A New Optimized Version of a Colorectal Cancer-Targeted Immunotoxin Based on a Non-Immunogenic Variant of the Ribotoxin α-Sarcin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
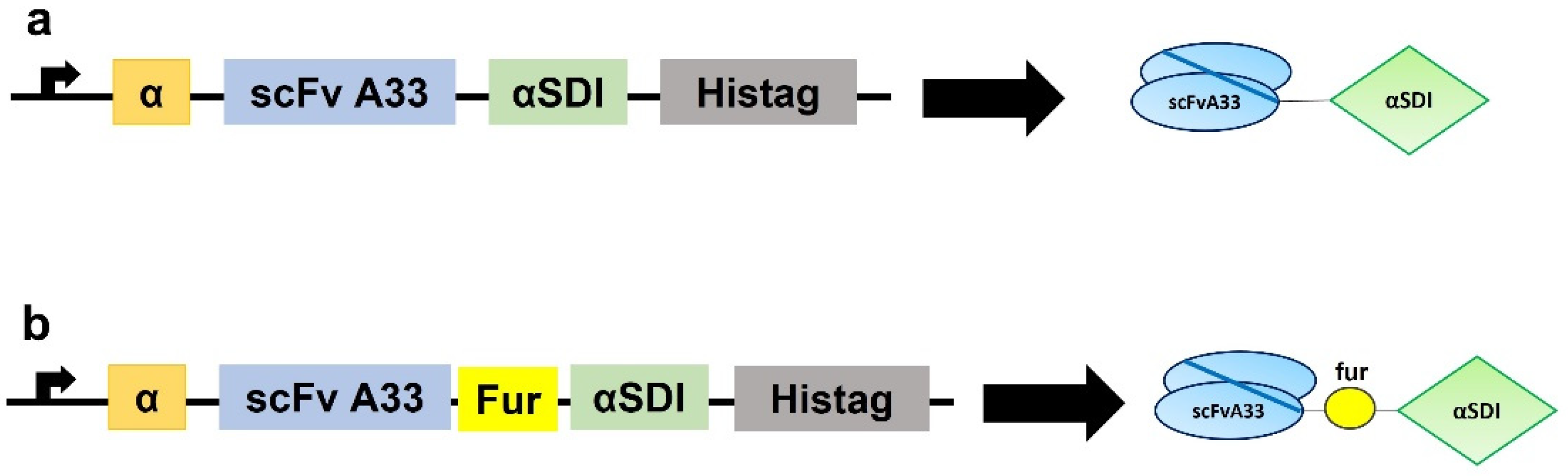
2.1. Plasmid Design
2.2. Cell Line Cultures
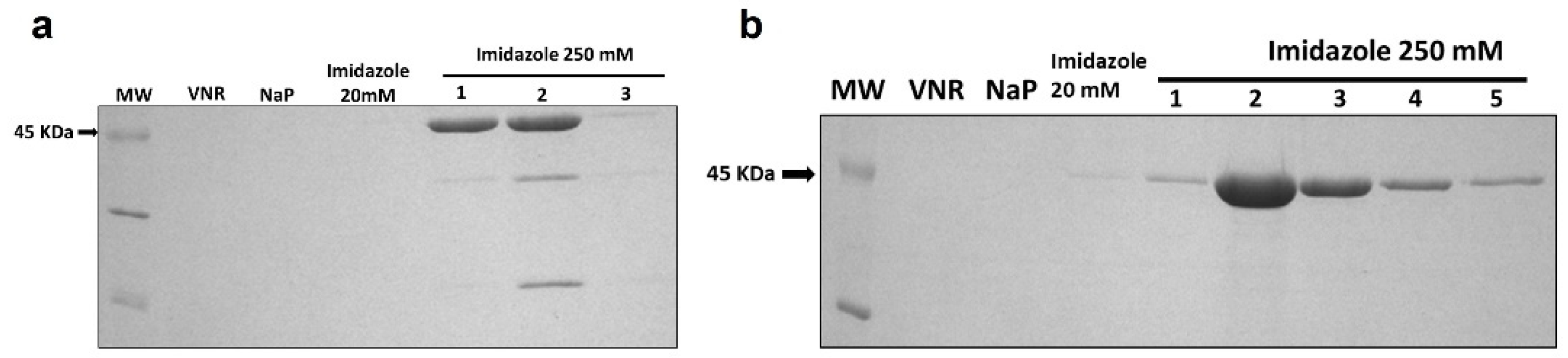
2.3. Protein Production and Purification
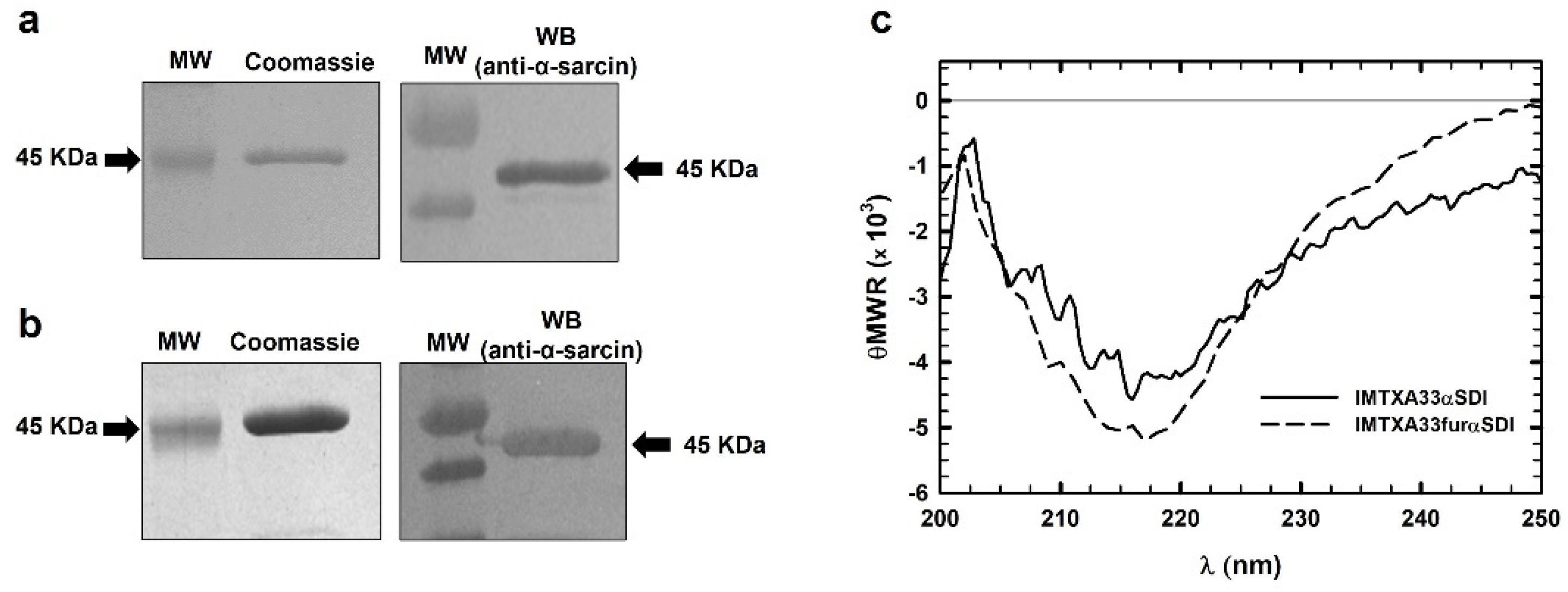
2.4. Structural Characterization
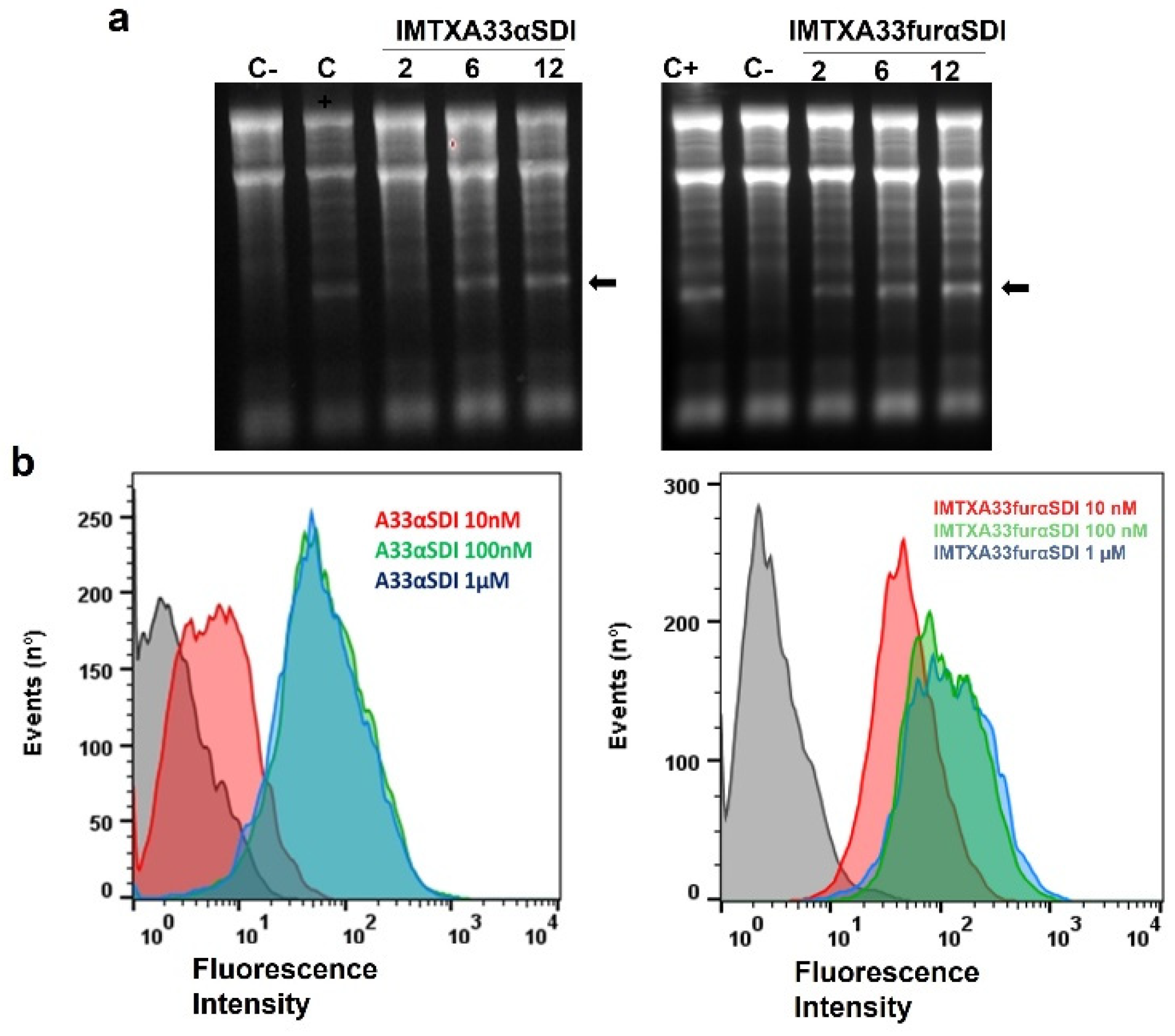
2.5. Ribonucleolytic Activity Assays
2.6. Flow Cytometry Studies
2.7. MTT Viability Assay
2.8. Immunological Assays
2.8.1. Monocytic Activation
2.8.2. PBMC Proliferation Assay
2.8.3. Cytokine Analysis
2.9. In vivo Antitumor Assays
2.10. Statistical Analysis
3. Results
3.1. Generation, Production, and Purification of Immunotoxins
3.2. Structural Characterization
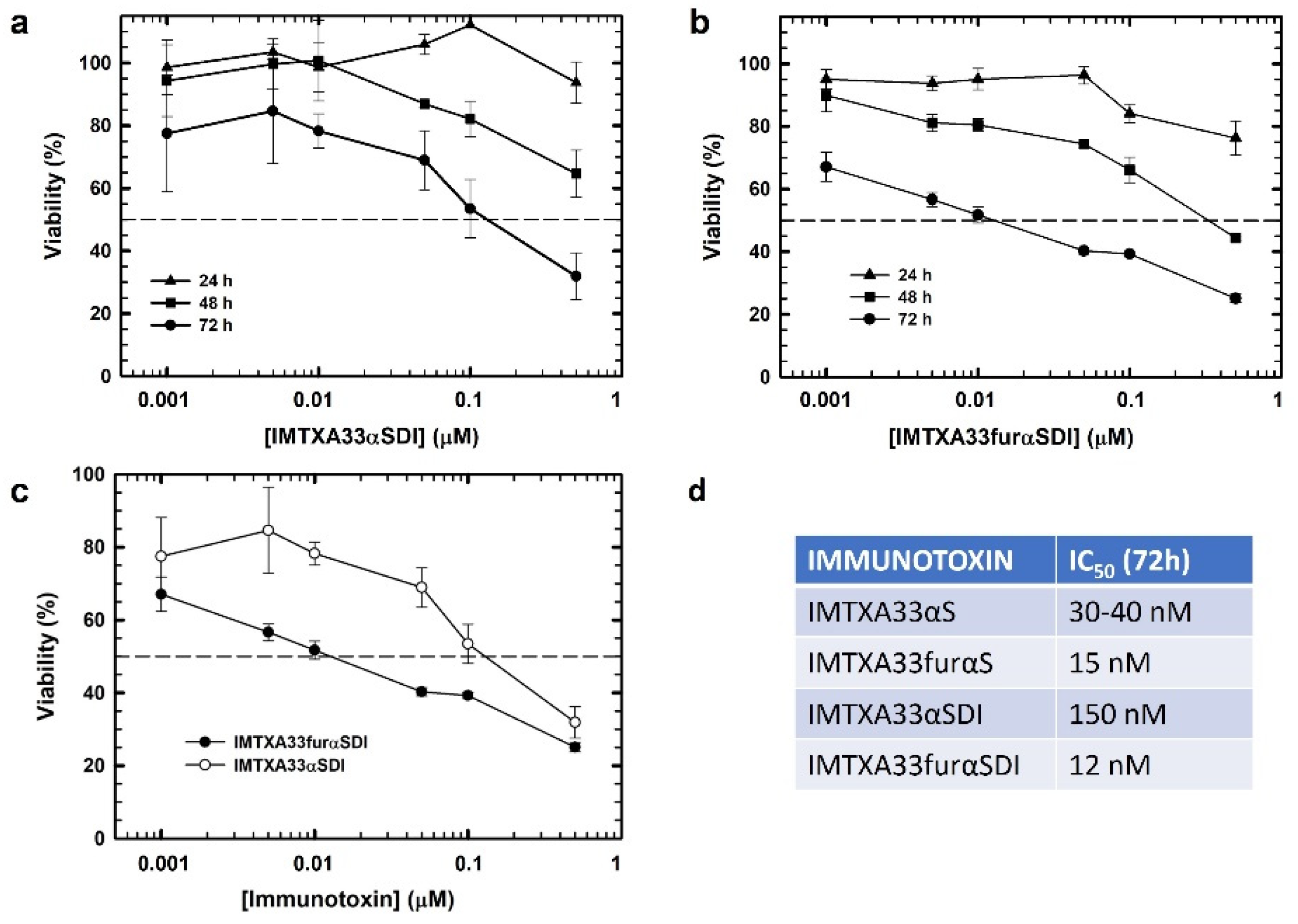
3.3. In Vitro Functional Characterizations of IMTXA33αSDI and IMTXA33furαSDI
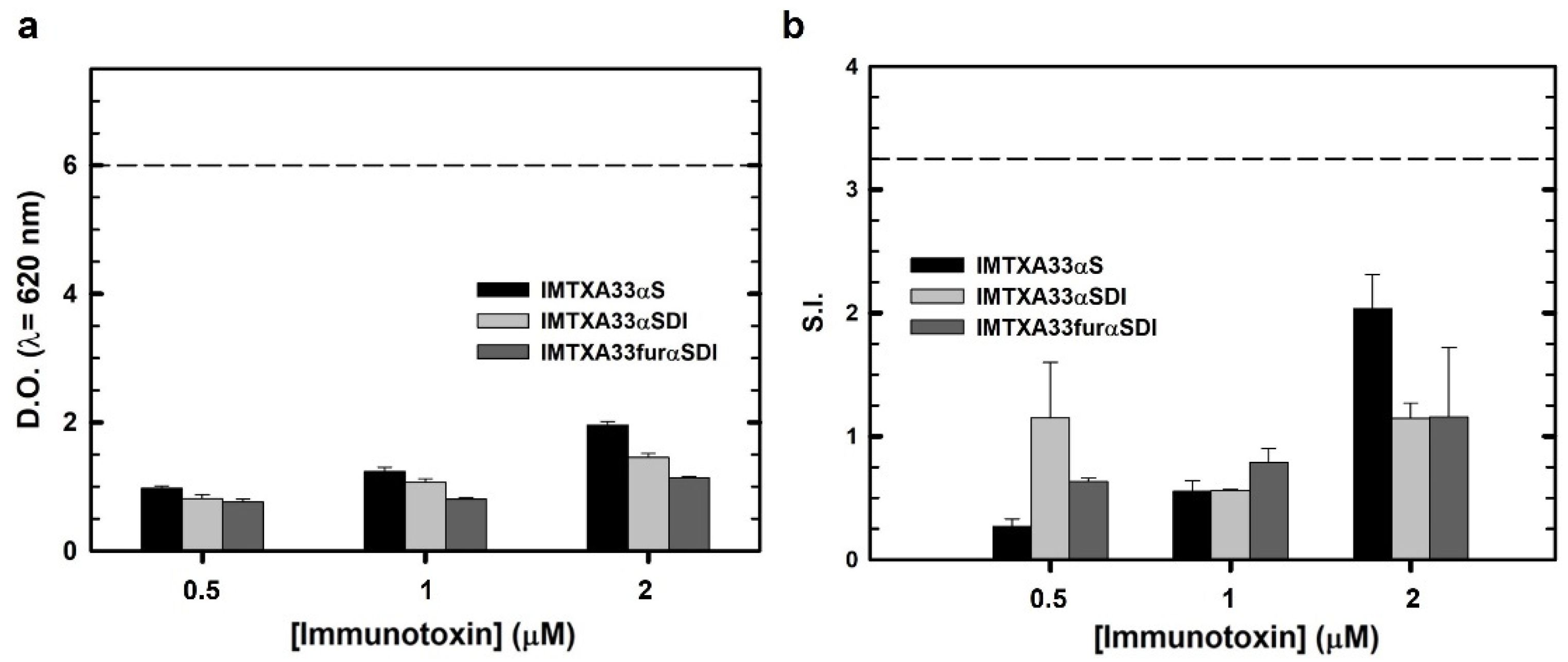
3.4. Immunological Characterization of Immunotoxins
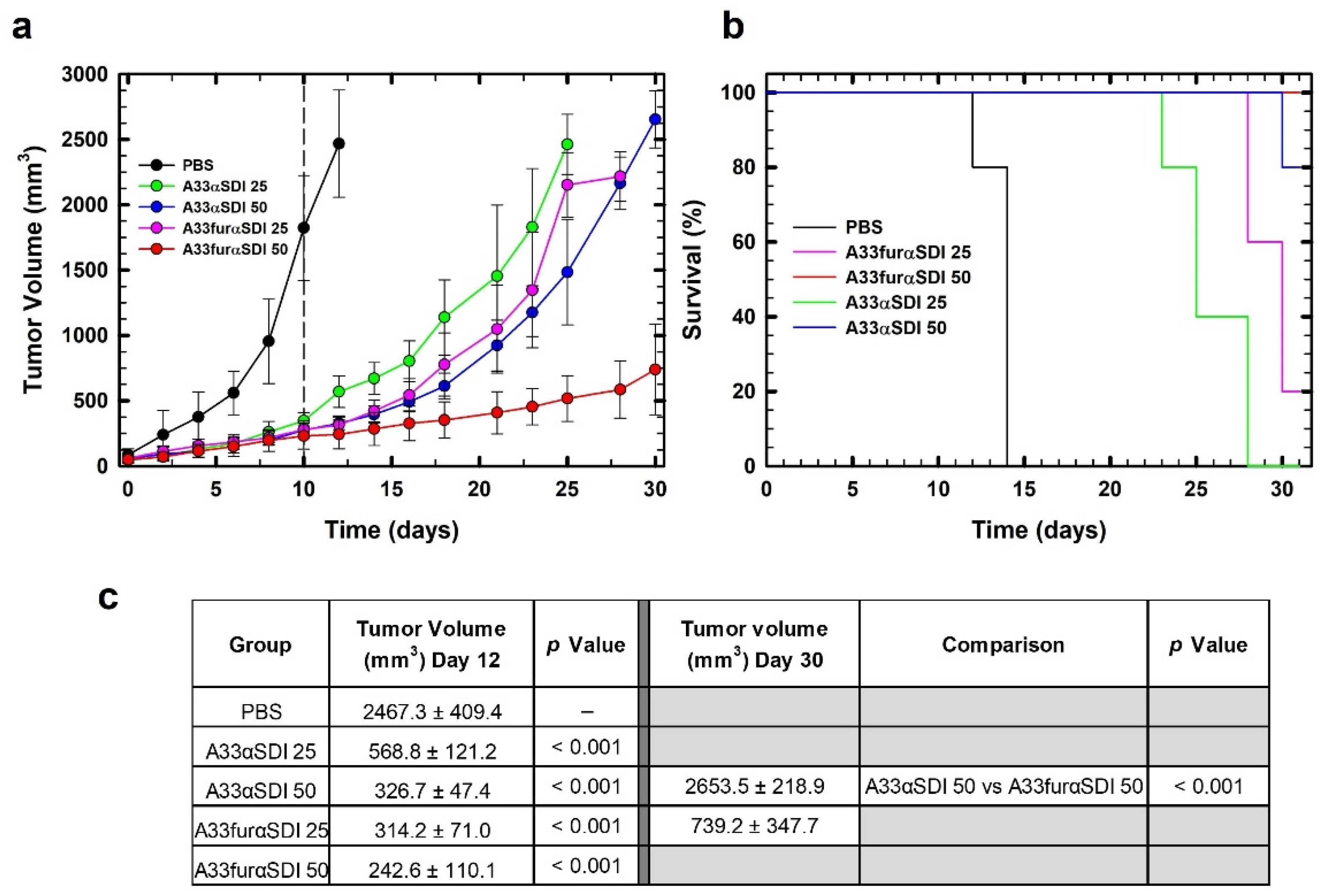
3.5. In Vivo Antitumor Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), 22–40. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Prim. 2016, 1, 15065. [Google Scholar] [CrossRef]
- Tintelnot, J.; Stein, A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges, and novel approaches. World J. Gastroenterol. 2019, 25, 3920–3928. [Google Scholar]
- Fercher, C.; Keshvari, S.; AMcGuckin, M.; Barnard, R.T. Evolution of the magic bullet: Single chain antibody fragments for the targeted delivery of immunomodulatory proteins. Exp. Biol. Med. 2018, 243, 166–183. [Google Scholar]
- Antignani, A.; Hei-Ho, E.C.; Bilotta, M.T.; Qiu, R.; Sarnvosky, R.; FitzGerald, D.J. Targeting receptors on cancer cells with protein toxins. Biomolecules 2020, 10, 1331. [Google Scholar] [CrossRef]
- Sanz, L.; Ibáñez-Pérez, R.; Guerrero-Ochoa, P.; Lacadena, J.; Anel, A. Antibody-based immunotoxins for colorectal cancer therapy. Biomedicines 2021, 9, 1729. [Google Scholar] [CrossRef]
- Rust, A.; Partridge, L.J.; Davletov, B.; Hautberg, G.M. The use of plant-derived ribosome inactivating proteins in immunotoxin development: Past, present and future generations. Toxins 2017, 9, 344. [Google Scholar] [CrossRef]
- Alewine, C.; Hassan, R.; Pastan, I. Advances in anticancer immunotoxin therapy. Oncologist 2015, 20, 176–185. [Google Scholar]
- Kim, J.S.; Jun, S.J.; Kim, Y.S. Critical Issues in the development of immunotoxins for anticancer therapy. J. Pharm. Sci. 2020, 109, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Catimel, B.; Ritter, G.; Welt, S.; Old, L.J.; Cohen, L.; Nerrie, M.A.; White, S.J.; Heath, J.K.; Demediuk, B.; Domagala, T.; et al. Purification and characterization of a novel restricted antigen expressed by normal and transformed human colonic epithelium. J. Biol. Chem. 1996, 271, 25664–25670. [Google Scholar]
- Heath, J.K.; White, S.J.; Johnstone, C.N.; Catimel, B.; Simpson, R.J.; Moritz, R.L.; Tu, G.F.; Ji, H.; Whitehead, R.H.; Groenen, L.C.; et al. The human A33 antigen is a transmembrane glycoprotein and a novel member of the immunoglobulin superfamily. Biochemistry 1997, 94, 469–474. [Google Scholar]
- Ackerman, M.E.; Chalouni, C.; Schmidt, M.M.; Raman, V.V.; Ritter, G.; Old, L.J.; Mellman, I.; Wittrup, K.D. A33 antigen displays persistent surface expression. Cancer Immunol. Immunother. 2008, 57, 1017–1027. [Google Scholar] [CrossRef]
- Carreras-Sangrà, N.; Tomé-Amat, J.; García-Ortega, L.; Batt, C.A.; Oñaderra, M.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Production and characterization of a colon cancer-specific immunotoxin based on the fungal ribotoxin α-sarcin. Protein Eng. Des. Sel. 2012, 25, 425–435. [Google Scholar] [PubMed]
- Tomé-Amat, J.; Menéndez-Méndez, A.; García-Ortega, L.; Batt, C.A.; Oñaderra, M.; Martínez-del-Pozo, Á.; Gavilanes, J.G.; Lacadena, J. Production and characterization of scFvA33T1, an immunoRNase targeting colon cancer cells. FEBS J. 2012, 279, 3022–3032. [Google Scholar] [CrossRef]
- Tomé-Amat, J.; Ruiz de la Herrán, J.; Martínez del Pozo, A.; Gavilanes, J.G.; Lacadena, J. α-Sarcin and RNase T1 based immunoconjugates: The role of intracellular trafficking in cytotoxic efficiency. FEBS J. 2015, 282, 673–684. [Google Scholar]
- Tomé-Amat, J.; Herrero-Galán, E.; Oñaderra, M.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Preparation of an engineered safer immunotoxin against colon carcinoma based on the ribotoxin hirsutellin A. FEBS J. 2015, 282, 2131–2141. [Google Scholar]
- Tomé-Amat, J.; Olombrada, M.; Ruiz-de-la-Herrán, J.; Pérez-Gómez, E.; Andradas, C.; Sánchez, C.; Martínez, L.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Efficient in vivo antitumor effect of an immunotoxin based on ribotoxin α-sarcin in nude mice bearing human colorectal cancer xenografts. SpringerPlus Med. 2015, 4, 168. [Google Scholar] [CrossRef]
- Ruiz-de-la-Herrán, J.; Tomé-Amat, J.; Lázaro-Gorines, R.; Gavilanes, J.G.; Lacadena, J. Inclusion of a furin cleavage site enhances antitumor efficacy against colorectal cancer cells of ribotoxin α-sarcin-or RNase T1-based immunotoxins. Toxins 2019, 11, 593. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Lagassé, D.; Pedras-Vasconcelos, J.; Golding, B.; Rosenberg, A.S. Evaluating and mitigating the immunogenicity of therapeutic proteins. Trends Biotechnol. 2018, 36, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; King, E.M.; Pastan, I. Strategies to reduce the immunogenicity of recombinant immunotoxins. Am. J. Pathol. 2018, 188, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Pastan, I. Immunogenicity of immunotoxins containing Pseudomonas exotoxin A: Causes, consequences, and mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Gotte, G.; Menegazzi, M. Biological activities of secretory RNases: Focus on their oligomerization to design antitumor drugs. Front. Immunol. 2019, 10, 2626. [Google Scholar] [CrossRef]
- Mungra, N.; Jordaan, S.; Hlongwane, P.; Naran, K.; Chetty, S.; Barth, S. Targeted human cytolytic fusion proteins at the cutting edge: Harnessing the apoptosis-inducing properties of human enzymes for the selective elimination of tumor cells. Oncotarget 2019, 10, 897–915. [Google Scholar]
- Lacadena, J.; Álvarez-García, E.; Carreras-Sangrà, N.; Herrero-Galán, E.; Alegre-Cebollada, J.; García-Ortega, L.; Oñaderra, M.; Gavilanes, J.G.; Martínez del Pozo, A. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237. [Google Scholar] [CrossRef]
- Olombrada, M.; Martínez-del-Pozo, A.; Medina, P.; Budia, F.; Gavilanes, J.G.; García-Ortega, L. Fungal ribotoxins: Natural protein-based weapons against insects. Toxicon 2014, 83, 69–74. [Google Scholar]
- Olombrada, M.; Lázaro-Gorines, R.; López-Rodríguez, J.C.; Martínez-del-Pozo, A.; Oñaderra, M.; Maestro-López, M.; Lacadena, J.; Gavilanes, J.G.; García-Ortega, L. Fungal ribotoxins: A review of potential biotechnological applications. Toxins 2017, 9, 71. [Google Scholar] [CrossRef]
- Jones, T.D.; Hearn, A.R.; Holgate, R.; Kozub, D.; Fogg, M.H.; Carr, F.J.; Baker, M.P.; Lacadena, J.; Gehlsen, K.R. A deimmunized form of the ribotoxin α-sarcin, lacking CD4+ T cell epitopes and its use as an immunotoxin warhead. Protein Eng. Des. Sel. 2016, 29, 531–540. [Google Scholar] [PubMed]
- Mazor, R.; Eberle, J.A.; Hu, X.; Vassall, A.N.; Onda, M.; Beers, R.; Lee, E.C.; Kreitman, R.J.; Lee, B.; Baker, D.; et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc. Natl. Acad. Sci. USA 2014, 111, 8571–8576. [Google Scholar]
- Ritter, G.; Cohen, L.S.; Williams, C.J.R.; Richards, E.C.; Old, L.J.; Welt, S. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res. 2001, 61, 6851–6859. [Google Scholar]
- Scott, A.M.; Lee, F.T.; Jones, R.; Hopkins, W.; MacGregor, D.; Cebon, J.S.; Hannah, A.; Chong, G.U.P.; Papenfuss, A.; Rigopoulos, A.; et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: Biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin. Cancer Res. 2005, 11, 4810–4817. [Google Scholar]
- Damasceno, L.; Lee, F.; Ritter, G.; Old, L.; Batt, C. High-level expression of a phage display-derived scFv in Pichia pastoris. Methods Mol. Biol. 2009, 562, 225–236. [Google Scholar]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 753–766. [Google Scholar]
- Weldon, J.E.; Skarzynski, M.; Therres, J.A.; Ostovitz, J.R.; Zhou, H.; Kreitman, R.J.; Pastan, I. Designing the furin–cleavable linker in recombinant immunotoxins based on Pseudomonas exotoxin A. Bioconj. Chem. 2015, 26, 1120–1128. [Google Scholar]
- Kao, R.; Martínez-Ruiz, A.; Martínez-del-Pozo, Á.; Crameri, R.; Davies, J. Mitogillin and related fungal ribotoxins. Methods Enzymol. 2001, 341, 324–335. [Google Scholar]
- Martínez-Ruiz, A.; García-Ortega, L.; Kao, R.; Lacadena, J.; Oñaderra, M.; Mancheño, J.M.; Davies, J.; Martínez-del-Pozo, Á.; Gavilanes, J.G. RNase U2 and α-sarcin: A study of relantionships. Methods Enzymol. 2001, 341, 335–351. [Google Scholar]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Corkum, C.P.; Ings, D.P.; Burgess, C.; Karwowska, S.; Kroll, W.; Michalak, T.I. Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPT) and standard density gradient. Immunology 2015, 16, 48. [Google Scholar] [CrossRef]
- Choi, P.; Reiser, H. IL-4: Role in disease and regulation of production. Clin. Exp. Immunol. 1998, 113, 317–319. [Google Scholar]
- Mata-Espinosa, D.; Hernándes-Pando, R. Interferón gamma: Aspectos básicos, importancia clínica y usos terapéuticos. Rev. Investig. Clin. 2008, 60, 421–431. [Google Scholar]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R.A. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.S.; Liu, X.L.; Hui, Q.; Lu, S.Y.; Qu, L.L.; Li, Y.S.; Zhou, Y.; Ren, H.L.; Hu, P. Clinical targeting recombinant immunotoxins for cancer therapy. Oncotargets Ther. 2017, 10, 3645–3665. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Onda, M.; Nagata, S.; Lee, B.; Kreitman, R.J.; Pastan, I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-tac(fv)-pe38 (lmb-2) improves antitumour activity and reduces animal toxicity and immunogenicity. Proc. Natl. Acad. Sci. USA 2000, 97, 8548–8553. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.M.; Steinberg, S.M.; Fowler, D.H.; et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci. Transl. Med. 2013, 5, 208ra147. [Google Scholar] [CrossRef]
- Rosenberg, A.S.; Sauna, Z.E. Immunogenicity assessment during the development of protein therapeutics. J. Pharm. Pharmacol. 2018, 70, 584–594. [Google Scholar]
- Williams, J.M.; Tsai, B. Intracellular trafficking of bacterial toxins. Curr. Opin. Cell Biol. 2016, 41, 51–56. [Google Scholar] [CrossRef]
- Goyal, A.; Batra, J.K. Inclusion of a furin-sensitive spacer enhances the cytotoxicity of ribotoxin restrictocin containing recombinant single-chain immunotoxins. Biochem. J. 2000, 345, 247–254. [Google Scholar] [CrossRef]
- Van de Ven, W.J.; Creemers, J.W.; Roebroek, A.J. Furin: The prototype mammalian subtilisin-like proprotein processing enzyme. Endoproteolytic cleavage at paired basic residues of proproteins of the eukaryotic secretory pathway. Enzyme 1991, 45, 257–270. [Google Scholar]
- Gruenberg, J. Lipids in endocytic membrane transport and sorting. Curr. Opin. Cell Biol. 2003, 15, 382–388. [Google Scholar]
- Zhao, P.; Liu, F.; Zhang, B.; Liu, X.; Wang, B.; Gong, J.; Yu, G.; Ma, M.; Lu, Y.; Sun, J.; et al. MAIGO2 is involved in abscisic acid-mediated response to abiotic stresses and Golgi-to-ER retrograde transport. Physiol. Plant. 2013, 148, 2246–2260. [Google Scholar] [CrossRef]
- Yeung, V.P.; Chang, J.; Miller, J.; Barnett, C.; Stickler, M.; Hardling, F.A. Elimination of an inmunodominant CD4+ T cell epitope in human IFN-beta does not result in an in vivo response directed at the subdominant epitope. J. Immunol. 2004, 172, 6658–6665. [Google Scholar] [CrossRef]
- Mazor, R.; Crown, D.; Addissie, S.; Jang, Y.; Kaplan, G.; Pastan, I. Elimination of murin and human T-cell epitopes in recombinant immunotoxin eliminates neutralizing and anti-drug antibodies in vivo. Cell Mol. Immunol. 2017, 14, 432–442. [Google Scholar] [CrossRef]
- Tassignon, J.; Burny, W.; Dahmani, S.; Zhou, L.; Stordeur, P.; Byl, B.; De Groote, D. Monitoring of cellular responses after vaccination against tetanus toxoid: Comparison of the measurement of IFN-γ production by ELISA, ELISPOT, flow cytometry and real-time PCR. J. Immunol. Methods 2005, 305, 188–198. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of inteleukin-10. J. Exp. Med. 2019, 217, e20190418. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narbona, J.; Gordo, R.G.; Tomé-Amat, J.; Lacadena, J. A New Optimized Version of a Colorectal Cancer-Targeted Immunotoxin Based on a Non-Immunogenic Variant of the Ribotoxin α-Sarcin. Cancers 2023, 15, 1114. https://doi.org/10.3390/cancers15041114
Narbona J, Gordo RG, Tomé-Amat J, Lacadena J. A New Optimized Version of a Colorectal Cancer-Targeted Immunotoxin Based on a Non-Immunogenic Variant of the Ribotoxin α-Sarcin. Cancers. 2023; 15(4):1114. https://doi.org/10.3390/cancers15041114
Chicago/Turabian StyleNarbona, Javier, Rubén G. Gordo, Jaime Tomé-Amat, and Javier Lacadena. 2023. "A New Optimized Version of a Colorectal Cancer-Targeted Immunotoxin Based on a Non-Immunogenic Variant of the Ribotoxin α-Sarcin" Cancers 15, no. 4: 1114. https://doi.org/10.3390/cancers15041114
APA StyleNarbona, J., Gordo, R. G., Tomé-Amat, J., & Lacadena, J. (2023). A New Optimized Version of a Colorectal Cancer-Targeted Immunotoxin Based on a Non-Immunogenic Variant of the Ribotoxin α-Sarcin. Cancers, 15(4), 1114. https://doi.org/10.3390/cancers15041114






