Gastric Cancer (GC) with Peritoneal Metastases (PMs): An Overview of Italian PSM Oncoteam Evidence and Study Purposes
Abstract
Simple Summary
Abstract
1. Introduction
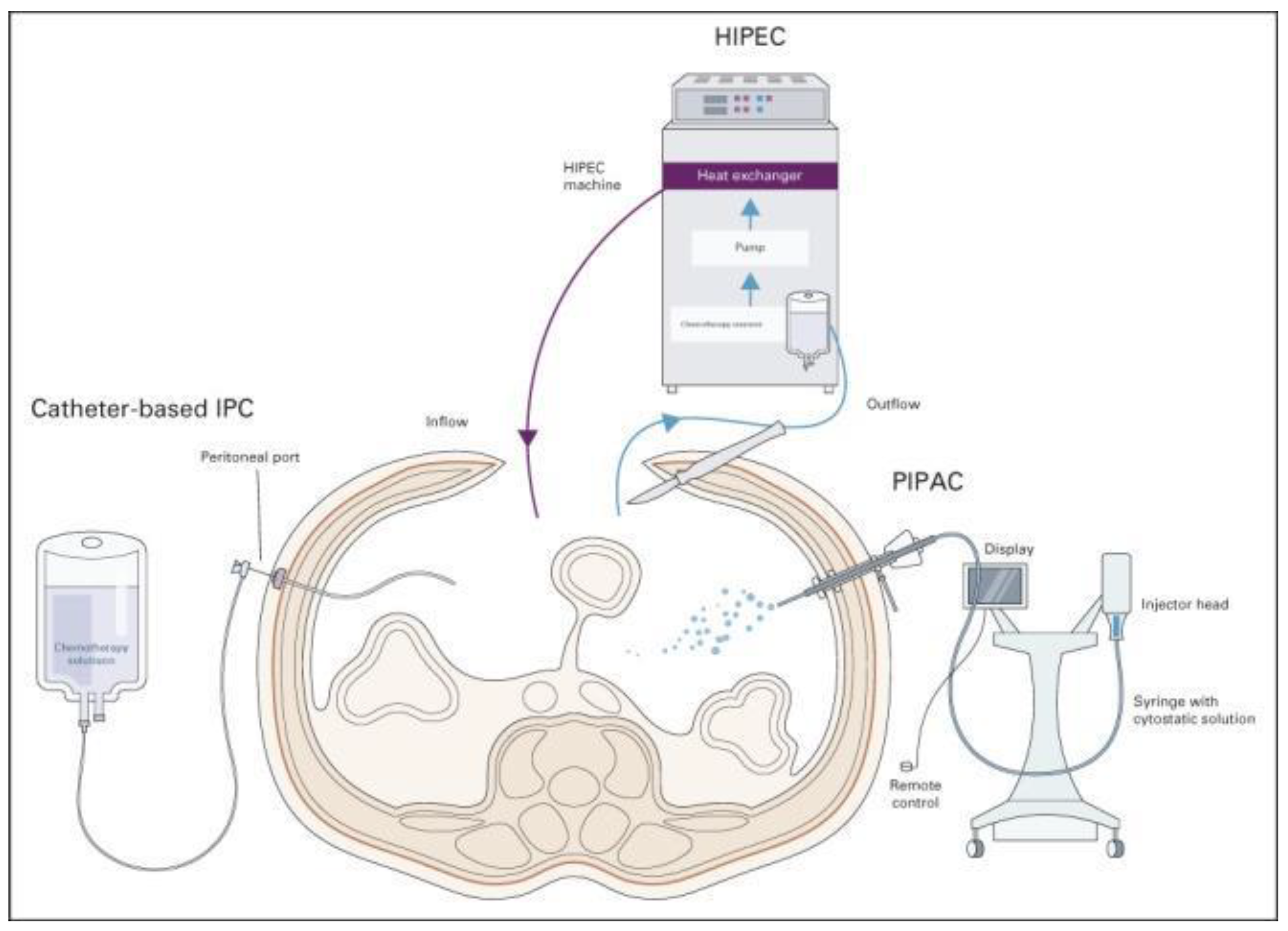
2. Neoadjuvant Chemotherapy for Patients with GC and Synchronous PMs
3. CRS Combined with HIPEC in Patients with GC and PMs
4. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
5. Metastatic GC (MGC): An Overview
6. Experience and Proposals of the Italian PSM Oncoteam
7. Sapienza Phase II NIPS Study (Grant Sapienza n RG12117A807F5D85)
8. PIPAC VEROne—A Randomized Multicenter Phase III Trial: Trial Registration EUDRACT 2021-000830-33; NCT 05303714
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study. E Clin. Med. 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, K.; Yang, H.-K.; Mizusawa, J.; Kim, Y.-W.; Terashima, M.; Han, S.-U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, version 2.2022, NCCN clinical practice Guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar]
- Kitayama, J.; Ishigami, H.; Yamaguchi, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Lefor, A.K.; Sata, N. Treatment of patients with peritoneal metastases from gastric cancer. Ann. Gastroenterol. Surg. 2018, 2, 116–123. [Google Scholar] [CrossRef]
- Nakamura, K.; Eto, K.; Iwagami, S.; Ogawa, K.; Sawayama, H.; Ishimoto, T.; Iwatsuki, M.; Baba, Y.; Miyamoto, Y.; Yoshida, N.; et al. Clinicopathological characteristics and prognosis of poorly cohesive cell subtype of gastric cancer. Int. J. Clin. Oncol. 2022, 27, 512–519. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, X.; Ji, C.; Shi, H.; Wang, Y.; Li, L.; Zhou, Z. Gastric poorly cohesive carcinoma: Differentiation from tubular adenocarcinoma using nomograms based on CT findings in the 40 s late arterial phase. Eur. Radiol. 2021, 31, 5768–5778. [Google Scholar] [CrossRef]
- Ishigami, H.; Yamaguchi, H.; Yamashita, H.; Asakage, M.; Kitayama, J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 2017, 20, 128–134. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, J.; Wang, T.; Chen, J.; Xu, H. Benefits of surgery after neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal metastasis: A meta-analysis. J. Surg. Res. 2020, 245, 234–243. [Google Scholar] [CrossRef]
- Kinoshita, J.; Yamaguchi, T.; Moriyama, H.; Fushida, S. Current status of conversion surgery for stage IV gastric cancer. Surg. Today 2021, 51, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.L. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin. Oncol. 1985, 12, 1–6. [Google Scholar] [PubMed]
- Yan, T.D.; Cao, C.Q.; Munkholm-Larsen, S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J. Gastrointest. Oncol. 2010, 2, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Solaß, W.; Hetzel, A.; Nadiradze, G.; Sagynaliev, E.; Reymond, M.A. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg. Endosc. 2012, 26, 1849–1855. [Google Scholar] [CrossRef]
- Brandl, A.; Yonemura, Y.; Glehen, O.; Sugarbaker, P.; Rau, B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: A multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 2021, 47, 172–180. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Terashima, M.; Mizusawa, J.; Katayama, H.; Nakamura, K.; Katai, H.; Yoshikawa, T.; Ito, S.; Kaji, M.; Kimura, Y.; et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): An open-label, phase 3, randomized controlled trial. Gastric Cancer 2021, 24, 492–502. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takashima, A.; Nagashima, K.; Terashima, M.; Aizawa, M.; Ohashi, M.; Tanaka, R.; Yamada, T.; Kinoshita, T.; Matsushita, H.; et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): A multi-institutional retrospective study. Gastric Cancer 2021, 24, 701–709. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer. JAMA Oncol. 2017, 3, 1237. [Google Scholar] [CrossRef]
- Bonnot, P.-E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.-M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with synchronous peritoneal metastases: Multicenter study of “Italian Peritoneal Surface Malignancies Oncoteam-S.i.c.o.”. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtröder, C.; Schüle, S.; Schlitt, H.-J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, I.; Pereira, F.; Rihuete-Caro, C.; Pérez-Viejo, E.; Serrano, Á.; Calvo, A.G.; Regueira, F.M.; Casado-Adam, Á.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: Multicenter study of Spanish group of peritoneal oncologic surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Brandl, A.; Thuss-Patience, P.; Bergner, F.; Raue, W.; Arnold, A.; Horst, D.; Pratschke, J.; Biebl, M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer 2019, 22, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, B. Regional therapy trials in peritoneal metastases: The path to standardization of care for gastric cancer. J. Surg. Oncol. 2022, 125, 64–68. [Google Scholar] [CrossRef]
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Ho, L.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J.A. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 3338–3344. [Google Scholar] [CrossRef]
- Yonemura, Y.; Ishibashi, H.; Hirano, M.; Mizumoto, A.; Takeshita, K.; Noguchi, K.; Takao, N.; Ichinose, M.; Liu, Y.; Li, Y. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann. Surg. Oncol. 2017, 24, 478–485. [Google Scholar] [CrossRef]
- Beeharry, M.K.; Ni, Z.T.; Yang, Z.Y.; Zheng, Y.N.; Feng, R.H.; Liu, W.T.; Yan, C.; Yao, X.X.; Li, C.; Yan, M.; et al. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): Dragon II trial. BMC Cancer 2020, 20, 224. [Google Scholar]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center. Paclitaxel for the Treatment of Gastric or Gastroesophageal Cancer. Clinicaltrials.gov Identifier NCT04220827. Available online: https://clinicaltrials.gov/ct2/show/NCT04220827 (accessed on 7 January 2020).
- Vatandoust, S.; Bright, T.; Roy, A.C.; Watson, D.; Gan, S.; Bull, J.; Abbas, M.N.; Karapetis, C.S. Phase I open-label trial of intraperitoneal paclitaxel in combination with intravenous cisplatin and oral capecitabine in patients with advanced gastric cancer and peritoneal metastases (IPGP study): Study protocol. BMJ Open 2019, 9, e026732. [Google Scholar] [CrossRef]
- Yonemura, Y.; Bandou, E.; Sawa, T.; Yoshimitsu, Y.; Endou, Y.; Sasaki, T.; Sugarbaker, P. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur. J. Surg. Oncol. 2006, 32, 661–665. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Intraperitoneal paclitaxel: Pharmacology, clinical results and future prospects. J. Gastrointest. Oncol. 2021, 12, S231–S239. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H.; Kitayama, J.; Kaisaki, S.; Kato, M.; Yamaguchi, H.; Otani, K.; Kamei, T.; Nagawa, H. Gastrectomy in combination with S-1, intravenous, and intraperitoneal paclitaxel: A novel multidisciplinary treatment strategy for gastric cancer with peritoneal metastasis. J. Clin. Oncol. 2010, 28, e14527. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takiguchi, S.; Nakajima, K.; Miyata, H.; Yamasaki, M.; Kurokawa, Y.; Mori, M.; Doki, Y. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J. Surg. Oncol. 2012, 105, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Fushida, S.; Kinoshita, J.; Kaji, M.; Hirono, Y.; Goda, F.; Yagi, Y.; Oyama, K.; Sudo, Y.; Watanabe, Y.; Fujimura, T.; et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother. Pharmacol. 2013, 71, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Kitayama, J.; Ishigami, H.; Emoto, S.; Yamashita, H.; Watanabe, T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer 2013, 119, 3354–3358. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H.; Kinoshita, J.; Kaji, M.; Hirono, Y.; Goda, F.; Yagi, Y.; Oyama, K.; Sudo, Y.; Watanabe, Y.; Fujimura, T.; et al. Phase III study of intraperitoneal paclitaxel plus s-1/paclitaxel compared with s-1/cisplatin in gastric cancer patients with peritoneal metastasis: PHOENIX-GC trial. J. Clin. Oncol. 2016, 34, 4014. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Ishigami, H.; Miwa, H.; Tanaka, T.; Kodera, Y.; Imamoto, H.; Imano, M.; Fukushima, R.; Hidemura, A.; Ueda, S.; et al. Phase II study of intraperitoneal paclitaxel plus S-1/oxaliplatin for gastric cancer with peritoneal metastasis: SOX+IP PTX trial. J. Clin. Oncol. 2016, 15, 4040. [Google Scholar] [CrossRef]
- Fukushima, R.; Ishigami, H.; Miwa, H.; Imano, M.; Kobayashi, D.; Tsuji, Y.; Hidemura, A.; Kusumoto, T.; Omori, T.; Yabusaki, H.; et al. Phase II study of intraperitoneal docetaxel plus capecitabine/cisplatin for gastric cancer with peritoneal metastasis: XP+IP DOC trial. J. Clin. Oncol. 2017, 15, 4039. [Google Scholar] [CrossRef]
- Cho, H.; Ryu, M.-H.; Kim, K.-P.; Ryoo, B.-Y.; Park, S.R.; Kim, B.S.; Lee, I.-S.; Kim, H.-S.; Yoo, M.-W.; Yook, J.H.; et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer 2017, 20, 970–977. [Google Scholar] [CrossRef]
- Shinkai, M.; Imano, M.; Chiba, Y.; Hiraki, Y.; Kato, H.; Iwama, M.; Shiraishi, O.; Yasuda, A.; Tsubaki, M.; Nishida, S.; et al. Intraperitoneal and systemic chemotherapy for patients with gastric cancer with peritoneal metastasis: A phase II trial. Anticancer Res. 2018, 38, 5975–5981. [Google Scholar] [CrossRef]
- Yonemura, Y.; Prabhu, A.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; Nishihara, K.; et al. Long term survival after cytoreductive surgery combined with perioperative chemotherapy in gastric cancer patients with peritoneal metastasis. Cancers 2020, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamaguchi, H.; Ohzawa, H.; Miyato, H.; Kanamaru, R.; Kurashina, K.; Hosoya, Y.; Lefor, A.K.; Sata, M.; Kitayama, J. Intraperitoneal administration of paclitaxel combined with S-1 plus oxaliplatin as induction therapy for patients with advanced gastric cancer with peritoneal metastases. Ann. Surg. Oncol. 2021, 28, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Mezhir, J.J.; Shah, M.A.; Jacks, L.M.; Brennan, M.; Coit, D.G.; Strong, V.E. Positive peritoneal cytology in patients with gastric cancer: Natural history and outcome of 291 patients. Ann. Surg. Oncol. 2010, 17, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J. Clin. Oncol. 2018, 36, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, H. Reply to Z. Li et al. J. Clin. Oncol. 2019, 37, 167–168. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Huang, H.; Yang, D.; Wang, P.; Huang, X.; Hu, Z.; Zhang, Y.; Yan, R.; Zhu, Z.; et al. Neoadjuvant intraperitoneal and systemic chemotherapy versus neoadjuvant systemic chemotherapy with docetaxel, oxaliplatin and S1 for gastric cancer with peritoneal metastasis: A propensity score matched analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211036310. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.-Y.; Yan, C.; Liu, W.-T.; Ni, Z.-T.; Yao, X.-X.; Hua, Z.-C.; Feng, R.-H.; Zheng, Y.-N.; Wang, Z.-Q.; et al. A phase III trial of neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis. Future Oncol. 2022, 18, 1175–1183. [Google Scholar] [CrossRef]
- Chan, D.Y.S.; Syn, N.L.-X.; Yap, R.; Phua, J.N.S.; Soh, T.I.P.; Chee, C.E.; Nga, M.E.; Shabbir, A.; So, J.B.Y.; Yong, W.P. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J. Gastrointest. Surg. 2017, 21, 425–433. [Google Scholar] [CrossRef]
- Kang, S.H.; Min, S.-H.; Kim, J.W.; Lee, E.; Park, S.W.; Lee, S.; Oh, H.J.; Park, Y.S.; Lee, Y.J.; Ahn, S.-H.; et al. Safety and efficacy of intraperitoneal paclitaxel plus intravenous fluorouracil, leucovorin, and oxaliplatin (FOLFOX) for gastric cancer with peritoneal metastasis. Ann. Surg. Oncol. 2022, 29, 5084–5091. [Google Scholar] [CrossRef]
- Yang, X.-J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.-L.; Zhou, Y.-F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Lintis, A.; Mercier, F.; Benzerdjeb, N.; Passot, G.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; et al. Prognosis of poorly cohesive gastric cancer after complete cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CYTO-CHIP study). Br. J. Surg. 2021, 108, 1225–1235. [Google Scholar] [CrossRef]
- Ji, Z.-H.; Yu, Y.; Liu, G.; Zhang, Y.-B.; An, S.-L.; Li, B.; Li, X.-B.; Yan, G.-J.; Li, Y. Peritoneal cancer index (PCI) based patient selecting strategy for complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in gastric cancer with peritoneal metastasis: A single-center retrospective analysis of 125 patients. Eur. J. Surg. Oncol. 2021, 47, 1411–1419. [Google Scholar] [CrossRef]
- Martins, M.; Santos-Sousa, H.; Araújo, F.; Nogueiro, J.; Sousa-Pinto, B. Impact of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer with peritoneal carcinomatosis: A systematic review and meta-analysis. Ann. Surg. Oncol. 2022, 29, 7528–7537. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef]
- D’Angelica, M.; Gonen, M.; Brennan, M.F.; Turnbull, A.D.; Bains, M.; Karpeh, M.S. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 2004, 240, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Squires, M.H.; Poultsides, G.A.; Fields, R.C.; Schmidt, C.; Weber, S.M.; Votanopoulos, K.; Maithel, S.K.; et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J. Am. Coll. Surg. 2014, 219, 664–675. [Google Scholar] [CrossRef]
- Beeharry, M.K.; Zhu, Z.L.; Liu, W.T.; Yao, X.X.; Yan, M.; Zhu, Z.G. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study. BMC Cancer 2019, 19, 932. [Google Scholar]
- Yarema, R.; Mielko, J.; Fetsych, T.; Ohorchak, M.; Skorzewska, M.; Rawicz-Pruszyński, K.; Mashukov, A.; Maksimovsky, V.; Jastrzębski, T.; Polkowski, W.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative Central-Eastern European study. Cancer Med. 2019, 8, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Hsu, C.-H.; Fan, H.-L.; Liao, G.-S.; Chen, T.-W.; Chan, D.-C. Prophylactic hyperthermic intraperitoneal chemotherapy for patients with clinical T4 gastric cancer. Eur. J. Surg. Oncol. 2022, 48, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef]
- Götze, T.O.; Piso, P.; Lorenzen, S.; Bankstahl, U.S.; Pauligk, C.; Elshafei, M.; Amato, G.; Reim, D.; Bechstein, W.O.; Königsrainer, A.; et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma—The phase III “PREVENT”-(FLOT9) trial of the AIO/CAOGI /ACO. BMC Cancer 2021, 21, 1158. [Google Scholar] [CrossRef]
- Rau, B.; Lang, H.; Königsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmüller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. 1376O The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM): A randomized multicentre phase III trial (GASTRIPEC-I-trial). Ann. Oncol. 2021, 32, S1040. [Google Scholar] [CrossRef]
- Van der Kaaij, R.T.; Wassenaar, E.C.E.; Koemans, W.J.; Sikorska, K.; Grootscholten, C.; Los, M.; Huitema, A.; Schellens, J.H.M.; Veenhof, A.A.F.A.; Hartemink, K.J.; et al. Treatment of peritoneal dissemination in stomach cancer patients with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): Rationale and design of the PERISCOPE study. JMIR Res. Protoc. 2017, 6, e136. [Google Scholar] [CrossRef]
- Van der Kaaij, R.T.; Wassenaar, E.C.E.; Koemans, W.J.; Sikorska, K.; Grootscholten, C.; Los, M.; Huitema, A.; Schellens, J.H.M.; Veenhof, A.A.F.A.; Hartemink, K.J.; et al. Treatment of PERItoneal disease in Stomach Cancer with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy: PERISCOPE I initial results. Br. J. Surg. 2020, 107, 1520–1528. [Google Scholar] [CrossRef]
- Koemans, W.J.; Kaaij, R.T.; Wassenaar, E.C.E.; Boerma, D.; Boot, H.; Sikorska, K.; Los, M.; Grootscholten, C.; Hartemink, K.J.; Veenhof, A.A.F.A.; et al. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the PERISCOPE I trial. J. Surg. Oncol. 2021, 123, 904–910. [Google Scholar] [CrossRef]
- Carneiro, F.; Lauwers, G.Y. Epithelial Tumours of the Stomach. In Morson and Dawson’s Gastrointestinal Pathology 180-222; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wu, H.; Rusiecki, J.A.; Zhu, K.; Potter, J.; Devesa, S.S. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1945–1952. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Hiraki, S.; Nomura, S.; Yamamoto, J.; Ueno, H. Diagnostic accuracy of T stage of gastric cancer from the view point of application of laparoscopic proximal gastrectomy. Mol. Clin. Oncol. 2018, 8, 773–778. [Google Scholar] [CrossRef]
- Koemans, W.J.; Van Der Kaaij, R.T.; Boot, H.; Buffart, T.; Veenhof, A.A.F.A.; Hartemink, K.J.; Grootscholten, C.; Snaebjornsson, P.; Retel, V.P.; Van Tinteren, H.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 2019, 19, 420. [Google Scholar] [CrossRef]
- Bao, D.; Yang, Z.; Chen, S.; Li, K.; Hu, Y. Construction of a nomogram model for predicting peritoneal dissemination in gastric cancer based on clinicopathologic features and preoperative serum tumor markers. Front. Oncol. 2022, 12, 844786. [Google Scholar] [CrossRef]
- Alyami, M.; Sgarbura, O.; Khomyakov, V.; Horvath, P.; Vizzielli, G.; So, J.; Torrent, J.; Delgadillo, E.X.; Martin, D.; Ceelen, W.; et al. Standardizing training for Pressurized Intraperitoneal Aerosol Chemotherapy. Eur. J. Surg. Oncol. 2020, 46, 2270–2275. [Google Scholar] [CrossRef]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Sindayigaya, R.; Dogan, C.; Demtröder, C.R.; Fischer, B.; Karam, E.; Buggisch, J.R.; Tempfer, C.B.; Lecomte, T.; Ouaissi, M.; Giger-Pabst, U. Clinical outcome for patients managed with low-dose cisplatin and doxorubicin delivered as pressurized intraperitoneal aerosol chemotherapy for unresectable peritoneal metastases of gastric cancer. Ann. Surg. Oncol. 2022, 29, 112–123. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Huang, L.; Jansen, L.; Verhoeven, R.H.; Ruurda, J.P.; Van Eycken, L.; De Schutter, H.; Johansson, J.; Lindblad, M.; Johannesen, T.B.; Zadnik, V.; et al. Largely varying patterns and trends of primary cancer-directed resection for gastric carcinoma with synchronous distant metastasis in Europe and the US: A population-based study calling for further standardization of care. Ther. Adv. Med. Oncol. 2021, 13, 17588359211027836. [Google Scholar] [CrossRef]
- Berger, Y.; Giurcanu, M.; Vining, C.C.; Schuitevoerder, D.; Posner, M.C.; Roggin, K.K.; Polite, B.N.; Liao, C.-Y.; Eng, O.S.; Catenacci, D.V.T.; et al. Cytoreductive surgery for selected patients whose metastatic gastric cancer was treated with systemic chemotherapy. Ann. Surg. Oncol. 2021, 28, 4433–4443. [Google Scholar] [CrossRef]
- Nohria, A.; Kaslow, S.R.; Hani, L.; He, Y.; Sacks, G.D.; Berman, R.S.; Lee, A.Y.; Correa-Gallego, C. Outcomes after surgical palliation of patients with gastric cancer. J. Surg. Res. 2022, 279, 304–311. [Google Scholar] [CrossRef]
- Davis, J.A.; Cui, Z.L.; Ghias, M.; Li, X.; Goodloe, R.; Wang, C.; Liepa, A.M.; Hess, L.M. Treatment heterogeneity and overall survival in patients with advanced/metastatic gastric or gastroesophageal junction adenocarcinoma in the United States. J. Gastrointest. Oncol. 2022, 13, 949–957. [Google Scholar] [CrossRef]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Rha, S.Y.; Bae, J.M.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Noh, S.H.; et al. International retrospective cohort study of conversion therapy for stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Li, Z.; Guan, G.; Liu, Z.; Li, J.; Ying, X.; Shan, F.; Li, Z. Predicting peritoneal carcinomatosis of gastric cancer: A simple model to exempt low-risk patients from unnecessary staging laparoscopy. Front. Surg. 2022, 9, 916001. [Google Scholar] [CrossRef] [PubMed]
- Solaini, L.; Bencivenga, M.; D’Ignazio, A.; Milone, M.; Marino, E.; De Pascale, S.; Rosa, F.; Sacco, M.; Romario, U.F.; Graziosi, L.; et al. Which gastric cancer patients could benefit from staging laparoscopy? A GIRCG multicenter cohort study. Eur. J. Surg. Oncol. 2022, 48, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Gęca, K.; Skórzewska, M.; Rawicz-Pruszyński, K.; Mlak, R.; Sędłak, K.; Pelc, Z.; Małecka-Massalska, T.; Polkowski, W.P. Prognostic value of molecular cytology by one-step nucleic acid amplification (OSNA) assay of peritoneal washings in advanced gastric cancer patients. Sci. Rep. 2022, 12, 12477. [Google Scholar] [CrossRef] [PubMed]
- Hasbahceci, M.; Akcakaya, A.; Guler, B.; Kunduz, E.; Malya, F.; Muslumanoglu, M. Use of peritoneal washing cytology for the detection of free peritoneal cancer cells before and after surgical treatment of gastric adenocarcinoma. J. Cancer Res. Ther. 2018, 14, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, H.; Elimova, E.; Slack, R.S.; Chen, H.-C.; Staerkel, G.A.; Sneige, N.; Shimodaira, Y.; Sagebiel, T.; Lee, J.H.; Bhutani, M.S.; et al. Prognosis of gastric adenocarcinoma patients with various burdens of peritoneal metastases. J. Surg. Oncol. 2016, 113, 29–35. [Google Scholar] [CrossRef]
- Kobayashi, H.; Honda, M.; Kawamura, H.; Takiguchi, K.; Muto, A.; Yamazaki, S.; Teranishi, Y.; Shiraso, S.; Kono, K.; Hori, S.; et al. Clinical impact of gastrectomy for gastric cancer patients with positive lavage cytology without gross peritoneal dissemination. J. Surg. Oncol. 2022, 125, 615–620. [Google Scholar] [CrossRef]
- Jamel, S.; Markar, S.R.; Malietzis, G.; Acharya, A.; Athanasiou, T.; Hanna, G.B. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: Systematic review and meta-analysis. Gastric Cancer 2018, 21, 10–18. [Google Scholar] [CrossRef]
- Casella, F.; Bencivenga, M.; Rosati, R.; Fumagalli, U.R.; Marrelli, D.; Pacelli, F.; Macrì, A.; Donini, A.; Torroni, L.; Pavarana, M.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in multimodal therapy for patients with oligometastatic peritoneal gastric cancer: A randomized multicenter phase III trial PIPAC VEROne. Pleura Peritoneum 2022, 7, 135–141. [Google Scholar] [CrossRef]
- Gwee, Y.X.; Chia, D.K.A.; So, J.; Ceelen, W.; Yong, W.P.; Tan, P.; Ong, C.-A.J.; Sundar, R. Integration of genomic biology into therapeutic strategies of gastric cancer peritoneal metastasis. J. Clin. Oncol. 2022, 40, 2830–2845. [Google Scholar] [CrossRef]

| Author, Year | IP Regimen | Systemic Regimen | Study | n | MST (mo) | 1 y OS (%) | Cytology Negative Conversion Rate (%) |
|---|---|---|---|---|---|---|---|
| Ishigami, 2010 [33] | PTX (20 mg/m2) | S-1 + PTX | P2 | 40 | 22.5 | 78 | 86 |
| Fujiwara, 2012 [34] | DTX (40~60 mg/m2) | S-1 | R/S | 18 | 24.6 | 76 | 78 |
| Fushida, 2013 [35] | DTX (45 mg/m2) | S-1 | P1/2 | 39 | 16.2 | 70.4 | 81 |
| Yamaguchi, 2013 [36] | PTX (20 mg/m2) | S-1 + PTX | P1 | 35 | 17.6 | 77.1 | 97 |
| Ishigami, 2016 [37] | PTX (20 mg/m2) | S-1 + PTX | P3 | 114 | 17.7 | 71.9 | 95 |
| Fujiwara, 2016 [38] | PTX (40 mg/m2) | S-1 + L-OHP | P2 | 60 | NR | 71.5 | 71 |
| Fukushima, 2017 [39] | DTX (10 mg/m2) | Cap + CDDP | P2 | 48 | NR | 75 | 76 |
| Cho, 2017 [40] | DTX (100 mg/m2) | Cap + CDDP | P1/2 | 39 | 15.1 | - | - |
| Shinkai, 2018 [41] | PTX (60 mg/m2) | S-1 + PTX + CDDP | P2 | 17 | 23.9 | 82.4 | - |
| Yonemura, 2020 [42] | DTX (30 mg/m2) + CDDP (30 mg/m2) | DTX + CDDP | R/S | 419 | 20.5 | 70 | 63.5 |
| Saito, 2021 [43] | PTX (40 mg/m2) | S-1 + L-OHP | R/S | 44 | 25.8 | 79.5 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammartino, P.; De Manzoni, G.; Marano, L.; Marrelli, D.; Biacchi, D.; Sommariva, A.; Scaringi, S.; Federici, O.; Guaglio, M.; Angrisani, M.; et al. Gastric Cancer (GC) with Peritoneal Metastases (PMs): An Overview of Italian PSM Oncoteam Evidence and Study Purposes. Cancers 2023, 15, 3137. https://doi.org/10.3390/cancers15123137
Sammartino P, De Manzoni G, Marano L, Marrelli D, Biacchi D, Sommariva A, Scaringi S, Federici O, Guaglio M, Angrisani M, et al. Gastric Cancer (GC) with Peritoneal Metastases (PMs): An Overview of Italian PSM Oncoteam Evidence and Study Purposes. Cancers. 2023; 15(12):3137. https://doi.org/10.3390/cancers15123137
Chicago/Turabian StyleSammartino, Paolo, Giovanni De Manzoni, Luigi Marano, Daniele Marrelli, Daniele Biacchi, Antonio Sommariva, Stefano Scaringi, Orietta Federici, Marcello Guaglio, Marco Angrisani, and et al. 2023. "Gastric Cancer (GC) with Peritoneal Metastases (PMs): An Overview of Italian PSM Oncoteam Evidence and Study Purposes" Cancers 15, no. 12: 3137. https://doi.org/10.3390/cancers15123137
APA StyleSammartino, P., De Manzoni, G., Marano, L., Marrelli, D., Biacchi, D., Sommariva, A., Scaringi, S., Federici, O., Guaglio, M., Angrisani, M., Cardi, M., Fassari, A., Casella, F., Graziosi, L., & Roviello, F. (2023). Gastric Cancer (GC) with Peritoneal Metastases (PMs): An Overview of Italian PSM Oncoteam Evidence and Study Purposes. Cancers, 15(12), 3137. https://doi.org/10.3390/cancers15123137









