10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Electronic Searches and Selection Criteria
2.2. Aims
2.3. Data Extraction
2.4. Risk of Bias and Quality Assessment
2.5. Data Synthesis and Statistical Analysis
3. Results
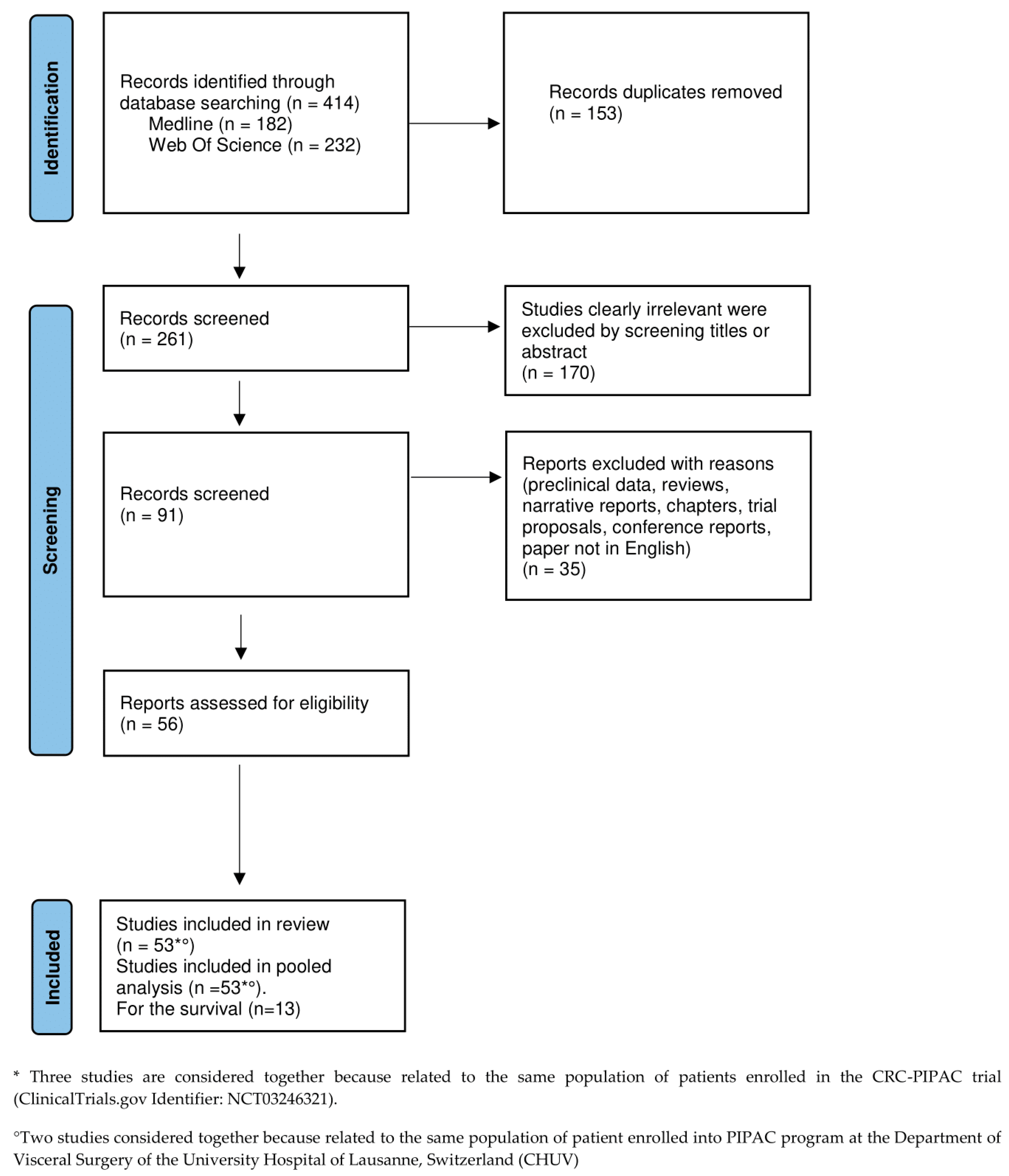
3.1. Study Selection
3.2. Quality Assessment
3.3. Study Characteristics
3.4. Patient Characteristics
3.5. Feasibility
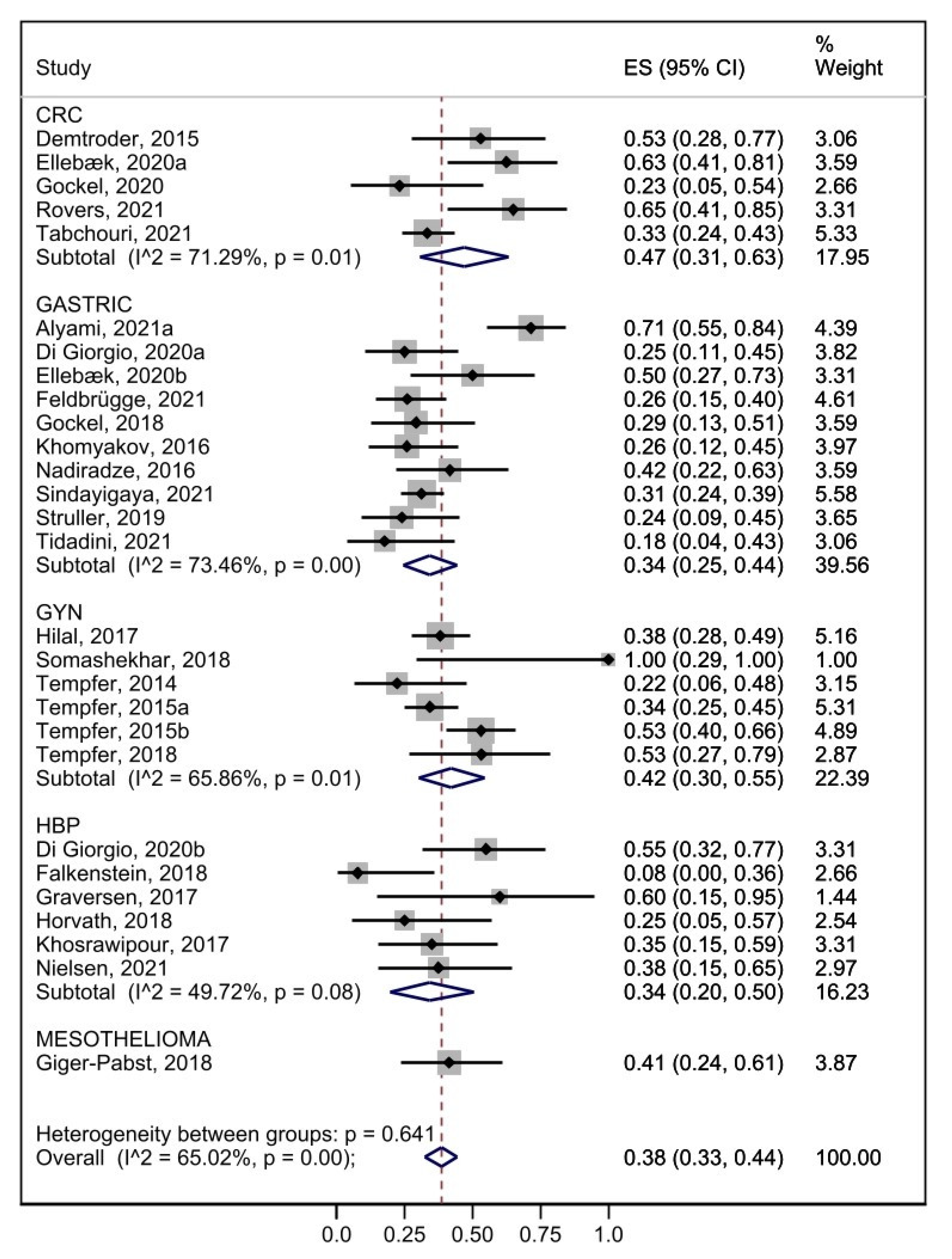
3.6. Safety
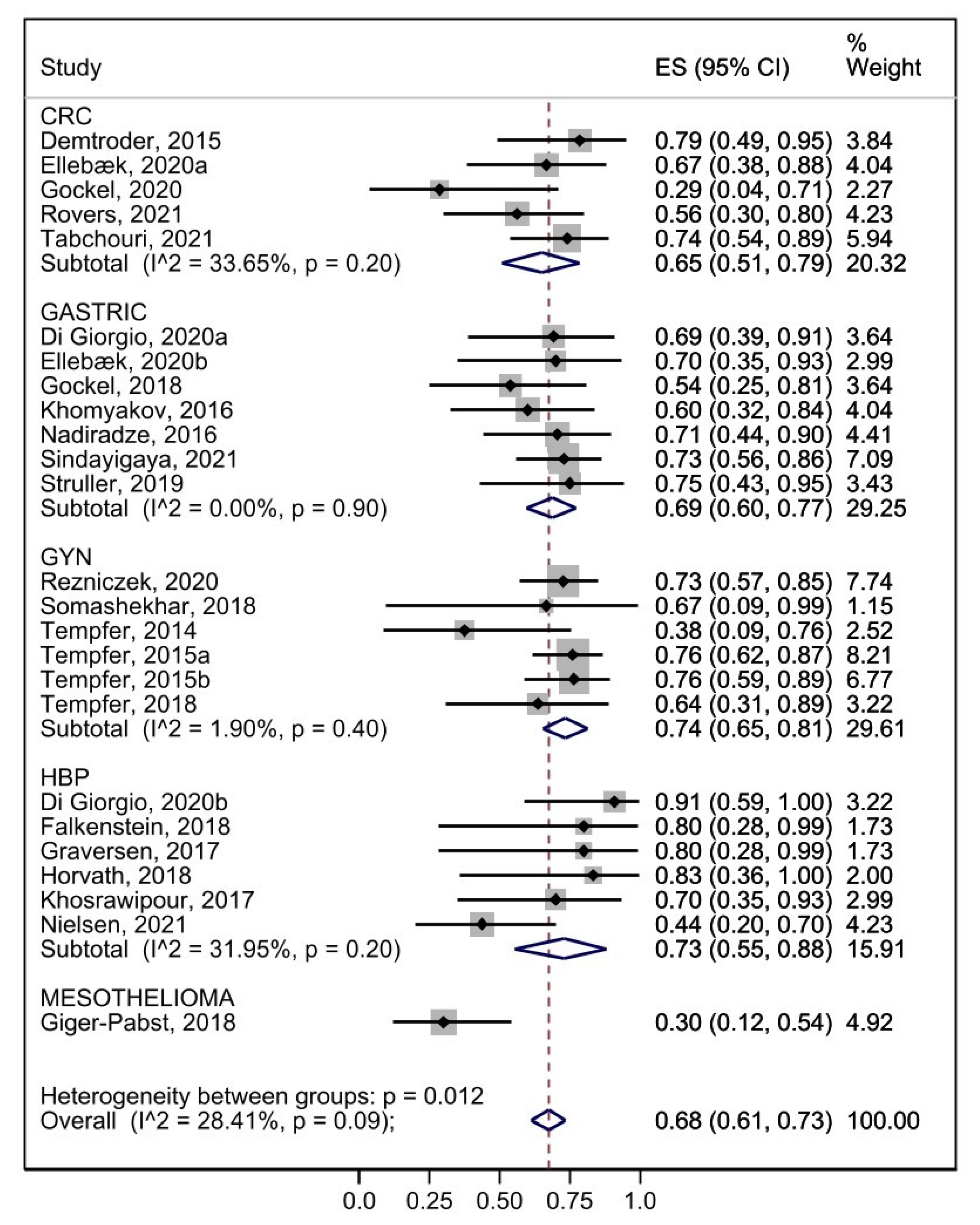
3.7. Efficacy
3.8. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised Intraperitoneal Aerosol Chemotherapy: Rationale, Evidence, and Potential Indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Higgins, J.; James, T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Available online: https://training.cochrane.org/handbook/current (accessed on 5 November 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 5 November 2022).
- Ferracci, F.; Di Giorgio, A.; Robella, M.; Macrì, A. 10 Years of Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastases: A Systematic Review and Meta- Analysis. PROSPERO 2022 CRD42022320389. PROSPERO Int. Prospect. Regist. Syst. Rev. 2022, 1–4. [Google Scholar]
- 2013 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Baltimore, Maryland, USA, 17–20 April 2013 Poster Presentation. Surg. Endosc. 2013, 27, 471. [CrossRef]
- Kleinbaum, D.G.; Klein, M. Survival Analysis: A Self-Learning Text (Statistics for Biology and Health), 2nd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Alyami, M.; Bonnot, P.E.; Mercier, F.; Laplace, N.; Villeneuve, L.; Passot, G.; Bakrin, N.; Kepenekian, V.; Glehen, O. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Unresectable Peritoneal Metastasis from Gastric Cancer. Eur. J. Surg. Oncol. 2021, 47, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Gagniere, J.; Sgarbura, O.; Cabelguenne, D.; Villeneuve, L.; Pezet, D.; Quenet, F.; Glehen, O.; Bakrin, N.; Passot, G. Multicentric Initial Experience with the Use of the Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in the Management of Unresectable Peritoneal Carcinomatosis. Eur. J. Surg. Oncol. 2017, 43, 2178–2183. [Google Scholar] [CrossRef]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) of Peritoneal Metastasis from Gastric Cancer: A Descriptive Cohort Study. Clin. Exp. Metastasis 2020, 37, 325–332. [Google Scholar] [CrossRef]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC)-Directed Treatment of Peritoneal Metastasis in End-Stage Colo-Rectal Cancer Patients. Pleura Peritoneum 2020, 5, 20200109. [Google Scholar] [CrossRef]
- Falkenstein, T.A.; Götze, T.O.; Ouaissi, M.; Tempfer, C.B.; Giger-Pabst, U.; Demtröder, C. First Clinical Data of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) as Salvage Therapy for Peritoneal Metastatic Biliary Tract Cancer. Anticancer. Res. 2018, 38, 373–378. [Google Scholar] [CrossRef]
- Feldbrügge, L.; Gronau, F.; Brandl, A.; Auer, T.A.; Oeff, A.; Thuss-Patience, P.; Pratschke, J.; Rau, B. Systemic Chemotherapy Including Ramucirumab in Combination with Pressurized Intra-Peritoneal Aerosol Chemotherapy Is a Safe Treatment Option for Peritoneal Metastasis of Gastric Cancer. Front. Oncol. 2021, 10, 610572. [Google Scholar] [CrossRef]
- Giger-Pabst, U.; Demtröder, C.; Falkenstein, T.A.; Ouaissi, M.; Götze, T.O.; Rezniczek, G.A.; Tempfer, C.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for the Treatment of Malignant Mesothelioma. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Girshally, R.; Demtröder, C.; Albayrak, N.; Zieren, J.; Tempfer, C.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) as a Neoadjuvant Therapy before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. World J. Surg. Oncol. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Jansen-Winkeln, B.; Haase, L.; Niebisch, S.; Moulla, Y.; Lyros, O.; Lordick, F.; Schierle, K.; Wittekind, C.; Thieme, R. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in Patients with Peritoneal Metastasized Colorectal, Appendiceal and Small Bowel Cancer. Tumori 2020, 106, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gockel, I.; Jansen-Winkeln, B.; Haase, L.; Rhode, P.; Mehdorn, M.; Niebisch, S.; Moulla, Y.; Lyros, O.; Lordick, F.; Schierle, K.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Gastric Cancer Patients with Peritoneal Metastasis (PM): Results of a Single-Center Experience and Register Study. J. Gastric Cancer 2018, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Bjerregaard, J.K.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Prospective, Single-Center Implementation and Response Evaluation of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis. Ther. Adv. Med. Oncol. 2018, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Bjerregaard, J.K.; Pfeiffer, P.; Mortensen, M.B. Peritoneal Metastasis from Pancreatic Cancer Treated with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Clin. Exp. Metastasis 2017, 34, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable Peritoneal Metastasis Treated by Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Leading to Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Ellebaek, S.B.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy with One Minute of Electrostatic Precipitation (EPIPAC) Is Feasible, but the Histological Tumor Response in Peritoneal Metastasis Is Insufficient. Eur. J. Surg. Oncol. 2019, 46, 155–159. [Google Scholar] [CrossRef]
- Graversen, M.; Lundell, L.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an Outpatient Procedure. Pleura Peritoneum 2018, 3, 20180128. [Google Scholar] [CrossRef]
- Hilal, Z.; Rezniczek, G.A.; Klenke, R.; Dogan, A.; Tempfer, C.B. Nutritional Status, Cachexia, and Anorexia in Women with Peritoneal Metastasis and Intraperitoneal Chemotherapy: A Longitudinal Analysis. J. Gynecol. Oncol. 2017, 28, e80. [Google Scholar] [CrossRef]
- Horvath, P.; Beckert, S.; Struller, F.; Königsrainer, A.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastases of Pancreas and Biliary Tract Cancer. Clin. Exp. Metastasis 2018, 35, 635–640. [Google Scholar] [CrossRef]
- Hübner, M.; Teixeira Farinha, H.; Grass, F.; Wolfer, A.; Mathevet, P.; Hahnloser, D.; Demartines, N. Feasibility and Safety of Pressurized Intraperitoneal Aerosol Chemotherapy for Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 6852749. [Google Scholar] [CrossRef]
- Katdare, N.; Prabhu, R.; Mishra, S.; Mehta, S.; Bhatt, A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Initial Experience from Indian Centers and a Review of Literature. Indian J. Surg. Oncol. 2019, 10, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Khomyakov, V.; Ryabov, A.; Ivanov, A.; Bolotina, L.; Utkina, A.; Volchenko, N.; Kaprin, A. Bidirectional Chemotherapy in Gastric Cancer with Peritoneal Metastasis Combining Intravenous XELOX with Intraperitoneal Chemotherapy with Low-Dose Cisplatin and Doxorubicin Administered as a Pressurized Aerosol: An Open-Label, Phase-2 Study (PIPAC-GA2). Pleura Peritoneum 2016, 1, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, T.; Khosrawipour, V.; Giger-Pabst, U. Pressurized Intra Peritoneal Aerosol Chemotherapy in Patients Suffering from Peritoneal Carcinomatosis of Pancreatic Adenocarcinoma. PLoS ONE 2017, 12, e0186709. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Tan, H.L.; Sundar, R.; Lieske, B.; Chee, C.E.; Ho, J.; Shabbir, A.; Babak, M.V.; Ang, W.H.; Goh, B.C.; et al. PIPAC-OX: A Phase I Study of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy in Patients with Peritoneal Metastases. Clin. Cancer Res. 2021, 27, 1875–1881. [Google Scholar] [CrossRef]
- Kurtz, F.; Struller, F.; Horvath, P.; Solass, W.; Bösmüller, H.; Königsrainer, A.; Reymond, M.A. Feasibility, Safety, and Efficacy of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Metastasis: A Registry Study. Gastroenterol. Res. Pract. 2018, 2018, 2743985. [Google Scholar] [CrossRef]
- Ceribelli, C.; Debs, T.; Chevallier, A.; Piche, M.A.; Bereder, J.M. Initial Experience of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in a French Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Expert Center. Surg. Endosc. 2020, 34, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Nielsen, M.; Graversen, M.; Ellebæk, S.B.; Kristensen, T.K.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B.; Detlefsen, S. Next-Generation Sequencing and Histological Response Assessment in Peritoneal Metastasis from Pancreatic Cancer Treated with PIPAC. J. Clin. Pathol. 2021, 74, 19–24. [Google Scholar] [CrossRef]
- Odendahl, K.; Solass, W.; Demtröder, C.; Giger-Pabst, U.; Zieren, J.; Tempfer, C.; Reymond, M.A. Quality of Life of Patients with End-Stage Peritoneal Metastasis Treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur. J. Surg. Oncol. 2015, 41, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Račkauskas, R.; Baušys, A.; Lukšta, M.; Jurgaitis, J.; Paškonis, M.; Strupas, K. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Malignancy: Initial Experience of the First Program in the Baltic Countries. World J. Surg. Oncol. 2021, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Giger-Pabst, U.; Thaher, O.; Tempfer, C.B. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Rare Gynecologic Indications: Peritoneal Metastases from Breast and Endometrial Cancer. BMC Cancer 2020, 20, 1122. [Google Scholar] [CrossRef] [PubMed]
- Robella, M.; De Simone, M.; Berchialla, P.; Argenziano, M.; Borsano, A.; Ansari, S.; Abollino, O.; Ficiarà, E.; Cinquegrana, A.; Cavalli, R.; et al. A Phase I Dose Escalation Study of Oxaliplatin, Cisplatin and Doxorubicin Applied as PIPAC in Patients with Peritoneal Carcinomatosis. Cancers 2021, 13, 1060. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; De Simone, M. Safety and Feasibility of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Associated with Systemic Chemotherapy: An Innovative Approach to Treat Peritoneal Carcinomatosis. World J. Surg. Oncol. 2016, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Wassenaar, E.C.E.; Lurvink, R.J.; Creemers, G.J.M.; Burger, J.W.A.; Los, M.; Huysentruyt, C.J.R.; van Lijnschoten, G.; Nederend, J.; Lahaye, M.J.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (Oxaliplatin) for Unresectable Colorectal Peritoneal Metastases: A Multicenter, Single-Arm, Phase II Trial (CRC-PIPAC). Ann. Surg. Oncol. 2021, 28, 5311–5326. [Google Scholar] [CrossRef]
- Sgarbura, O.; Hübner, M.; Alyami, M.; Eveno, C.; Gagnière, J.; Pache, B.; Pocard, M.; Bakrin, N.; Quénet, F. Oxaliplatin Use in Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Is Safe and Effective: A Multicenter Study. Eur. J. Surg. Oncol. 2019, 45, 2386–2391. [Google Scholar] [CrossRef]
- Siebert, M.; Alyami, M.; Mercier, F.; Gallice, C.; Villeneuve, L.; Laplace, N.; Passot, G.; Bakrin, N.; Glehen, O.; Kepenekian, V. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Association with Systemic Chemotherapy and Bevacizumab, Evaluation of Safety and Feasibility. A Single Center Comparative Study. Eur. J. Surg. Oncol. 2021, 47, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado Ayuso, M.; Cabañas Montero, J.; Priego Jiménez, P.; Corral Moreno, S.; Longo Muñoz, F.; Pachón Olmos, V.; Fernández Cebrián, J.M.; Galindo Álvarez, J. Initial Single-Center Experience of PIPAC in Patients with Unresectable Peritoneal Metastasis. Cirugía Española Engl. Ed. 2021, 99, 354–360. [Google Scholar] [CrossRef]
- Sindayigaya, R.; Dogan, C.; Demtröder, C.R.; Fischer, B.; Karam, E.; Buggisch, J.R.; Tempfer, C.B.; Lecomte, T.; Ouaissi, M.; Giger-Pabst, U. Clinical Outcome for Patients Managed with Low-Dose Cisplatin and Doxorubicin Delivered as Pressurized Intraperitoneal Aerosol Chemotherapy for Unresectable Peritoneal Metastases of Gastric Cancer. Ann. Surg. Oncol. 2021, 29, 112–123. [Google Scholar] [CrossRef]
- Solass, W.; Kerb, R.; Mürdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal Chemotherapy of Peritoneal Carcinomatosis Using Pressurized Aerosol as an Alternative to Liquid Solution: First Evidence for Efficacy. Ann. Surg. Oncol. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Ashwin, K.R.; Kumar, C.R.; Rauthan, A.; Rakshit, S.H. Pressurized Intraperitoneal Aerosol Chemotherapy Procedure for Nonresectable Peritoneal Carcinomatosis: First Indian Study. South Asian J. Cancer 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Somashekhar, S.P.; Rajagopal, A.K.; Zaveri, S.S.; Chandrashekhar, R.K.; Rauthan, A.; Rakshit, S.H. First Indian Study on Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Procedure for Advanced Peritoneal Carcinomatosis Secondary to Epithelial Ovarian Cancer. Indian J. Gynecol. Oncol. 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Königsrainer, A.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy with Low-Dose Cisplatin and Doxorubicin (PIPAC C/D) in Patients with Gastric Cancer and Peritoneal Metastasis: A Phase II Study. Ther. Adv. Med. Oncol. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Tabchouri, N.; Buggisch, J.; Demtröder, C.R.; Thiery, J.; Rezniczek, G.; Tempfer, C.B.; Fischer, B.; Dogan, C.; Lecomte, T.; Ouaissi, M.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy for Colorectal Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 5275–5286. [Google Scholar] [CrossRef] [PubMed]
- Taibi, A.; Teixeira Farinha, H.; Durand Fontanier, S.; Sayedalamin, Z.; Hübner, M.; Sgarbura, O. Pressurized Intraperitoneal Aerosol Chemotherapy Enhanced by Electrostatic Precipitation (EPIPAC) for Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3852–3860. [Google Scholar] [CrossRef]
- Tempfer, C.; Rezniczek, G.; Tsitas, M.; Ende, P.; Solass, W.; Demtroeder, C.; Reymond, M. Pressurized Intraperitoneal Aerosol Chemotherapy with Cisplatin and Doxorubicin in Women with Peritoneal Carcinomatosis: A Cohort Study. Anticancer Res. 2015, 35, 6723–6729. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Celik, I.; Solass, W.; Buerkle, B.; Pabst, U.G.; Zieren, J.; Strumberg, D.; Reymond, M.A. Activity of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Cisplatin and Doxorubicin in Women with Recurrent, Platinum-Resistant Ovarian Cancer: Preliminary Clinical Experience. Gynecol. Oncol. 2014, 132, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.B.; Giger-Pabst, U.; Seebacher, V.; Petersen, M.; Dogan, A.; Rezniczek, G.A. A Phase I, Single-Arm, Open-Label, Dose Escalation Study of Intraperitoneal Cisplatin and Doxorubicin in Patients with Recurrent Ovarian Cancer and Peritoneal Carcinomatosis. Gynecol. Oncol. 2018, 150, 23–30. [Google Scholar] [CrossRef]
- De Simone, M.; Vaira, M.; Argenziano, M.; Berchialla, P.; Pisacane, A.; Cinquegrana, A.; Cavalli, R.; Borsano, A.; Robella, M. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Oxaliplatin, Cisplatin, and Doxorubicin in Patients with Peritoneal Carcinomatosis: An Open-Label, Single-Arm, Phase II Clinical Trial. Biomedicines 2020, 8, 102. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Winnekendonk, G.; Solass, W.; Horvat, R.; Giger-Pabst, U.; Zieren, J.; Rezniczek, G.A.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy in Women with Recurrent Ovarian Cancer: A Phase 2 Study. Gynecol. Oncol. 2015, 137, 223–228. [Google Scholar] [CrossRef]
- Tidadini, F.; Abba, J.; Quesada, J.-L.; Baudrant, M.; Bonne, A.; Foote, A.; Faucheron, J.-L.; Glehen, O.; Villeneuve, L.; Arvieux, C. Effect of Pressurized Intraperitoneal Aerosol Chemotherapy on the Survival Rate of Patients with Peritoneal Carcinomatosis of Gastric Origin. J. Gastrointest. Cancer 2021, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Willaert, W.; Van de Sande, L.; Van Daele, E.; Van De Putte, D.; Van Nieuwenhove, Y.; Pattyn, P.; Ceelen, W. Safety and Preliminary Efficacy of Electrostatic Precipitation during Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Unresectable Carcinomatosis. Eur. J. Surg. Oncol. 2019, 45, 2302–2309. [Google Scholar] [CrossRef]
- Demtröder, C.; Solass, W.; Zieren, J.; Strumberg, D.; Giger-Pabst, U.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy with Oxaliplatin in Colorectal Peritoneal Metastasis. Color. Dis. 2015, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Schena, C.A.; El Halabieh, M.A.; Abatini, C.; Vita, E.; Strippoli, A.; Inzani, F.; Rodolfino, E.; Romanò, B.; Pacelli, F.; et al. Systemic Chemotherapy and Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Bidirectional Approach for Gastric Cancer Peritoneal Metastasis. Surg. Oncol. 2020, 34, 270–275. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Sgarbura, O.; Rotolo, S.; Schena, C.A.; Bagalà, C.; Inzani, F.; Russo, A.; Chiantera, V.; Pacelli, F. Pressurized Intraperitoneal Aerosol Chemotherapy with Cisplatin and Doxorubicin or Oxaliplatin for Peritoneal Metastasis from Pancreatic Adenocarcinoma and Cholangiocarcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Dumont, F.; Passot, C.; Raoul, J.L.; Kepenekian, V.; Lelièvre, B.; Boisdron-Celle, M.; Hiret, S.; Senellart, H.; Pein, F.; Blanc-Lapierre, A.; et al. A Phase I Dose-Escalation Study of Oxaliplatin Delivered via a Laparoscopic Approach Using Pressurised Intraperitoneal Aerosol Chemotherapy for Advanced Peritoneal Metastases of Gastrointestinal Tract Cancers. Eur. J. Cancer 2020, 140, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lurvink, R.J.; Tajzai, R.; Rovers, K.P.; Wassenaar, E.C.E.; Moes, D.J.A.R.; Pluimakers, G.; Boerma, D.; Burger, J.W.A.; Nienhuijs, S.W.; de Hingh, I.H.J.T.; et al. Systemic Pharmacokinetics of Oxaliplatin After Intraperitoneal Administration by Electrostatic Pressurized Intraperitoneal Aerosol Chemotherapy (EPIPAC) in Patients with Unresectable Colorectal Peritoneal Metastases in the CRC-PIPAC Trial. Ann. Surg. Oncol. 2020, 28, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lurvink, R.J.; Rovers, K.P.; Wassenaar, E.C.E.; Bakkers, C.; Burger, J.W.A.; Creemers, G.J.M.; Los, M.; Mols, F.; Wiezer, M.J.; Nienhuijs, S.W.; et al. Patient-Reported Outcomes during Repetitive Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy for Isolated Unresectable Colorectal Peritoneal Metastases in a Multicenter, Single-Arm, Phase 2 Trial (CRC-PIPAC). Surg. Endosc. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Teixeira Farinha, H.; Grass, F.; Kefleyesus, A.; Achtari, C.; Romain, B.; Montemurro, M.; Demartines, N.; Hübner, M. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017, 2017, 4596176. [Google Scholar] [CrossRef]
- Ceelen, W.; Sandra, L.; Van de Sande, L.; Graversen, M.; Mortensen, M.B.; Vermeulen, A.; Gasthuys, E.; Reynders, D.; Cosyns, S.; Hoorens, A.; et al. Phase I Study of Intraperitoneal Aerosolized Nanoparticle Albumin Based Paclitaxel (NAB-PTX) for Unresectable Peritoneal Metastases. EBioMedicine 2022, 82, 104151. [Google Scholar] [CrossRef]
- Grass, F.; Vuagniaux, A.; Teixeira-Farinha, H.; Lehmann, K.; Demartines, N.; Hübner, M. Systematic Review of Pressurized Intraperitoneal Aerosol Chemotherapy for the Treatment of Advanced Peritoneal Carcinomatosis. Br. J. Surg. 2017, 104, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Graversen, M.; Pfeiffer, P.; Mortensen, M.B. Bidirectional Treatment of Peritoneal Metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and Systemic Chemotherapy: A Systematic Review. BMC Cancer 2020, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Sgarbura, O.; Eveno, C.; Alyami, M.; Bakrin, N.; Cortes Guiral, D.; Ceelen, W.; Delgadillo, X.; Dellinger, T.; Di Giorgio, A.; Kefleyesus, A.; et al. Consensus Statement for Treatment Protocols in Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Peritoneum 2022, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- James, E.C.; Popoola, B.; Schiavone, F.; Badrock, J.; Fananapazir, F.; Cook, A.D.; Parmar, M.; Kaplan, R.; Ka, L.; Clamp, A.R.; et al. Weekly Dose-Dense Chemotherapy in First-Line Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer Treatment (ICON8): Overall Survival Results from an Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 919–930. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of Patients with Peritoneal Metastatic Colorectal Cancer given Systemic Therapy: An Analysis of Individual Patient Data from Prospective Randomised Trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) Database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined with Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Alyami, M.; Khomiakov, V.; Lintis, A.; Piso, P.; Eveno, C.; Glehen, O.; the ISSPP PIPAC cohort study group. ISSPP 2021 2nd Congress of the International Society for the Study of Pleura and Peritoneum. Pleura Peritoneum 2021, 6, eA1–eA77. [Google Scholar] [CrossRef]
- Balmer, A.; Clerc, D.; Toussaint, L.; Sgarbura, O.; Taïbi, A.; Hübner, M.; Farinha, H.T. Selection Criteria for Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Treatment in Patients with Peritoneal Metastases. Cancers 2022, 14, 2557. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Abatini, C.; Attalla El Halabieh, M.; Vita, E.; Vizzielli, G.; Gallotta, V.; Pacelli, F.; Rotolo, S. From Palliation to Cure: PIPAC for Peritoneal Malignancies. Minerva Med. 2019, 110, 385–398. [Google Scholar] [CrossRef] [PubMed]

| Medline | |||
|---|---|---|---|
| Set | Search Terms | Search Type | Results |
| #1 | peritoneal carcinosis OR peritoneal metastases OR peritoneal carcinomatosis | Advanced | 35,973 |
| #2 | pressurized intraperitoneal aerosol chemotherapy OR pipac | Advanced | 278 |
| #3 | #1 AND #2 | Advanced | 191 |
| #4 | #3 Filters: from 2011–2021 | Advanced | 182 |
| Web of Science | |||
| Set | Search Terms | Search Type | Results |
| #1 | TOPIC: peritoneal carcinosis OR peritoneal metastases OR peritoneal carcinomatosis | Advanced | 13,674 |
| #2 | TOPIC: pressurized intraperitoneal aerosol chemotherapy or pipac | Advanced | 292 |
| #3 | #1 AND #2 | Advanced | 235 |
| #4 | #3 Filters: from 2011–2021 | Advanced | 232 |
| Author | Year | Origin | Drugs | PIPAC Dose mg/m2 |
|---|---|---|---|---|
| Tempfer | 2015 | OC | Doxorubicin + Cisplatin | 2.1 + 10.5 |
| Dumont | 2020 | GC, CRC, SBC | Oxaliplatin | 90 |
| Kim | 2021 | GC, CRC, HBPC, AC | Oxaliplatin | 120 |
| Robella | 2021 | GC, OC, PMP | Doxorubicin + Cisplatin | 6 + 30 |
| CRC | Oxaliplatin | 135 | ||
| Celeen | 2022 * | GC, CRC, HBPC, AC, OC | Nabpaclitaxel | 112.5 |
| First Author, Year of Publication | Study Design | Primary Tumor | Sample Size | Number of PIPAC | ECOG at 1st PIPAC * | PCI at 1st PIPAC * | PCI Post * | Ascites at 1st PIPAC (%) | Previous Systemic Chemotherapy (%) | Concomitant Systemic Chemotherapy (%) | Non-Access (%) | ≥3 PIPAC (%) | CTCAE 3–4 (%) | CTCAE 5 (%) |
| Demtroder, 2015 | Retrospective | CRC | 17 | 48 | 1.00 | 16.0 | 18.0 | 94.1 | 64.7 | 0.0 | 52.9 | 8.3 | 0.0 | |
| Ellebæk, 2020a | Retrospective | CRC | 24 | 74 | 0.83 | 10.7 § | 29.0 | 91.7 | 12.5 | 62.5 | 2.7 | 0.0 | ||
| Gockel, 2020 | Prospective | CRC | 13 | 26 | 1.00 § | 14.0 § | 23.0 | 92.3 | 38.5 | 18.8 | 23.1 | 0.0 | 0.0 | |
| Rovers, 2021 | Phase II | CRC | 20 | 59 | 0.75 | 29.0 § | 60.0 | 0.0 | 6.5 | 65.0 | 22.0 | 5.0 | ||
| Tabchouri, 2021 | Prospective | CRC | 102 | 185 | 13.0 § | 19.0 § | 42.5 | 97.1 | 56.9 | 16.4 | 33.3 | 3.8 | 0.5 | |
| Subtotal, sum | 176 | 392 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 90 | 30 | 8 | 47 | 6 | ND | ||||
| Alyami, 2021a | Prospective | GASTRIC | 42 | 163 | 17.0 § | 100 | 100 | 71.4 | 3.1 | 4.7 | ||||
| Di Giorgio, 2020a | Prospective | GASTRIC | 28 | 46 | 1.00 | 20.0 § | 20.6 | 38.4 | 92.9 | 92.9 | 4.2 | 25.0 | 4.3 | 0.0 |
| Ellebæk, 2020b | Prospective | GASTRIC | 20 | 52 | 0.90 | 13.0 | 57.0 | 100 | 50.0 | 1.9 | 0.0 | |||
| Feldbrügge, 2021 | Retrospective | GASTRIC | 50 | 90 | 0.78 | 19.0 § | 100 | 100 | 4.4 | 26.0 | 5.6 | 0.0 | ||
| Gockel, 2018 | Prospective | GASTRIC | 24 | 46 | 1.00 § | 14.0 § | 83.3 | 41.7 | 9.6 | 29.2 | 0.0 | 0.0 | ||
| Khomyakov, 2016 | Phase II | GASTRIC | 31 | 56 | 16.0 § | 38.7 | 22.6 | 100 | 0.0 | 25.8 | 1.8 | 0.0 | ||
| Nadiradze, 2016 | Retrospective | GASTRIC | 24 | 60 | 16.0 | 79.2 | 33.3 | 6.3 | 41.7 | 11.7 | 4.2 | |||
| Sindayigaya, 2021 | Prospective | GASTRIC | 144 | 296 | 15.0 § | 15.0 § | 50.3 | 91.0 | 23.6 | 6.5 | 31.3 | 2.4 | 1.4 | |
| Struller, 2019 | Phase II | GASTRIC | 25 | 43 | 1.00 | 15.3 | 12.2 § | 100 | 0.0 | 2.3 | 24.0 | 7.0 | 0.0 | |
| Tidadini, 2021 | Retrospective | GASTRIC | 17 | 42 | 1.00 | 18.0 | 100 | 100 | 0.0 | 17.6 | ||||
| Subtotal, sum | 405 | 894 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 92 | 73 | 4 | 34 | 3 | ND | ||||
| Hilal, 2017 | Prospective | GYN | 84 | 18.9 | 100 | 7.1 | 38.1 | 0.0 | ||||||
| Rezniczek, 2020 | Retrospective | GYN | 44 | 150 | 0.70 | 68.2 | 4.7 | 8.0 | 0.0 | |||||
| Somashekhar, 2018 | Prospective | GYN | 3 | 9 | 2.00 § | 19.6 | 66.6 | 100 | 100 | 0.0 | 0.0 | |||
| Tempfer, 2014 | Case series | GYN | 18 | 34 | 17.3 | 76.2 | 100 | 8.1 | 22.2 | 14.7 | 0.0 | |||
| Tempfer, 2015a | Retrospective | GYN | 99 | 252 | 1.00 § | 16.6 | 45.0 | 100 | 6.7 | 34.3 | 7.9 | 0.0 | ||
| Tempfer, 2015b | Phase II | GYN | 64 | 130 | 0.43 | 16.3 | 42.0 | 82.8 | 0.0 | 7.8 | 53.1 | 6.9 | 0.0 | |
| Tempfer, 2018 | Phase I | GYN | 15 | 34 | 1.00 | 16.3 | 14.9 | 20.0 | 100 | 0.0 | 5.9 | 53.3 | 2.9 | 0.0 |
| Subtotal, sum | 327 | 609 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 100 | 11 | 6 | 42 | 7 | ND | ||||
| Di Giorgio, 2020b | Retrospective | HBP | 20 | 45 | 1.15 | 17.0 § | 40.0 | 100 | 55.0 | 0.0 | 55.0 | 0.0 | 0.0 | |
| Falkenstein, 2018 | Prospective | HBP | 13 | 17 | 1.57 | 20.0 | 11.4 | 53.8 | 23.1 | 10.5 | 7.7 | 0.0 | 0.0 | |
| Graversen, 2017 | Prospective | HBP | 5 | 16 | 0.60 | 20.0 | 100 | 20.0 | 0.0 | 60.0 | 0.0 | 0.0 | ||
| Horvath, 2018 | Prospective | HBP | 12 | 23 | 1.18 | 10.2 | 7.0 § | 25.0 | 83.3 | 0.0 | 25.0 | 0.0 | 0.0 | |
| Khosrawipour, 2017 | Prospective | HBP | 20 | 41 | 0.70 | 15.2 | 14.9 | 80.0 | 100 | 7.3 | 35.0 | 0.0 | 0.0 | |
| Nielsen, 2021 | Retrospective | HBP | 16 | 0.68 | 100 | 37.5 | 37.5 | |||||||
| Subtotal, sum | 86 | 142 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 95 | 37 | 2 | 34 | 0 | ND | ||||
| Giger-Pabst, 2018 | Retrospective | MESOTHELIOMA | 29 | 74 | 0.70 | 19.1 § | 95.0 | 72.4 | 24.1 | 12.3 | 41.4 | 4.1 | 3.4 | |
| Subtotal, sum | 29 | 74 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 72 | 24 | 12 | 41 | 4 | ND | ||||
| Alyami, 2017 | Prospective | VARIOUS | 73 | 164 | 19.0 § | 15.0 § | 47.9 | 100 | 87.7 | 3.0 | 42.5 | 9.8 | 6.8 | |
| Alyami, 2021b | Prospective | VARIOUS | 26 | 437 | 16.0 § | 100 | 100 | 0.0 | 0.0 | |||||
| Ceribelli, 2020 | Prospective | VARIOUS | 43 | 71 | 100 | 11.6 | 6.6 | 25.6 | 1.4 | 0.0 | ||||
| Cuadrado Ayuso, 2021 | Prospective | VARIOUS | 5 | 9 | 27.6 § | 27.5 | 55.0 | 100 | 100 | 0.0 | 40.0 | 0.0 | 0.0 | |
| De Simone, 2020 | Phase II | VARIOUS | 40 | 100 | 0.60 | 72.5 | 50.0 | 1.6 | 50.0 | 3.0 | 0.0 | |||
| Dumont, 2020 | Phase I | VARIOUS | 10 | 33 | 22.0 § | 16.5 § | 100 | 20.0 | 27.3 | 0.0 | ||||
| Girshally, 2016 | Prospective | VARIOUS | 21 | 12 | 11.5 | 42.9 | ||||||||
| Graversen, 2018a | Prospective | VARIOUS | 41 | 106 | 90.2 | 19.5 | 48.8 | 0.9 | 0.9 | |||||
| Graversen, 2018b | Prospective | VARIOUS | 35 | 129 | 14.1 | 37.1 | 91.4 | 14.3 | 0.0 | 77.1 | 3.9 | 0.0 | ||
| Graversen, 2019 | Prospective | VARIOUS | 33 | 65 | 8.6 | 97.0 | 36.4 | 4.6 | 36.4 | 0.0 | 0.0 | |||
| Hubner, 2017 | Retrospective | VARIOUS | 42 | 91 | 0.86 | 10.0 § | 95.2 | 2.4 | 3.2 | 42.9 | 1.1 | 0.0 | ||
| Katdare, 2019 | Retrospective | VARIOUS | 16 | 17 | 0.76 | 25.1 § | 76.4 | 81.3 | 12.5 | 10.5 | 0.0 | 11.8 | 5.8 | |
| Kim, 2021 | Phase I | VARIOUS | 16 | 24 | 0.87 | 17.0 § | 12.0 § | 68.8 | 100 | 4.0 | 0.0 | 4.2 | 0.0 | |
| Kurtz, 2018 | Prospective | VARIOUS | 71 | 142 | 19.3 | 35.2 | 84.5 | 59.2 | 7.7 | 28.2 | 3.5 | 1.4 | ||
| Odendahl, 2015 | Retrospective | VARIOUS | 91 | 158 | 1.00 | 16.0 | 85.7 | 5.7 | 2.0 | |||||
| Račkauskas, 2021 | Retrospective | VARIOUS | 15 | 34 | 8.0 § | 5.0 § | 70.0 | 100 | 53.3 | 8.8 | 0.0 | |||
| Robella, 2016 | Prospective | VARIOUS | 14 | 40 | 17.0 § | 100 | 92.9 | 0.0 | 71.4 | 0.0 | 0.0 | |||
| Robella, 2021 | Phase I-II | VARIOUS | 13 | 13 | 0.70 | 14.0 § | 38.0 | 0.0 | 0.0 | 38.5 | 0.0 | |||
| Sgarbura, 2019 | Retrospective | VARIOUS | 101 | 251 | 19.0 § | 46.0 | 92.1 | 46.5 | 3.2 | 47.5 | 6.4 | 1.0 | ||
| Siebert, 2021 | Prospective | VARIOUS | 134 | 397 | 18.0 | 100 | 3.5 | 3.5 | ||||||
| Solass, 2014 | Case series | VARIOUS | 3 | 12 | 3.00 | 12.0 | 100 | 0.0 | 66.7 | 8.3 | 0.0 | |||
| Somashekhar, 2019 | Prospective | VARIOUS | 7 | 21 | 0.92 | 17.4 | 27.0 | 100 | 100 | 0.0 | 0.0 | |||
| Taibi, 2021 | Retrospective | VARIOUS | 69 | 147 | 16.3 § | 16.0 § | 100 | 78.3 | 0.0 | 31.9 | 7.5 | 0.0 | ||
| Willaert, 2019 | Prospective | VARIOUS | 48 | 135 | 21.2 § | 37.5 | 89.6 | 58.3 | 2.2 | 58.3 | 12.6 | 0.0 | ||
| Subtotal, sum | 967 | 2608 | ||||||||||||
| Subtotal, weighted means | NA | NA | NA | ND | 96 | 48 | 2 | 40 | 4 | ND | ||||
| Total, sum | 1990 | 4719 | ||||||||||||
| Total, weighted means | ||||||||||||||
| with various | NA | NA | NA | ND | 95 | 46 | 4 | 39 | 4 | ND | ||||
| without various | NA | NA | NA | ND | 94 | 44 | 5 | 38 | 4 | ND |
| First Author, Year of Publication | Primary Tumor | Histological Response (%) | Radiological Response (%) | Overall Survival (Median) |
| Demtroder, 2015 | CRC | 78.6 | 15.7 # | |
| Ellebæk, 2020a | CRC | 66.7 | 37.6 | |
| Gockel, 2020 | CRC | 28.6 | 10.1 # | |
| Rovers, 2021 | CRC | 56.3 | 0.0 | 8.0 |
| Tabchouri, 2021 | CRC | 74.1 | 13.0 | |
| Subtotal, weighted means | 65 | 0 | NA | |
| Alyami, 2021a | GASTRIC | 19.1 | ||
| Di Giorgio, 2020a | GASTRIC | 69.2 | 12.3 # | |
| Ellebæk, 2020b | GASTRIC | 70.0 | 4.7 # | |
| Feldbrügge, 2021 | GASTRIC | |||
| Gockel, 2018 | GASTRIC | 53.8 | 4.0 # | |
| Khomyakov, 2016 | GASTRIC | 60.0 | ||
| Nadiradze, 2016 | GASTRIC | 70.6 | 15.4 | |
| Sindayigaya, 2021 | GASTRIC | 73.0 | 11.0 | |
| Struller, 2019 | GASTRIC | 75.0 | 12.0 | 6.7 |
| Tidadini, 2021 | GASTRIC | 12.8 | ||
| Subtotal, weighted means | 69 | 12 | NA | |
| Hilal, 2017 | GYN | |||
| Rezniczek, 2020 | GYN | 72.7 | BC 42.9, EC 25.0 | 19.6 # |
| Somashekhar, 2018 | GYN | 66.7 | ||
| Tempfer, 2014 | GYN | 37.5 | ||
| Tempfer, 2015a | GYN | 76.0 | 14.1 # | |
| Tempfer, 2015b | GYN | 76.5 | 3.2 | |
| Tempfer, 2018 | GYN | 63.6 | ||
| Subtotal, weighted means | 74 | 21 | NA | |
| Di Giorgio, 2020b | HBP | 90.9 | 10.3 # | |
| Falkenstein, 2018 | HBP | 80.0 | 2.8 # | |
| Graversen, 2017 | HBP | 80.0 | 0.0 | 14.0 |
| Horvath, 2018 | HBP | 83.3 | 13.9 # | |
| Khosrawipour, 2017 | HBP | 70.0 | 8.4 # | |
| Nielsen, 2021 | HBP | 43.8 | 9.9 # | |
| Subtotal, weighted means | 73 | 0 | NA | |
| Giger-Pabst, 2018 | MESOTHELIOMA | 30.0 | 26.6 # | |
| Subtotal, weighted means | 30 | - | NA | |
| Alyami, 2017 | VARIOUS | |||
| Alyami, 2021b | VARIOUS | |||
| Ceribelli, 2020 | VARIOUS | 42.9 | ||
| Cuadrado Ayuso, 2021 | VARIOUS | 50.0 | ||
| De Simone, 2020 | VARIOUS | 17.9 | 17.5 | 18.1 |
| Dumont, 2020 | VARIOUS | 20.0 | ||
| Girshally, 2016 | VARIOUS | 100 | 77.8 | |
| Graversen, 2018a | VARIOUS | |||
| Graversen, 2018b | VARIOUS | 66.7 | ||
| Graversen, 2019 | VARIOUS | 60.0 | ||
| Hubner, 2017 | VARIOUS | |||
| Katdare, 2019 | VARIOUS | |||
| Kim, 2021 | VARIOUS | 66.7 | 0.0 | |
| Kurtz, 2018 | VARIOUS | 25.6 | 11.8 # | |
| Odendahl, 2015 | VARIOUS | |||
| Račkauskas, 2021 | VARIOUS | 25.0 # | ||
| Robella, 2016 | VARIOUS | 35.7 | ||
| Robella, 2021 | VARIOUS | 0.0 | ||
| Sgarbura, 2019 | VARIOUS | 102.0 | ||
| Siebert, 2021 | VARIOUS | |||
| Solass, 2014 | VARIOUS | 100 | 6.2 | |
| Somashekhar, 2019 | VARIOUS | 57.1 | ||
| Taibi, 2021 | VARIOUS | 53.8 | ||
| Willaert, 2019 | VARIOUS | |||
| Subtotal, weighted means | 53 | 20 | NA | |
| Total, weighted means | ||||
| with various | 62 | 15 | NA | |
| without various | 68 | 11 | NA |
| Survival at: | 3 Months | 6 Months | 9 Months | 12 Months |
| ES (95% CI) | ES (95% CI) | ES (95% CI) | ES (95% CI) | |
|---|---|---|---|---|
| CRC | 0.87 (0.71–0.97) | 0.73 (0.56–0.88) | 0.60 (0.41–0.77) | 0.53 (0.35–0.71) |
| Demtroder, 2015 | 0.88 (0.64–0.99) | 0.76 (0.50–0.93) | 0.65 (0.38–0.86) | 0.59 (0.33–0.82) |
| Gockel, 2020 | 0.85 (0.55–0.98) | 0.69 (0.39–0.91) | 0.54 (0.25–0.81) | 0.46 (0.19–0.75) |
| GASTRIC | 0.71 (0.52–0.87) | 0.49 (0.25–0.73) | 0.35 (0.12–0.62) | 0.25 (0.05–0.51) |
| Di Giorgio, 2020a | 0.86 (0.67–0.96) | 0.71 (0.51–0.87) | 0.61 (0.41–0.78) | 0.50 (0.31–0.69) |
| Ellebæk, 2020b | 0.65 (0.41–0.85) | 0.40 (0.19–0.64) | 0.25 (0.09–0.49) | 0.15 (0.03–0.38) |
| Gockel, 2018 | 0.58 (0.37–0.78) | 0.33 (0.16–0.55) | 0.21 (0.07–0.42) | 0.13 (0.03–0.32) |
| GYN | 0.88 (0.82–0.93) | 0.77 (0.70–0.84) | 0.67 (0.59–0.75) | 0.59 (0.51–0.67) |
| Rezniczek, 2020 | 0.91 (0.78–0.97) | 0.82 (0.67–0.92) | 0.73 (0.57–0.85) | 0.66 (0.50–0.80) |
| Tempfer, 2015a | 0.86 (0.77–0.92) | 0.75 (0.65–0.83) | 0.65 (0.54–0.74) | 0.56 (0.45–0.66) |
| HBP | 0.76 (0.63–0.87) | 0.59 (0.42–0.75) | 0.46 (0.27–0.66) | 0.37 (0.21–0.54) |
| Di Giorgio, 2020b | 0.80 (0.56–0.94) | 0.65 (0.41–0.85) | 0.55 (0.32–0.77) | 0.45 (0.23–0.68) |
| Falkenstein, 2018 | 0.46 (0.19–0.75) | 0.23 (0.05–0.54) | 0.08 (0.00–0.36) | 0.08 (0.00–0.36) |
| Horvath, 2018 | 0.83 (0.52–0.98) | 0.75 (0.43–0.95) | 0.67 (0.35–0.90) | 0.58 (0.28–0.85) |
| Khosrawipour, 2017 | 0.80 (0.56–0.94) | 0.60 (0.36–0.81) | 0.50 (0.27–0.73) | 0.35 (0.15–0.59) |
| Nielsen, 2021 | 0.81 (0.54–0.96) | 0.69 (0.41–0.89) | 0.56 (0.30–0.80) | 0.44 (0.20–0.70) |
| MESOTELIOMA | - | - | - | - |
| Giger-Pabst, 2018 | 0.93 (0.77–0.99) | 0.86 (0.68–0.96) | 0.79 (0.60–0.92) | 0.72 (0.53–0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giorgio, A.; Macrì, A.; Ferracci, F.; Robella, M.; Visaloco, M.; De Manzoni, G.; Sammartino, P.; Sommariva, A.; Biacchi, D.; Roviello, F.; et al. 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1125. https://doi.org/10.3390/cancers15041125
Di Giorgio A, Macrì A, Ferracci F, Robella M, Visaloco M, De Manzoni G, Sammartino P, Sommariva A, Biacchi D, Roviello F, et al. 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis. Cancers. 2023; 15(4):1125. https://doi.org/10.3390/cancers15041125
Chicago/Turabian StyleDi Giorgio, Andrea, Antonio Macrì, Federica Ferracci, Manuela Robella, Mario Visaloco, Giovanni De Manzoni, Paolo Sammartino, Antonio Sommariva, Daniele Biacchi, Franco Roviello, and et al. 2023. "10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis" Cancers 15, no. 4: 1125. https://doi.org/10.3390/cancers15041125
APA StyleDi Giorgio, A., Macrì, A., Ferracci, F., Robella, M., Visaloco, M., De Manzoni, G., Sammartino, P., Sommariva, A., Biacchi, D., Roviello, F., Pastorino, R., Pires Marafon, D., Rotolo, S., Casella, F., & Vaira, M. (2023). 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis. Cancers, 15(4), 1125. https://doi.org/10.3390/cancers15041125