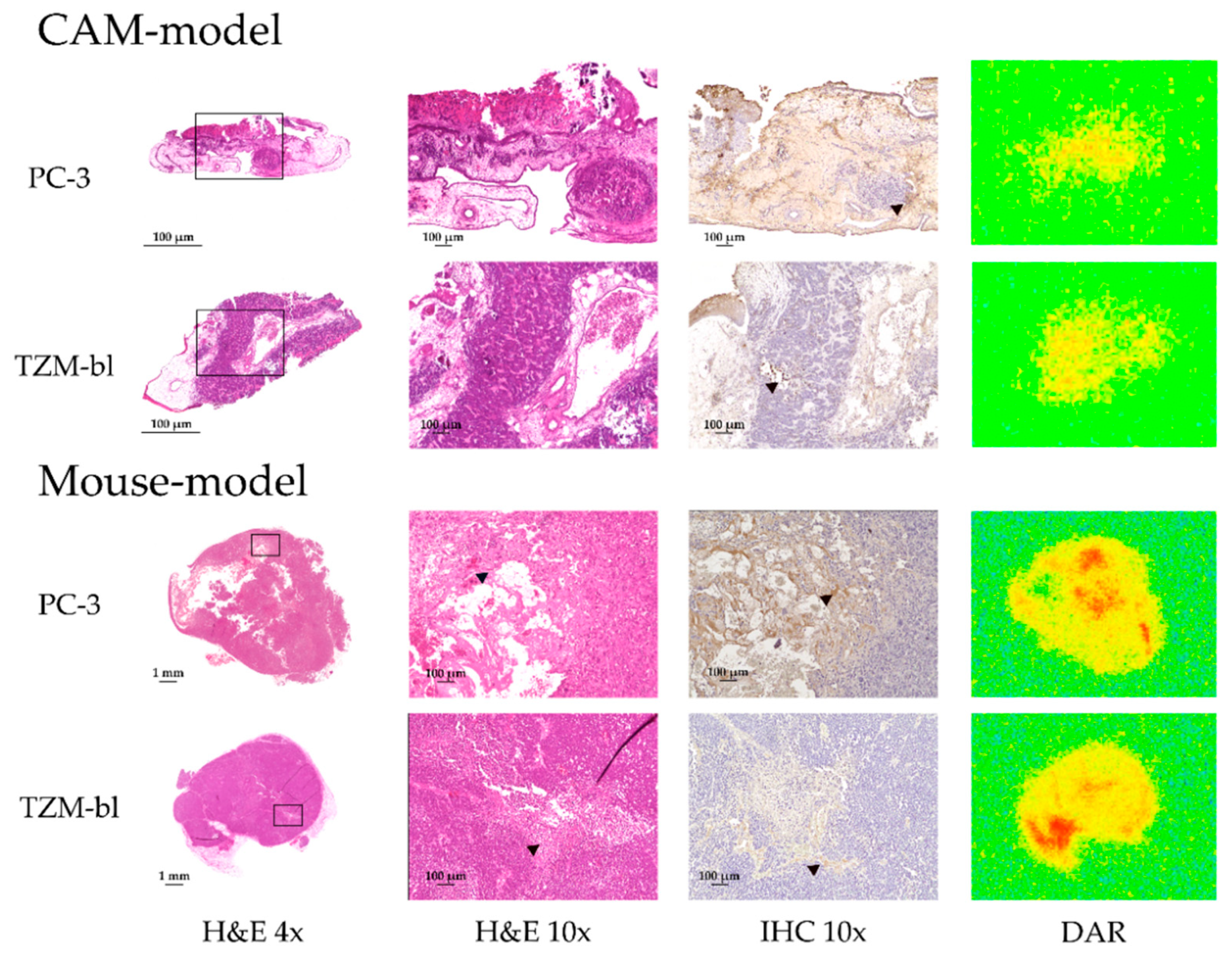
3.5. Immunohistochemical Analysis, H&E Staining and Digital Autoradiography
4. Discussion
4.1. Radiolabeling and In Vitro Studies
4.2. In Vivo Biodistribution in CAM and Mouse Model
4.3. In Vivo Tumor Accumulation in CAM and Mouse Model
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurunnabi, M.; Khatun, Z.; Moon, W.C.; Lee, G.; Lee, Y.K. Heparin based nanoparticles for cancer targeting and noninvasive imaging. Quant. Imaging Med. Surg. 2012, 2, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.; Wilby, D.; Kelber, A. Quantitative studies of animal colour constancy: Using the chicken as model. Proc. Biol. Sci. 2016, 283, 20160411. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co Ltd.: London, UK, 1959; p. 238. [Google Scholar]
- Kroeger, M. How omics technologies can contribute to the ‘3R’ principles by introducing new strategies in animal testing. Trends Biotechnol. 2006, 24, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Koch, A.B.F.; Löffler, J.; Linden, M.; Solbach, C.; Abaei, A.; Li, H.; Glatting, G.; Beer, A.J.; Rasche, V. Multi-Modal PET and MR Imaging in the Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) Model for Initial in Vivo Testing of Target-Specific Radioligands. Cancers 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Löffler, J.; Hamp, C.; Scheidhauer, E.; Di Carlo, D.; Solbach, C.; Abaei, A.; Hao, L.; Glatting, G.; Beer, A.J.; Rasche, V.; et al. Comparison of Quantification of Target-Specific Accumulation of [18F]F-siPSMA-14 in the HET-CAM Model and in Mice Using PET/MRI. Cancers 2021, 13, 4007. [Google Scholar] [CrossRef]
- Löffler, J.; Herrmann, H.; Scheidhauer, E.; Wirth, M.; Wasserloos, A.; Solbach, C.; Glatting, G.; Beer, A.J.; Rasche, V.; Winter, G. Blocking Studies to Evaluate Receptor-Specific Radioligand Binding in the CAM Model by PET and MR Imaging. Cancers 2022, 14, 3870. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Heneweer, C.; Holland, J.P.; Divilov, V.; Carlin, S.; Lewis, J.S. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J. Nucl. Med. 2011, 52, 625–633. [Google Scholar] [CrossRef]
- Ding, D.; Yang, C.; Lv, C.; Li, J.; Tan, W. Improving Tumor Accumulation of Aptamers by Prolonged Blood Circulation. Anal. Chem. 2020, 92, 4108–4114. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Hinrichsen, P.F. Decay of 78.4 H 89Zr. Nucl. Phys. A 1968, A118, 538–544. [Google Scholar] [CrossRef]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Liu, Y.; Xie, S.; Wang, Y.; Luo, K.; Wang, Y.; Cai, Y. Liquiritigenin inhibits tumor growth and vascularization in a mouse model of HeLa cells. Molecules 2012, 17, 7206–7216. [Google Scholar] [CrossRef]
- Xiao, D.; Lew, K.L.; Kim, Y.A.; Zeng, Y.; Hahm, E.R.; Dhir, R.; Singh, S.V. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin. Cancer Res. 2006, 12, 6836–6843. [Google Scholar] [CrossRef]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human prostate cancer PC-3 xenografts in nude mice: Evaluation of tumor growth and immunohistochemistry. Vivo 2005, 19, 179–183. [Google Scholar]
- Arai, T.; Okamoto, K.; Ishiguro, K.; Terao, K. HeLa cell-tumor in nude mice and its response to antitumor agents. GANN Jpn. J. Cancer Res. 1976, 67, 493–503. [Google Scholar]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- Tomaszewski, M.R.; Gonzalez, I.Q.; O’Connor, J.P.; Abeyakoon, O.; Parker, G.J.; Williams, K.J.; Gilbert, F.J.; Bohndiek, S.E. Oxygen enhanced optoacoustic tomography (OE-OT) reveals vascular dynamics in murine models of prostate cancer. Theranostics 2017, 7, 2900–2913. [Google Scholar] [CrossRef]
- Kramer, L.; Winter, G.; Baur, B.; Kuntz, A.J.; Kull, T.; Solbach, C.; Beer, A.J.; Lindén, M. Quantitative and correlative biodistribution analysis of 89Zr-labeled mesoporous silica nanoparticles intravenously injected into tumor-bearing mice. Nanoscale 2017, 9, 9743–9753. [Google Scholar] [CrossRef]
- Bain, M.M.; Fagan, A.J.; Mullin, J.M.; McNaught, I.; McLean, J.; Condon, B. Noninvasive monitoring of chick development in ovo using a 7T MRI system from day 12 of incubation through to hatching. J. Magn. Reson. Imaging 2007, 26, 198–201. [Google Scholar] [CrossRef]
- Zuo, Z.; Syrovets, T.; Wu, Y.; Hafner, S.; Vernikouskaya, I.; Liu, W.; Ma, G.; Weil, T.; Simmet, T.; Rasche, V. The CAM cancer xenograft as a model for initial evaluation of MR labelled compounds. Sci. Rep. 2017, 7, 46690. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Syrovets, T.; Genze, F.; Abaei, A.; Ma, G.; Simmet, T.; Rasche, V. High-resolution MRI analysis of breast cancer xenograft on the chick chorioallantoic membrane. NMR Biomed. 2015, 28, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.S.; Im, K.C.; Kim, S.J.; Kim, S.Y.; Lee, D.S.; Moon, D.H. Performance measurement of the microPET focus 120 scanner. J. Nucl. Med. 2007, 48, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, N.; Shioda, S.; Niehoff, M.L.; Banks, W.A. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J. Pharmacol. Exp. Ther. 2003, 306, 948–953. [Google Scholar] [CrossRef]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef]
- Miller, L.; Winter, G.; Baur, B.; Witulla, B.; Solbach, C.; Reske, S.; Lindén, M. Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale 2014, 6, 4928–4935. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Casulleras, M.; Flores-Costa, R.; Duran-Guell, M.; Alcaraz-Quiles, J.; Sanz, S.; Titos, E.; Lopez-Vicario, C.; Fernandez, J.; Horrillo, R.; Costa, M.; et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 2020, 12, eaax5135. [Google Scholar] [CrossRef]
- Fritzsche, T.; Schnolzer, M.; Fiedler, S.; Weigand, M.; Wiessler, M.; Frei, E. Isolation and identification of heterogeneous nuclear ribonucleoproteins (hnRNP) from purified plasma membranes of human tumour cell lines as albumin-binding proteins. Biochem. Pharmacol. 2004, 67, 655–665. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.L. The Avian Embryo. Structural and Functional Development; Macmillan: New York, NY, USA, 1960; p. 1305. [Google Scholar]
- Freeman, B.M.; Vince, M.A. Development of the Avian Embryo: A Behavioural and Physiological Study; Chapman and Hall: London, UK, 1974; p. 362. [Google Scholar]
- Yoshizaki, N.; Ito, Y.; Hori, H.; Saito, H.; Iwasawa, A. Absorption, transportation and digestion of egg white in quail embryos. Dev. Growth Differ. 2002, 44, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Labas, V.; Nys, Y.; Réhault-Godbert, S. Investigating proteins and proteases composing amniotic and allantoic fluids during chicken embryonic development. Poult. Sci. 2017, 96, 2931–2941. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Low, B.E.; Christianson, G.J.; Proetzel, G.; Sproule, T.J.; Wiles, M.V. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs 2015, 7, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Bertossi, M. The development of the blood-brain barrier in the chick. Studies with evans blue and horseradish peroxidase. Ann. Anat. 1993, 175, 85–88. [Google Scholar] [CrossRef]
- Meechai, T.; Tepmongkol, S.; Pluempitiwiriyawej, C. Partial-volume effect correction in positron emission tomography brain scan image using super-resolution image reconstruction. Br. J. Radiol. 2015, 88, 20140119. [Google Scholar] [CrossRef]
- Soret, M.; Bacharach, S.L.; Buvat, I. Partial-Volume Effect in PET Tumor Imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef]
- Rousset, O.G.; Ma, Y.; Evans, A.C. Correction for partial volume effects in PET: Principle and validation. J. Nucl. Med. 1998, 39, 904–911. [Google Scholar]
- Khalil, A.A.; Horsman, M.R.; Overgaard, J. The importance of determining necrotic fraction when studying the effect of tumour volume on tissue oxygenation. Acta Oncol. 1995, 34, 297–300. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Chiche, J.; Pouyssegur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilbrig, C.; Löffler, J.; Fischer, G.; Scheidhauer, E.; Solbach, C.; Huber-Lang, M.; Beer, A.J.; Rasche, V.; Winter, G. Evaluation of the EPR Effect in the CAM-Model by Molecular Imaging with MRI and PET Using 89Zr-Labeled HSA. Cancers 2023, 15, 1126. https://doi.org/10.3390/cancers15041126
Hilbrig C, Löffler J, Fischer G, Scheidhauer E, Solbach C, Huber-Lang M, Beer AJ, Rasche V, Winter G. Evaluation of the EPR Effect in the CAM-Model by Molecular Imaging with MRI and PET Using 89Zr-Labeled HSA. Cancers. 2023; 15(4):1126. https://doi.org/10.3390/cancers15041126
Chicago/Turabian StyleHilbrig, Colmar, Jessica Löffler, Gabriel Fischer, Ellen Scheidhauer, Christoph Solbach, Markus Huber-Lang, Ambros J. Beer, Volker Rasche, and Gordon Winter. 2023. "Evaluation of the EPR Effect in the CAM-Model by Molecular Imaging with MRI and PET Using 89Zr-Labeled HSA" Cancers 15, no. 4: 1126. https://doi.org/10.3390/cancers15041126
APA StyleHilbrig, C., Löffler, J., Fischer, G., Scheidhauer, E., Solbach, C., Huber-Lang, M., Beer, A. J., Rasche, V., & Winter, G. (2023). Evaluation of the EPR Effect in the CAM-Model by Molecular Imaging with MRI and PET Using 89Zr-Labeled HSA. Cancers, 15(4), 1126. https://doi.org/10.3390/cancers15041126






